Abstract
Background
In diabetes foot ulceration may result from increased skin fragility. Retinoids can reverse some diabetes-induced deficits of skin structure and function but their clinical utility is limited by skin irritation. The effect of diabetes and MDI 301 a non-irritating synthetic retinoid and retinoic acid have been evaluated on matrix metalloproteinases (MMPs), procollagen expression and skin structure in skin biopsies from non-diabetic volunteers and diabetic subjects at risk of foot ulceration using organ culture techniques.
Methods
Zymography and ELISA were utilized for analysis of MMP-1,-2,-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1) and immunohistochemisty for type I procollagen protein abundance. Collagen structure parameters were assessed in formalin-fixed, paraffin-embedded tissue sections.
Results
The % of active MMP-1 and -9 was higher and TIMP-1 abundance lower in subjects with diabetes. Type 1 procollagen abundance was reduced and skin structural deficits were increased in diabetes. Three μm MDI 301 reduced active MMP-1 and -9 abundance by 29% (p<0.05) and 40% (p<0.05), respectively and increased TIMP-1 by 45% (p=0.07). MDI 301 increased type 1 procollagen abundance by 40% (p<0.01) and completely corrected structural deficit scores. Two μm retinoic acid reduced MMP-1 but did not significantly affect skin structure.
Conclusions
These data indicate that diabetic patients at risk of foot ulceration have deficits of skin structure and function. MDI 301 offers potential for repairing this skin damage complicating diabetes.
Keywords: Diabetes, skin, retinoids, foot ulceration
Introduction
Diabetic foot ulceration remains a cause of considerable morbidity (Edmonds et al., 1999 & 2004; Boulton, Kirsner & Vileikyte, 2004; Pecoraro, Reiber & Burgess, 1990). Although early identification of foot insensitivity, vascular insufficiency and deformities have helped reduced the incidence of foot complications, chronic ulceration remains one of the most common and most serious consequences of diabetes. Progressive atrophy of dermal connective tissue has been identified as an important factor predisposing to increased skin fragility and ultimately wound formation (Teno et al., 1999; Loots, Lamme, Mekkes, Bos & Middelkoop, 1999; Lateef, Aslam, Stevens & Varani, 2005; Lateef, Stevens & Varani, 2004; Kang et al., 1995). Pathologically, a number of deficits have been identified in chronic skin wounds which fail to heal including reduced fibroblast numbers and proliferative capacity, reduced procollagen synthesis and increased levels of connective tissue-degrading matrix metalloproteinases (MMPs) (Teno et al., Lateef, Stevens & Varani, 2004; Varani et al., 2000; Bizot-Foulon, Bouchard, Homebeck, Dubertret, Bertaux, 1995).
The salutary effects of biologically active retinoids on overall appearance of skin damaged by ultraviolet radiation has been recognised for many years (Weis et al., 1988; Kligman, Dogadkina & Lavker, 1993). Treatment of aged skin with retinoids stimulates proliferation of dermal fibroblasts and reduces the expression of a wide range of matrix MMPs. These include MMP-1 (interstitial collagenase), MMP-3 (stromelysin-1) and MMP-9 (92-kd gelatinase B) (Varani, Perone, Fligiel, Inman & Voorhees, 1994; Varani, Fisher, Kang & Voorhees, 1998). In concert, retinoids upregulate tissue inhibitor of MMP (TIMP-1) (Braunhut & Moses, 1994; Bigg, McLeod, Waters, Cawston & Clark, 2000) and stimulate procollagen synthesis (Varani, Perone, Fligiel, Inman, Voorhees, 1994). We have found that there are many parallels between the structural and functional defects identified in aged skin with that in diabetes. For example in diabetes, there is dermal atrophy of skin in the lower limb which is associated with increased elaboration of MMPs (Lateef , Stevens & Varani, 2004).
However retinoids are irritating (Griffiths et al., 1995; Philips et al., 2002) which could lead to discontinuation of use or lowering of dose to levels that become clinically ineffective. Also significant irritation may attenuate any beneficial effects of retinoids on skin structure and could even provoke skin ulceration in susceptible individuals. Indeed subjects with diabetes and foot complications such as charcot neuroarthropathy exhibit a range of skin vascular and inflammatory complications which may predispose them to augmented skin inflammatory responses and irritation (Stevens, Edmonds, Foster & Watkins, 1992). Determining the efficacy and safety of retinoids in such high risk subjects is therefore critical.
Molecular Design International Inc. MDI 301 is a synthetic retinoid which retains the ability to inhibit MMPs and stimulate collagen synthesis (Varani et al., 2003; Appelyard et al., 2004) but has less potential to produce irritation. These properties could be particularly beneficial in the delicate high-risk skin that complicates diabetes. We report the effects of diabetes and its complications on skin structure and function and the impact of MDI 301 on these deficits in these high risk subjects.
Material and methods
Human skin organ cultures
Organ cultures of human skin were prepared as described previously (Lateef, Stevens & Varani, 2004). In brief, four 3 mm full-thickness punch biopsies of upper leg skin were obtained which were immersed in MCDB-153 (Sigma-Aldrich, Gillingham, Dorset, UK) culture medium containing 1.4mM CaCl2 and 6 mM glucose. The biopsies were incubated in a 24-well dish containing 260 ul of Ca2+ supplemented MCDB-153 medium, or addition of 1uM, 3uM MDI 301, or 2uM RA (Sourced by Nova Laboratories, Leicester, UK from BR Biopharma Ltd, Hertfordshire, UK). Cultures were incubated at 37°C in an atmosphere of 95% air and 5% CO2 for 8 days and the cultured media were collected and replaced every second day. The participation of human subjects in this project was approved by the local ethics committees, and all subjects provided written informed-consent prior to their inclusion in the study.
MMP assays
Organ cultured media were assayed for MMP-1, MMP-2 (72-kDa gelatinase) and MMP-9 by casein and gelatine zymography, respectively as described previously (Gibbs et al, 1999). Zymographic images were digitized and quantified by scanning densitometry. Quantitative values for MMP-1, MMP-2 and MMP-9 were obtained and normalised against MMPs normalisation standards.
Tissue inhibitor of Metalloproteinase-1
Culture fluids were assayed for TIMP-1 by ELISA using a commercially-available assay kit (R&D systems, Abingdon, Oxfordshire, UK).
Formalin-fixed, paraffin-embedded tissue
After culture, tissue was fixed in 10% formal saline and processed for paraffin histology. Sections (4 μm) were stained with hematoxylin & eosin and blinded. To asses collagen structure four parameters were evaluated. These included: 1) fibril thickness, 2) space between fibres, 3) degree of organisation and 4) depth of any disorganisation, using a scale of 1-9 for each parameter, where 1 was normal and 9 is maximal fibre damage. These parameters have proven useful in the past to document connective tissue fiber damage in aged skin (Varani et al., 2000).
Immunohistochemistry
Paraffin sections (4μm) were de-waxed and endogenous peroxidases removed. After antigen retrieval, non specific staining was blocked followed by incubation with antibodies to type 1 pro-collagen (Millipore, Watford, Hertfordshire, UK) (1: 400) overnight at 4°C. After washing, the immunoreactivity was revealed using a Vectastain universal elite ABC kit (Vector Laboratories Ltd, Peterborough, Cambridgeshire, UK) and diaminobenzidine (DAKO, Ely, Cambridgeshire, UK). Sections are counterstained with Mayers Haematoxylin, dehydrated and mounted. The area and density of staining within an area of 1000 X 1000 pixels was determined by image analysis after subtraction of background staining and the product calculated and presented as the overall abundance.
Statistical Analysis
Data analysis was performed using SPSS 15.0 for Windows (LEAD Technologies Inc.). Continuous data are presented as mean ± SD. The two-tailed independent t test was used to assess the impact of diabetes on the study outcomes. To assess the impact of interventions on the study outcomes across the multiple independent groups, one way analysis of variance (ANOVA) was used. Equality of group variances was tested using the homogeneity of variance test. If the assumption of equal variance was violated, the Welch statistic was used to test for the equality of group means. Post-hoc analysis was performed using the Tukey method if equal variances assumed and the Games-Howell method if equal variances not assumed. A p value ≤0.05 was considered significant.
Results
Patients
Twenty seven high-risk subjects with diabetes (mean age 58 ± 8 years range 42-70 years, 18 male, 9 female) were recruited. The mean HbA1c was 8.5 ± 1.2%. All subjects underwent a comprehensive neurological assessment and the Michigan Neuropathy Screening Instrument examination score was 5.1 ± 1.9 consistent with the presence of clinically significant peripheral neuropathy (Feldman et al., 1994). Eleven subjects had a history of recurrent foot ulcers. Eight patients had a history of charcot arthropathy confirmed by radiological examination. A group of 12 nondiabetic control subjects (mean age 45 ± 15 years, range 27-70 years, 6 male, 6 female) was recruited for comparison. All the subjects underwent skin biopsy from the upper leg.
Effect of diabetes on MMP-1, MMP-2 and MMP-9 abundance and activity
Initially we examined the effect of diabetes on MMP abundance and activity. Levels of total (active and latent forms) MMP-1, -2 and -9 were not significantly different in the culture fluid from skin biopsies taken from non-diabetic and diabetic subjects (Table 1). However the diabetic skin exhibited marked activation of the degrading MMPs (Table 1). For example, in nondiabetic subjects, 33% of skin MMP-1 was found to be in the active form (i.e. 67% was inactive). In our subjects with diabetes, the % of activated MMP-1 had significantly increased to 51% (p<0.05). In a similar fashion, in nondiabetic subjects, 27% of MMP-2 was active and in subjects with diabetes, this had increased to 36% (p=0.1). Finally, 30% of MMP-9 was active in subjects without diabetes, compared to 47% (p<0.05) in diabetic subjects.
Table 1.
Effect of diabetes on MMP abundance and activity and type 1 procollagen abundance
| MMP-1 (% active) |
MMP-2 (% active |
MMP-9 (% active |
TIMP-1 (ng/ml) |
Type 1 Procollagen |
|
|---|---|---|---|---|---|
|
Non-
diabetic |
0.8±0.5 (33±12) | 2.4±1.1 (27±14) | 1.3±0.8 (30±28) | 157±44 | 720 ± 103 |
| Diabetic | 0.8±0.4 (51±21*) | 2.8±1.1 (36±13) | 1.3±0.8 (47±32*) | 125±76 | 495 ± 175** |
p<0.05 versus non-diabetic control,
p<0.001 vs non-diabetic control
Effect of diabetes on TIMP-1 abundance
Increased expression of MMPs in response to diabetes may reflect a reduction in TIMP. We therefore explored the effect of diabetes on the abundance of TIMP-1 which is the major MMP inhibitor in skin. Levels of TIMP-1 were reduced by 19% (p=0.07) in high-risk diabetic subjects (Table 1).
Effect of diabetes on type 1 procollagen abundance
Type I collagen protein is the most abundant skin structural protein (Talwar, Griffiths, Fisher, Hamilton & Voorhees, 1995). We therefore sought to explore the effect of diabetes on type 1 procollagen abundance using immunohistochemistry. Diabetes was found to reduce the abundance of type 1 procollagen by 31% (p=0.001) (Table 1).
Skin Structural Deficit Scores in Healthy Controls and Subjects with Diabetes
Four parameters of collagen structure were assessed in formalin-fixed, paraffin-embedded tissue sections. Diabetes was found to significantly disrupt all measured parameters of skin structure (Table 2). The mean skin structural deficit score was significantly higher in subjects with diabetes than healthy controls (4.0 ± 0.9) vs (2.7 ± 0.7, p=0.002, respectively).
Table 2.
Effect of diabetes, synthetic retinoid and retinoic acid on skin structure
| Fibril Thickness |
Space Between Fibrils |
Degree of organisation |
Depth of disorganisation |
Mean Score | |
|---|---|---|---|---|---|
| Non- diabetic |
|||||
| Untreated | 2.2 ± 0.6 | 2.5 ± 0.9 | 3.1 ± 1.1 | 3.0 ± 0.9 | 2.7 ± 0.7 |
| 1 μm MDI | 2.1 ± 0.8 | 2.0 ± 0.8 | 3.8 ± 1.9 | 3.7 ± 1.9 | 2.9 ± 1.3 |
| 3 μm MDI | 1.9 ± 0.5 | 1.9 ± 0.5 | 3.0 ± 1.0 | 2.7 ± 0.8 | 2.4 ± 0.6 |
| 2μm RA | 2.2 ± 0.9 | 2.2 ± 0.8 | 3.1 ± 1.2 | 3.1 ± 1.0 | 2.6 ± 0.9 |
| Diabetic | |||||
| Untreated | 3.0 ± 0.7** | 3.7 ± 1.3* | 4.7 ± 1.2** | 4.5 ± 1.4** | 4.0 ± 0.9** |
| 1 μm MDI | 2.8 ± 0.9 | 2.8 ± 0.9† | 4.0 ±1.2 | 3.8 ± 1.2 | 3.3 ± 0.8† |
| 3 μm MDI | 2.4 ± 0.5‡§ | 2.8 ± 0.8† | 3.3 ± 0.7‡§ | 3.3 ± 0.9‡ | 3.0± 0.6‡§ |
| 2 μm RA | 3.3 ± 0.9 | 3.1 ± 1.0 | 4.4 ± 1.0 | 4.1 ± 1.3 | 3.8 ± 0.9 |
p<0.05 vs untreated non-diabetic
p<0.01 vs untreated non-diabetic
p<0.05 vs untreated diabetic
p<0.01 vs untreated diabetic
p<0.01 vs RA
Effect of MDI 301 and RA on MMPs and TIMP-1
MMP-1, MMP-2, MMP-9 and TIMP-1
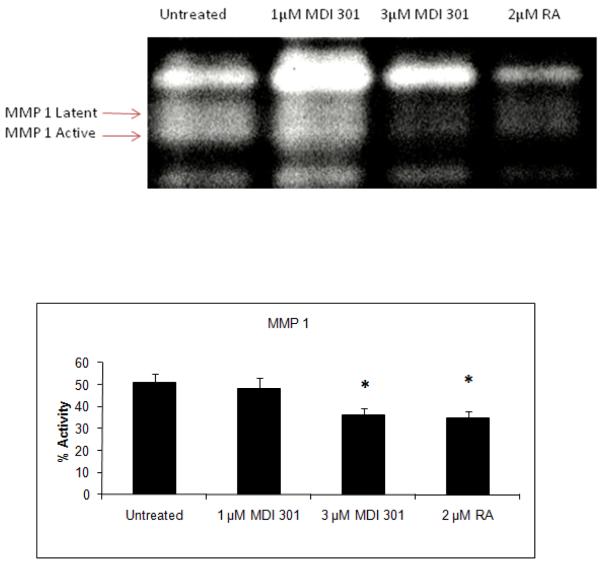
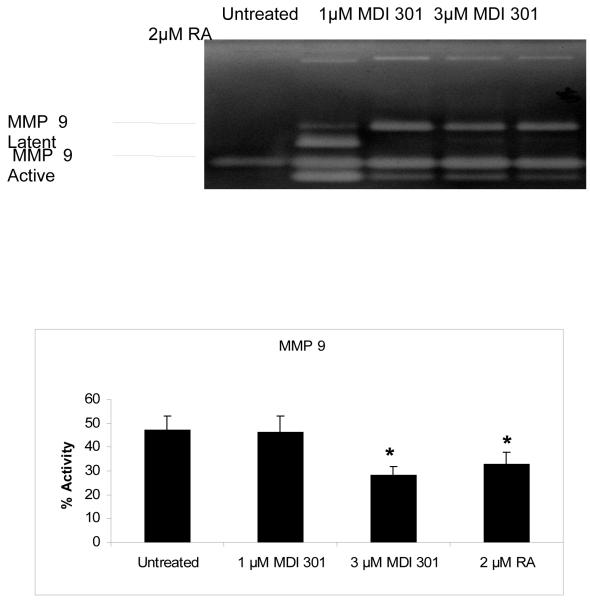
Incubation of skin biopsies from the diabetic subjects with 3 μm MDI 301 significantly reduced active MMP-1 abundance with levels being reduced by 29% (p<0.05) (Figure 1). One μM MDI 301 however only reduced active MMP-1 by 6%. In concert, 2 μM RA resulted in a 31% reduction in MMP-1 (p<0.05). Levels of active MMP-9 were decreased 40% (p<0.05) by 3 μM MDI 301 whereas the 30% reduction by 2 μM RA did not achieve statistical significance (p=0.2) (Figure 2). One μM MDI 301 did not affect levels of active MMP-9. In contrast, levels of active MMP-2 were unaffected by MDI 301 at any concentration or RA. There were however, no significant differences detected between the effect of 3 μM MDI 301 and RA. Exposure of tissue to MDI 301 or RA did not affect levels of total MMPs (data not shown). No significant effects of MDI 301 at any dose or RA were noted on MMP abundance or activity in samples from non-diabetic subjects (data not shown).
Figure 1. Effect of MDI 301 and retinoic acid on MMP-1 activity in organ culture.
Organ culture fluid from untreated, 1 μM and 3 μM MDI 301-treated or 2 μM RA-treated skin was collected on day 4 and assayed for MMP-1 by β–casein zymography. Zymographic images were scanned and digitized, and negative images quantified. Upper Panel: Representative β-casein zymogram demonstrating MMP-1 in organ culture fluid from untreated diabetic skin and skin treated with 1 μM and 3 μM MDI-301 and 2 μM retinoic acid (RA). A higher percentage of MMP-1 in the active form can be seen in culture fluid from untreated skin as compared to MDI 301 and RA treated skin. Lower Panel. Active enzyme expressed as a percentage of total enzyme (densitometry values from active forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures from 12 normal and 27 diabetic volunteers. Statistical significance of the differences among the four groups was determined by analysis of variance followed by paired-group comparisons.
*p<0.05 vs untreated samples
Figure 2. Effect of MDI 301 and retinoic acid on MMP-9 activity in organ culture.
Organ culture fluid from untreated, 1 μM and 3 μM MDI 301-treated or 2 μM RA-treated skin was collected on day 4 and assayed for MMP-9 by gelatin zymography. Zymographic images were scanned and digitized, and negative images quantified. Upper Panel: Representative gelatin zymogram demonstrating MMP-9 (and MMP-2) in organ culture fluid from untreated diabetic skin and skin treated with 1 μM and 3 μM MDI-301 and 2 μM retinoic acid (RA). A higher percentage of MMP-9 in the active form can be seen in culture fluid from untreated skin as compared to MDI 301 and RA-treated skin. MMP-2 latent and active forms can also be seen and there is a reduction of active MMP-2 in MDI-301 and RA-treated samples. Lower Panel. Active enzyme expressed as a percentage of total enzyme (densitometry values from active forms divided by values from active + latent forms). Values shown are means and standard errors based on organ cultures from 12 normal and 27 diabetic volunteers. Statistical significance of the differences among the four groups was determined by analysis of variance followed by paired-group comparisons.
*p<0.05 vs untreated samples
The culture fluids were examined for TIMP-1 levels by ELISA. In the diabetic skin samples, TIMP-1 protein abundance increased dose-dependently in response to MDI 301 with levels increasing by 25% (125±76 ng/ml vs 156±73 ng/ml [ns]) and by 45% (125±76 ng/ml vs 181±99 ng/ml [p=0.07]) in 1 and 3 μM MDI 301, respectively. TIMP-1 was also increased by 26% by RA although this failed to achieve statistical significance (p=0.6). In non-diabetic skin samples, TIMP-1 abundance was non-significantly increased by both 3 μm MDI 301 and RA (data not shown).
Effect of MDI 301 and RA on Type 1 Procollagen Abundance
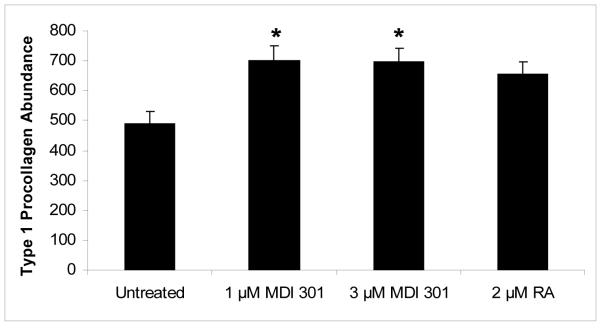
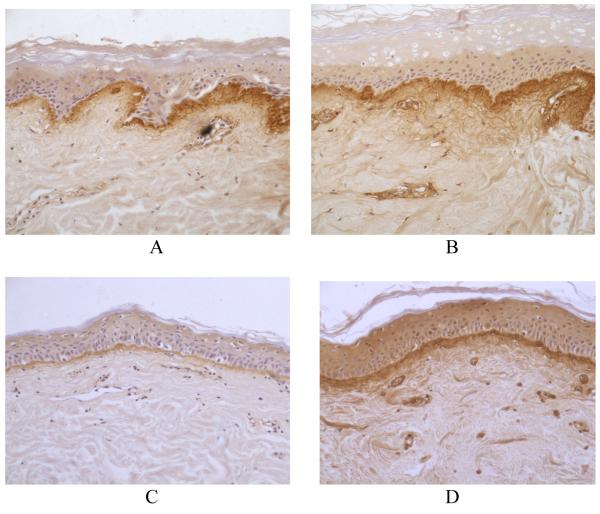
Type 1 procollagen abundance was increased in diabetic skin samples by ~40% (p<0.01) by both 1 and 3 μM MDI 301. The 31% increase in response to RA did not however achieve statistical significance (p=0.08) (Figure 3). Figure 4 shows representative tissue sections from a nondiabetic patient (Panels A and B) and sections from a diabetic subject (Panels C and D) cultured without or with 3 μM MDI 301. The reduction of type 1 procollagen abundance is clearly evident in untreated diabetes and is significantly augmented by the retinoid. Culture of non-diabetic skin samples with 3 μM MDI 301 significantly increased type 1 procollagen abundance (720±103 vs 888±75, p<0.01). The 14% and 11% increase in type 1 procollagen abundance observed by culture in 1 μM MDI 301 and 2 μM RA, respectively, did not achieve statistical significance.
Figure 3. Effect of MDI 301 and retinoic acid on type I procollagen abundance.
Skin tissue from non-diabetic and diabetic subjects was maintained in organ culture for 8 days under serum-free, growth factor-free conditions in the absence or presence of 1 μM and 3 μM MDI 301 or 2 μM RA. At the end of the incubation period, sections were dewaxed blocked with 10% horse serum and incubated with antibodies to type 1 pro-collagen and the immunoreactivity revealed using a Vectastain universal elite ABC kit (Vector Laboratories, Orton Southgate, Peterborough, Cambridgeshire, UK) and diaminobenzidine (DAKO, Ely, Cambridgeshire, UK). The area and density of staining within an area of 1000X1000 pixels was determined by image analysis after subtraction of background staining and the product calculated and presented as the overall abundance (in intensity units). Values shown are means and standard errors based on sections from 12 normal and 27 diabetic volunteers. Statistical significance of the differences among the four groups was determined by analysis of variance followed by paired-group comparisons.
RA= Retinoic Acid. Results expressed as mean ± SEM. *p<0.01 vs untreated samples
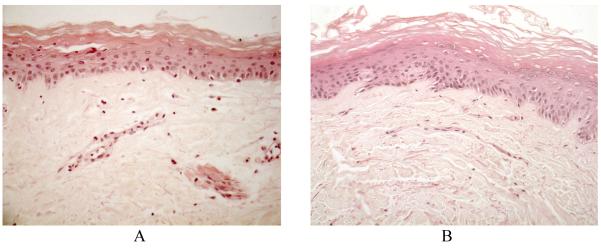
Figure 4. Immunohistochemcial staining for type 1 procollagen in untreated and MDI 301-treated non-diabetic and diabetic skin in organ culture.
Skin tissue from non-diabetic and diabetic subjects was maintained in organ culture for 8 days under serum-free, growth factor-free conditions in the absence or presence of 3 μM MDI 301. At the end of the incubation period, sections are dewaxed blocked with 10% horse serum and incubated with antibodies to type 1 pro-collagen and the immunoreactivity revealed using a Vectastain universal elite ABC kit (Vector Laboratories, Orton Southgate, Peterborough, Cambridgeshire, UK) and diaminobenzidine (DAKO, Ely, Cambridgeshire, UK). A: Normal control. B: Normal 3 μM MDI 301-treated. C: Diabetic control. D: Diabetic 3 μM MDI-301-treated. Reduced abundance of type 1 procollagen can be seen in the untreated diabetic skin which is increased by 3 μM MDI 301 treatment (x200)
Skin Structural Deficit Scores
MDI 301 improved the mean structural deficit score by 13% and by 21% (p<0.01), in 1 and 3 μM MDI 301, respectively (Table 2). All aspects of skin structure were found to be significantly improved by 3 μM MDI 301. In contrast, no discernable effect of RA on any parameter of skin structure was observed. Figure 5 shows representative tissue sections from a nondiabetic patient (Panels A and B) and sections from a diabetic subject (Panels C and D) cultured without or with 3 μM MDI 301. Epidermal hyperplasia can clearly be seen in the 3 μM MDI 301 treated diabetic skin.
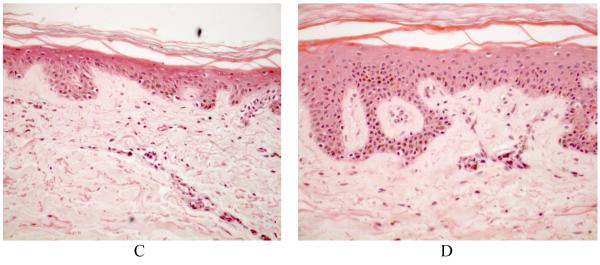
Figure 5. Histological features of untreated and MDI 301-treated non-diabetic and diabetic skin in organ culture.
Skin tissue from non-diabetic and diabetic subjects was maintained in organ culture for 8 days under serum-free, growth factor-free conditions in the absence or presence of 3 μM MDI 301. At the end of the incubation period, the tissue was fixed in 10% buffered formalin and examined at the light microscopy level after sectioning and staining with hematoxylin and eosin. A: Normal control. B: Normal 3 μM MDI 301-treated. C: Diabetic control. D: Diabetic 3 μM MDI-301-treated. Epidermal hyperplasia can be seen in the 3 μM MDI 301 treated diabetic skin (x200).
Discussion
In diabetes, the lifetime risk of developing a foot ulcer is estimated to be 5-15% (Reiber, 1996; Lavery, Armstrong, Wunderlich, Tredwell & Boulton, 2003). Foot ulceration causes substantial emotional, physical, productivity, and financial losses (Vileikyte 2001). Additional approaches aimed at reducing the risk of ulceration or improving healing rates once ulceration has occurred are urgently required.
The % of active MMP-1, -2 and -9 were higher and TIMP-1 abundance lower in our high-risk subjects with diabetes. Eleven of our current cohort had a history of foot ulceration and 8 had charcot arthropathy, clinical characteristics which are consistent with the very high risk population that we would wish to target for treatment. Seven subjects were without neuropathy or foot complications. Nevertheless we did not find differences in baseline activity levels of MMPs in these different diabetic groups (data not shown). Increased elaboration of activated MMPs is thought to precede changes in skin structure which is then followed by a reduction in collagen synthesis and wide-spread collagen destruction (Griffiths et al., 1993; Varani et al., 2002). Levels of pro-MMP-2 and pro-MMP3 have been reported to be increased in fibroblasts from diabetic patients (Wall, Sampson, Levell & Murphy, 2003).
In order to quantify the degree of damage to the skin and assess collagen structure a previously reported (Varani et al., 2000) skin structural deficit score was applied for the first time to subjects with diabetes. All parameters of collagen structure were greatly disrupted in the skin of the diabetic subjects compared to the healthy controls. In general, the extent of the skin structural damage correlated with the severity of diabetic foot complications since deficits were most advanced in subjects with charcot arthropathy. In concert, we report for the first time that type 1 procollagen abundance is also significantly reduced in diabetic subjects with foot complications. These data are consistent with the concept that in the high risk subject with diabetes, skin structural deficits in concert with foot deformities, insensitivity and vascular insufficiency represent a potential therapeutic target.
Topical retinoids have been proposed as a means to reverse many of the functional and structural deficits of the skin caused by aging or diabetes (Lateef, Stevens & Varani, 2004; Griffiths et al., 1993). However, clinically, the use of retinoids is limited by skin irritation (Griffiths et al., 1995; Philips et al., 2002). Topical retinoids are known to stimulate epidermal hyperplasia (Varani et al 2001) which is thought to reflect pro-inflammatory changes in the vasculature and upregulation of pro-inflammatory cytokines. This can lead to induction of adhesion molecules on microvascular endothelial cells and recruitment of inflammatory cells (Mulligan et al., 1991). Retinoids can also reduce epidermal cohesion (Varani et al., 1991). Therefore the altered vascular responsiveness of subjects with diabetes and in particular charcot neuroarthropathy (Stevens, Edmonds, Foster & Watkins, 1992) could predispose to increased retinoid-induced irritation which may negate any skin functional and structural improvements and ultimately may paradoxically predispose to wound formation. Indeed our pilot studies suggest that the use of topical retinoic acid in subjects with diabetes improves skin biochemistry but not structure (MJS observations). An alternative approach which is equally as potent but much less irritating is clearly desirable in these subjects.
We explored ability of MDI 301, a picolinic acid-substituted 9-cis RA ester to reproduce its salutary effects of skin collagen synthesis and MMP activation (Varani et al., 2003; Appelyard et al., 2004) in skin biopsies from subjects with diabetes and advanced complications. Levels of active MMP-1 and 9 were significantly reduced by 3 μM MDI 301 whereas 2 μM RA (a concentration found previously to be the most effective in inhibiting skin MMP activity (Lateef, Stevens & Varani, 2004) only significantly reduced levels of MMP-1. The attenuated response reported herein may reflect the more advanced damage to the skin in this cohort of subjects. In concert MDI 301 exceeded RA in augmentation of TIMP-1 protein abundance, consistent with overall greater potency.
In preclinical studies, pretreatment of diabetic hairless rats with topical MDI 301 can accelerate wound-closure time (Warner et al., 2008) a finding which is consistent with our previous report utilising RA in this model (Varani et al., 2002). MDI 301 possesses significantly less potential to irritate the skin when compared to RA (Varani et al., 2003; Appelyard et al., 2004). RA causes up-regulation of several pro-inflammatory cytokines including IL-1α and -1β, IL-6, IL-8 and MCP-1 and increases ICAM-1 and E-selectin (Varani, Fay & Perone, 2007). In the diabetic rodent model, MDI 301 was found to reverse the effect of diabetes on activation of skin MMP-1 and -9 and in concert restore abundance of TIMP-1 and type 1 procollagen (Warner et al., 2008). Although this effect may partly be due to attenuation of collagen breakdown secondary to inhibitory effects on MMPs, increased procollagen synthesis may also be involved. This would be consistent with reports of retinoid-induced increased cellular mRNA and protein of type I procollagen in tissue sections of aged/photoaged skin (Griffiths et al., 1993). These effects have been proposed to reflect a retinoid-mediated reduction in signaling through the JNK pathway, leading to reduced c-jun activation (Fisher et al., Chung, Kang, Varani, Lin, Fisher, Voorhees, 2000) and modulation of signaling via TGF-β, which is a potent inducer of collagen production (Fisher et al., 2000).
MDI 301 significantly reduced the mean deficit score to levels that were not significantly different from non-diabetic values. In nondiabetic skin, RA as well as MDI 301 can stimulate epidermal thickening and increase procollagen synthesis, while reducing MMP-1 abundance and augment the production of TIMP-1 (Varani, Fay & Perone, 2007). However in diabetic skin the effects of RA on MMP-9, TIMP-1, type 1 procollagen and skin structure are attenuated compared to MDI 301. This suggests that augmented potency of MDI 301 coupled with its reduced capacity for irritation may be advantage in these high-risk diabetic subjects.
A limitation to our study was the slightly younger age of our nondiabetic control group. We do not feel however that this will have significantly impacted our results since the increase of MMP levels that are observed with age is more than offset with the reduction in cellularity which affects both the epidermis and dermis (Varani et al., 2000). Thus if our two groups of subjects were more closely age matched, the differences in MMP levels might have been augmented. Since retinoid treatment increases dramatically the cellularity of the skin, the reduction of enzyme levels per cell in MDI 301 treated skin could be expected to be greater than measured herein.
In conclusion diabetes complicated by foot ulceration and charcot arthropathy exhibits skin structural and functional deficits which may predispose to fragility and ulceration. The synthetic retinoid MDI 301 appears to have greater potency than RA to reverse activation of connective tissue-degrading MMPs, increase type 1 procollagen abundance and ameliorate skin structural deficits in these high risk patients. If these effects can be reproduced by topical application on the lower limb skin of diabetic subjects without inducing inflammation, this approach may be of benefit in the prevention of chronic diabetic foot ulceration.
Acknowledgements
We would like to thank Molecular Design International for providing MDI 301.
Sources of Support: This work is supported by National Institutes of Health Grants GM77724 and RO1AT002146, Eli Lilly and the G.J.W. Turner Trust
Footnotes
No conflicts of interest
References
- Appelyard VCL, O’Neill MAO, Murray KE, Bray SE, Varani J, Zhang J, Thompson AM. Activity of MDI-301, a novel, synthetic retinoid, in xenografts. Anticancer Res. 2004;15:991–996. doi: 10.1097/00001813-200411000-00009. [DOI] [PubMed] [Google Scholar]
- Bigg HF, McLeod R, Waters JG, Cawston TE, Clark TE. Mechanisms of induction of human tissue inhibitor of metalloproteinases-1 (timp-1) gene expression by all-trans retinoic acid in combination with basic fibroblast growth factor. Eur J Biochem. 2000;267:4150–4156. doi: 10.1046/j.1432-1327.2000.01459.x. [DOI] [PubMed] [Google Scholar]
- Bizot-Foulon V, Bouchard B, Hornebeck W, Dubertret L, Bertaux B. Uncoordinate expressions of type i and iii collagens, collagenase and tissue inhibitor of matrix metalloproteinase 1 along in vitro proliferative life span of human skin fibroblasts. Regulation by all-trans retinoic acid. Cell Biol Int. 1995;19:129–135. doi: 10.1006/cbir.1995.1053. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Eng J Med. 2004;351:48–55. doi: 10.1056/NEJMcp032966. [DOI] [PubMed] [Google Scholar]
- Braunhut SJ, Moses MA. Retinoids modulate endothelial cell production of matrix-degrading proteases and tissue inhibitors of metalloproteinases (timp) J Biol Chem. 1994;269:13472–13479. [PubMed] [Google Scholar]
- Chung JH, Kang S, Varani J, Lin J, Fisher GJ, Voorhees JJ. Decreased extracellular signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000;114:177–182. doi: 10.1046/j.1523-1747.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Edmonds ME. Progress in care of the diabetic foot. Lancet. 1999;354:270–272. doi: 10.1016/s0140-6736(99)90012-0. [DOI] [PubMed] [Google Scholar]
- Edmonds ME. The diabetic foot. Diab Metab Res Rev. 2004;20((Suppl 1):S9–S1. doi: 10.1002/dmrr.458. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabet Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta S, Wang ZQ, Li X-Y, Quan T, Chung JH, Kang S, Voorhees JJ. C-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DF, Shanley TP, Warner RO, Murphy HS, Varani J, Johnson KJ. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury. Amer J Respir Cell Molec Biol. 1999;20:1145–1154. doi: 10.1165/ajrcmb.20.6.3482. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Kang S, Ellis CN, Kim KJ, Finkel LJ, Ortiz-Ferrer LC, White GM, Hamilton TA, Voorhees JJ. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin cream. Arch Dermatol. 1995;131:1037–1044. [PubMed] [Google Scholar]
- Kang S, Duell EA, Fisher GJ, Datta SC, Wang Z-Q, Reddy AP, Tavakkol A, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid-binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:49–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Dogadkina D, Lavker RM. Effects of topical tretinoin on non-sun-exposed protected skin of the elderly. J Am Acad Dermatol. 1993;29:25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- Lateef H, Aslam MN, Stevens MJ, Varani J. Pretreatment of diabetic rats with lipoic acid improves healing of subsequently-induced abrasion wounds. Arch Dermatol Res. 2005;297:75–83. doi: 10.1007/s00403-005-0576-6. [DOI] [PubMed] [Google Scholar]
- Lateef H, Stevens MJ, Varani J. All-trans retinoic acid suppresses matrix metalloproteinase production/activation and increases collagen synthesis in diabetic skin in organ culture. Am J Path. 2004;165:167–174. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabet Care. 2003;26:1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Derm Res. 1999;291:93–99. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Varani J, Dame MK, Lane CL, Smith CW, Anderson DC, Ward PA. Role of endothelial cell-leukocyte adhesion molecule-1 (E-selectin in neutrophil-mediated lung injury in rats. J Clin Invest. 1991;88:1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabet Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Gottlieb AB, Leyden JJ, Lowe NJ, Lew-Kaya DA, Sefton J, Walker PS, Gibson JR. Efficacy of 0.1% Tazarotene cream for the treatment of photodamage. Arch Dermatol. 2002;138:1486–1493. doi: 10.1001/archderm.138.11.1486. [DOI] [PubMed] [Google Scholar]
- Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. 1996;13(Suppl 1):S6–11. [PubMed] [Google Scholar]
- Stevens MJ, Edmonds ME, Foster AVM, Watkins PJ. Selective neuropathy and preserved vascular responses in the diabetic Charcot foot. Diabetologia. 1992;35:148–154. doi: 10.1007/BF00402547. [DOI] [PubMed] [Google Scholar]
- Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- Teno S, Kanno H, Oga S, Kumakura S, Kanamuro R, Iwamoto Y. Increased activity of membrane glycoprotein pc-1 in the fibroblasts from non-insulin-dependent diabetes mellitus patients with insulin resistance. Diabetes Res Clini Prac. 1999;45:25–30. doi: 10.1016/s0168-8227(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Varani J, Fay K, Perone P. MDI-301:A non-irritating retinoid, induces changes in organ-cultured human skin that underlie repair. Arch Dermatol Res. 2007;298:439. doi: 10.1007/s00403-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Fisher GJ, Kang S, Voorhees JJ. Molecular mechanisms of intrinsic skin aging and retinoid-induced repair and reversal. J Investig Dermatol Symp Proc. 1998;3:57–60. [PubMed] [Google Scholar]
- Varani J, Fligiel H, Zhang J, Aslam MN, Lu Y, Dehne LA, Keller ET. Separation of retinoid-induced epidermal and dermal thickening from skin irritation. Arch Dermatol Res. 2003;295:255–262. doi: 10.1007/s00403-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Gibbs DF, Inman DR, Shah B, Fligiel SE, Voorhees JJ. Inhibition of epithelial cell adhesion by retinoic acid: Relationship to reduced extracellular matrix production and alterations in Ca2+ levels. Amer J Pathol. 1991;138:887–895. [PMC free article] [PubMed] [Google Scholar]
- Varani J, Perone P, Fligiel SE, Inman DR, Voorhees JJ. All-trans-retinoic acid preserves viability of fibroblasts and keratinocytes in full-thickness human skin and fibroblasts in isolated dermis in organ culture. Arch Dermatol Res. 1994;286:443–447. doi: 10.1007/BF00371569. [DOI] [PubMed] [Google Scholar]
- Varani J, Perpone P, Merfert MG, Moon SE, Larkin D, Stevens MJ. All-trans retinoic acid improves the structure and function of diabetic rat skin in culture. Diabetes. 2002;51:3510–351632. doi: 10.2337/diabetes.51.12.3510. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Phan SH, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Zeigler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ, Stoll SW, Elder JT. Heparin-binding epidermal growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol. 2001;117:1335–1341. doi: 10.1046/j.0022-202x.2001.01564.x. [DOI] [PubMed] [Google Scholar]
- Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17:246–249. doi: 10.1002/dmrr.216. [DOI] [PubMed] [Google Scholar]
- Wall SJ, Sampson MJ, Levell N, Murphy G. Elevated matrix metalloproteinase-2 and n -3 production from human diabetic dermal fibroblasts. Br J Dermatol. 2003;149:13–16. doi: 10.1046/j.1365-2133.2003.05262.x. [DOI] [PubMed] [Google Scholar]
- Warner RL, Bhagavathula N, Nerusu K, Hanosh A, McClintock SD, Naik M, Johnson KJ, Varani J. MDI 301, a nonirritating retinoid, improves abrasion would healing in damaged/atrophic skin. Wound Rep Regen. 2008;16:117–124. doi: 10.1111/j.1524-475X.2007.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JS, Ellis CN, Headington JT, Tincoff T, Hamilton TA, Voorhees TJ. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988;259:527–532. [PubMed] [Google Scholar]