Abstract
Introduction
Endogenous dentin matrix metalloproteinases (MMPs) contribute to extracellular collagen matrix degradation in hybrid layers following adhesive dentin bonding procedures. Endodontic irrigants, including chlorhexidine (CHX) and ethylenediaminetetraacetic acid (EDTA) may help protect the hybrid layer from this process. The objective of the present study was to determine the exposure time necessary for EDTA to inactivate endogenous MMP activity in human dentin.
Methods
Dentin beams (2×1×3 mm) were prepared from mid-coronal dentin of extracted third molars. The beams were demineralized in 10 wt% phosphoric acid which also activated endogenous MMPs, and were divided into four experimental groups based on exposure time to 17% EDTA (0, 1, 2 or 5 min). A generic colorimetric MMP assay measured MMP activity via absorbance at 412 nm. Data were evaluated by Kruskal Wallis ANOVA, followed by Dunn’s pair-wise comparisons at α = 0.05.
Results
All exposure times resulted in significant inhibition (P<0.001) compared to unexposed controls. Specifically, percent inhibition for 1-, 2-, and 5-minute exposure times were 55.1±21.5%, 72.8±11.7%, and 74.7±19.7%, respectively.
Conclusions
17% EDTA significantly inhibits endogenous MMP activity of human dentin within 1–2 min. This may minimize hybrid layer degradation following resin bonding procedures in the root canal space.
Keywords: dentin, EDTA, hybrid layer, matrix metalloproteinases
INTRODUCTION
The first generation of methacrylate-based root canal sealers appeared in the 1970s [1–2]. The material, Hydron, was removed from the market because it absorbed too much water, swelled out of the confines of the root canal and leached 2-hydroxyethyl methacrylate into the periapex, causing chronic inflammation. The second generation [3–4] was designed to bond to NaOCl/EDTA-treated dentin. This dual-cured sealer, EndoREZ (Ultradent, South Jordan, UT), can be used with conventional gutta-percha or resin-coated gutta-percha points. The third generation utilizes a self-etching acidic primer to etch into the smear layer, followed by a dual-cured flowable composite. An example of such a sealer is Resilon (Resilon Research LLC, Madison, CT) [5]. The fourth generation is essentially a self-etching flowable composite (RealSeal SE, SybronEndo, Orange, CA) that combines an etchant, a primer, and a sealer into an all-in-one self-etching, self-adhesive composite [6]. As the etching ability of these sealers is sufficient to uncover and activate MMPs in apical dentin, the thin hybrid layers created by these sealers may be susceptible to MMP-induced degradation. Dental adhesives obtain their adhesion by flowing into the spaces between adjacent collagen fibrils. Once polymerized, this resin-infiltrated demineralized zone is called a “hybrid layer”. Hybrid layers provide micromechanical retention between overlying filling materials and the underlying mineralized dentin. The only continuous connection between mineralized dentin and filling materials are the collagen fibrils of the hybrid layer.
Dentin collagen fibrils contain inactive proforms of proteolytic enzymes called matrix metalloproteinases (MMPs) [7]. Once mineralized, the MMPs in the dentin matrix are inactive. They are exposed and activated by acid-etching during adhesive bonding procedures. If these matrix-bound, activated MMPs are not fully infiltrated with adhesive resin, they can slowly degrade the collagen fibrils that anchor the fillings to dentin. This can cause loss of adhesion [8] and gap formation. This has lead to a number of investigations on the use of MMP-inhibitors to inactivate exposed, activated MMPs [9,10]. The functions of MMPs are diverse but are mainly associated with degradation of the extracellular matrix including collagens. Dentin contains endogenous MMP-2, -8, -9, -20 [7] and are involved in degradation of resin-dentin bonds both in vivo and in vitro [8,10]. MMP-2 and -9 have traditionally been considered as gelatinases. However, more recent work showed that they also exhibit collagenolytic activity [11–13] When these inactive zinc- and calcium-dependent endopeptidases are exposed and activated by self-etch or total-etch adhesives [14–17], they can degrade type I collagen [18]. As collagen fibrils are incompletely resin-infiltrated during dentin bonding procedures [19], strategies to prevent bond degradation are necessary to increase the longevity of methacrylate resin-based root fillings and orifice barriers.
Several materials have been shown to inhibit MMPs, including some root canal irrigants. Both 2% chlorhexidine and 17 wt% ethylenediaminetetraacetic acid (EDTA) have been shown to inhibit MMP activity induced by self-etching adhesives [15,17]. Although the anti-MMP activity of EDTA is well-known, it is often used at only 0.34–0.68 wt% in anti-MMP assays. What is not known is how rapidly 17 wt% EDTA can inactivate matrix-bound MMPs when the latter is used in the context of a root canal irrigant. Thus, the aim of the present study was to determine the time necessary for 17 wt% EDTA to display anti-MMP effects on demineralized dentin. The null hypothesis tested was that different exposure times to 17 wt% EDTA has no effect on MMP activity of demineralized human dentin.
MATERIALS AND METHODS
Dentin Beams
Twenty-five extracted human third molars were obtained with patients’ informed consent using a protocol approved by the Human Assurance Committee of Georgia Health Sciences University. They were stored in 0.9% NaCl containing 0.02% sodium azide at 4°C to inhibit microbial growth. An ename l-free, 1-mm thick dentin disk was prepared from each tooth using a water-cooled, slow-speed diamond saw (Isomet, Buehler, Lake Bluff, IL). Each dentin disk was then used to prepare four 2×1×3 mm. The 100 mineralized dentin beams were completely demineralized in 10% phosphoric acid at 25°C, and radiographed with an aluminum ste p-wedge to confirm complete demineralization. The use of 10% phosphoric acid uncovers the endogenous proteases and activates MMP proforms [14]. The proforms of all MMPs have an intramolecular complex between the single cysteine residue in the propeptide and a zinc atom in the active site that blocks the active site. Acids dissociate cysteine from the active site, thereby activating MMPs by triggering the cysteine switch [20,21].
Generic MMP Assay
A 96-well plate was prepared for use with a generic MMP assay kit (Sensolyte generic MMP assay, Anaspec, San Jose, CA) [22]. The assay (Fig.1) involved incubating the treated dentin beams with a proprietary chromogenic substrate. The latter is a thiopeptide that is cleaved by the MMPs to release a sulfhydryl group. The sulfhydryl group reacts with 5,5′-dithiobis(2-nitrobenzoic acid) to produce the colored reaction product, 2-nitro-5-thiobenzoic acid, which can be detected at 412 nm. Human recombinant MMP-9 (rh-MMP-9) was used as the positive control in this study as a representative examplr of an MMP found in human dentin.
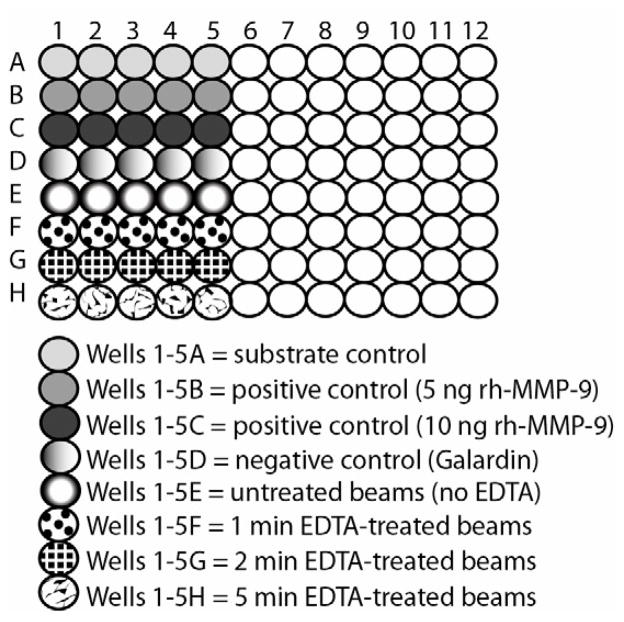
Figure 1.
Schematic of 96-well plate showing how 40 of the 96 wells was used to evaluate the time required for 17 wt% EDTA to inhibit the total endogenous MMPs in demineralized dentin beams.
Demineralized dentin beams were placed inside the wells of a 96-well plate containing generic MMP substrate and incubated for up to 3 hrs. The 96-well plate was placed in a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT) set to measure absorbance every 5 min. To avoid light-scattering by the 96-well plate, dentin beams were removed from their wells at 5 min intervals and placed on a Telfon surface, to permit measurement of the absorbance of the total endogenous MMP activity of the beam. The total time necessary to read the wells was 30 sec. Dentin beams were replaced in their respective wells and the incubation proceeded for the next 5 min before re-reading the absorbance. This procedure was continued every 5 min for 90 min, and then every 15 min for up to 3 hrs.
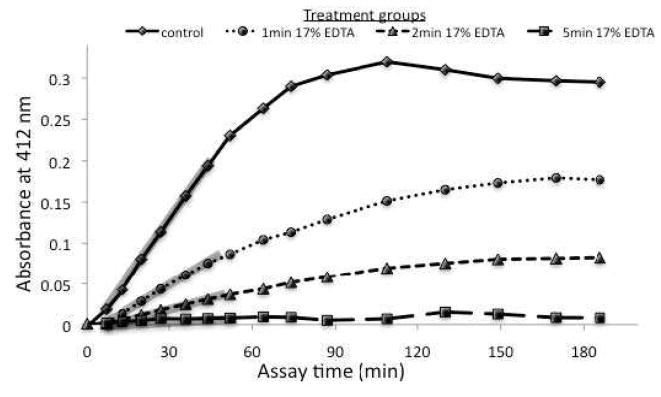
Demineralized dentin beams were divided into four groups with different EDTA exposure times: 0 min (substrate control), 1, 2 or 5 min (N=20). A solution of 17 wt% EDTA was prepared with deionized water, with pH adjusted to 7.4. To measure the ability of 17 wt% EDTA to inhibit the total MMP activity of dentin, demineralized dentin beams hanging from 30 gauge stainless steel needles were dipped into wells containing 250 μL of EDTA for 1, 2 or 5 min. After the designated time, the beams were removed from the EDTA and dipped into wells containing 250 μL of distilled water for 2 min to remove the absorbed EDTA, and then transferred to fresh wells containing 250 μL deionized water for another 2 min. Dentin beams from the zero time control group were placed individually into wells containing 250 μL of deionized water instead of EDTA, and then sequentially dipped in the water rinsing wells. These beams were then placed in generic MMP substrate to determine their residual MMP activity. Each plate contained 5 specimens per group. The early absorbance data (first 45 min) were fitted by linear regression to obtain the slopes of the lines for uninhibited dentin beams and EDTA-inhibited dentin beams (Fig. 2). The experiment was repeated four times to generate data derived from 20 beams for each group. The slopes of the lines of absorbance versus time were calculated for each specimen and then averaged. The maximum absorbance for each EDTA-inhibited specimen was expressed as a percentage of the mean absorbance of the uninhibited control.
Figure 2.
A plot depicting absorbance over time of control or 17% EDTA-treated dentin [N = 20]. Higher absorbance demonstrates greater MMP activity. Shaded regions indicate the initial linear portion of maximum MMP activity.
Data Analysis
The means of all the slopes for beams in the control and 3 EDTA experimental groups were analyzed using one-way ANOVA on ranks since the distribution of the data failed normality and equal variance tests. Dunn’s multiple comparison tests were used for pair-wise multiple comparisons. Statistical significance for all tests were preset at α=0.05.
RESULTS
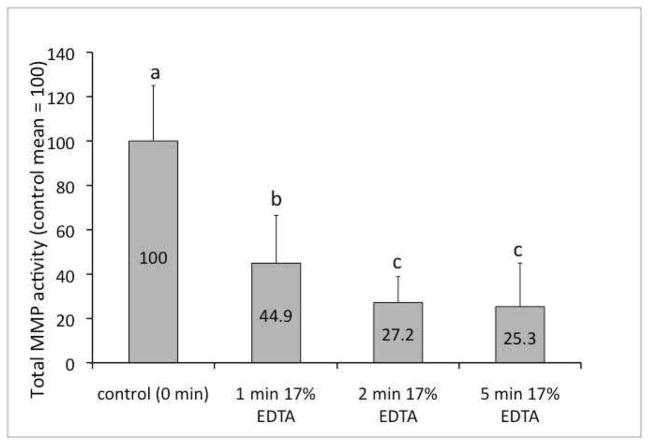
The effect of different EDTA exposure times on inhibition of endogenous MMPs was statistically significant (P<0.001). All three EDTA exposure times resulted in significant inhibition of MMP activity compared to the unexposed controls. When these absorbances were expressed as a percentage of the control, the percent inhibition for 1-min, 2-min and 5-min exposure times were 55.1±21.5%, 72.8±11.7%, and 74.7± 9.7%, respectively (Fig.3). The 2-min and 5-min EDTA exposures times were also significantly different from the 1-min exposure time (P=0.018 and 0.006, respectively) but were not different from each other.
Figure 3.
A bar chart showing the residual MMP activity of dentin blocks after different exposure times to 17% EDTA [N = 20]. Labels on each bar show the activity as a percentage of the control group mean. Groups labeled with different letters are significantly different (P < 0.05).
DISCUSSION
The results require rejection of the null hypothesis that 17 wt% EDTA exposure time has no effect on MMP activity of completely demineralized dentin beams. Ethylenediaminetetraacetic acid is used in endodontics to facilitate easier instrumentation of constricted canals [30], and to dissolve the inorganic portion of the smear layer created during shaping of the canal space [24]. The principle effect of EDTA on dentin is surface softening by chelation of calcium ions. In root canal treatment, removal of calcium by EDTA affects the superficial 20–30 μm of intraradicular dentin. However, its action is limited to 50 μm even after exposure times greater than 24 hrs [25].
The present study demonstrates an inhibitory effect of EDTA on the matrix-bound MMPs of demineralized dentin. As MMPs remain bound to the demineralized collagen matrix as they exist in vivo, the use of demineralized dentin beams permits simple screening of potential MMP inhibitors prior to engaging in more time- and resource-intensive studies.
We interpret the results as showing that 17 wt% EDTA pretreatment of dentin beams for as little as 1 min significantly lowered the endogenous MMP activity of completely demineralized dentin beams, by chelating both the calcium and zinc ions from the enzyme that are necessary for their optimum function [26]. Presumably, one could have allowed the beams pretreated with 17 wt% EDTA for 5 min to process the generic MMP substrate for 60 min and then transferred the beams to wells containing physiological levels of zinc (26 μM) or calcium (2.5 mM) or both to see if the activity of endogenous MMPs could recover after replacement of these critical ions. If the enzymes could recover, the slopes of those curves should increase to that of the control beams that were never exposed to EDTA. This is but one example of how one can use the generic MMP substrate with completely demineralized dentin beams to evaluate their sensitivity to experimental manipulation.
Although exogenous recombinant human MMP-9 (rh-MMP-9) was used in this study as a positive control for the assay, no direct comparisons can be made to the dentin beam samples that contain MMP-2, -8, -9, -20 and perhaps cathepsins [27]. Accurate absorbance measurements required that the dentin beams be removed from their wells, thus the cumulative incubation time in the colorimetric substrate was slightly less for the beams than for the soluble rh-MMP-9.
In conclusion, the present study demonstrated significant inhibition of dentin matrix-bound MMPs by 17 wt% EDTA within 1 min. This may help minimize hybrid layer degradation following resin-dentin bonding procedures within the root canal system [28]. Chlorhexidine has been recommended as an additional antibacterial irrigant following canal debridement with NaOCl and EDTA [29]. Although EDTA is an excellent MMP inhibitor, it is so water soluble that it may be rinsed off EDTA-treated dentin. Chlorhexidine (CHX) also inhibits MMPs [9] but binds to demineralized dentin very firmly [30,31] and may sustain MMP-inhibition much longer than EDTA. As there is no incompatibility between EDTA and CHX, they could be combined together. Further studies have to be performed to examine the combined effects of EDTA and CHX in inhibiting matrix bound MMPs in root canals.
Acknowledgments
Research supported by NIDCR grant R01 DE015306-06 (PI David Pashley)
The work was supported, in part, by R01 DE015306 from the NIDCR (PI: DHP). This work satisfied the requirement for a M.S. in Oral Biology degree to Dr. Thompson.
Footnotes
The authors deny any conflicts of interest.
The opinions or assertions contained herein are the private views of the author and not to be construed as official or as reflecting the views of the U.S. Army Medical Department, Department of the Army, or the Department of Defense. Citation of commercial organizations and trade names in the manuscript do not constitute any official Department of the Army of Department of Defense endorsement or approval of the products or services of these organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benkel BH, Rising DW, Goldman LB, Rosen H, Goldman M, Kronman JH. Use of a hydrophilic plastic as a root canal filling material. J Endod. 1976;2:196–202. doi: 10.1016/S0099-2399(76)80133-1. [DOI] [PubMed] [Google Scholar]
- 2.Kronman JH, Goldman M, Goldman LB, Coleman E, Kilment CK. Microbiologic evaluation of poly-HEMA root canal filling material. Oral Surg Oral Med Oral Pathol. 1979;48:175–7. doi: 10.1016/0030-4220(79)90057-4. [DOI] [PubMed] [Google Scholar]
- 3.DeMunck J, Vargas M, Van Landuyt K, Hikita K, Lambrechts P, Van Meerbeek B. Bonding of an auto-adhesive luting material to enamel and dentin. Dent Mater. 2004;30:963–71. doi: 10.1016/j.dental.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Zmener O, Pameijer CH, Serrano SA, Vidueira M, Macchi RH. Significance of moist root canal dentin with the use of methacrylate-based endodontic sealers: an in vitro coronal dye leakage study. J Endod. 2008;34:76–9. doi: 10.1016/j.joen.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Shipper G, Orstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon) J Endod. 2004;30:342–7. doi: 10.1097/00004770-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Lawson MS, Loushine B, Mai S, Weller RN, Pashley DH, Tay FR, et al. Resistance of a 4-META-containing, methacrylate-based sealer to dislocation in root canals. J Endod. 2008;34:833–7. doi: 10.1016/j.joen.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Carrilho MRO, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley DH. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 9.Gendron R, Greiner D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breschi L, Mazzoni A, Nato F, Carrilho R, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorgio EDS, Pashley DH. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent Mater. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnero P, Ferraras M, Karsdal M, Nicamhlaoibh R, Ristell J, Borel O, Qvist P, Delmas PD, Fogel NT, Delaisse JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Mineral Res. 2003;18:859–67. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 12.Patterson ML, Atkinson SJ, Knäuper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2 is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Letters. 2001;503:158–62. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 13.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley DH, Tay FR, Toledano M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Nishitani Y, Yoshiyama M, Wadgonkar B, Elrod D, Breschi L, Mannello F, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 16.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State-of-the-art of etch-and-rinse adhesives. Dent Mater. 2001;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity of radicular dentin. J Endod. 2006;32:862–8. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Carrilho MRO, Tay FR, Donnelly AM, Agee KA, Tjäderjame, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin stiffness associated with solubilization of collagen. J Biomed Mater Res Part B Appl Biomater. 2009;90B:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarada K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Ann Biomed Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagase H, Visse R, Murphy G. Structure and function of metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. Crit Rev Oral Biol Med. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 22.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003;36:810–30. doi: 10.1111/j.1365-2591.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microsopic comparison of a high volume final flush with several irrigating solutions. Part 3 J Endod. 1983;9:137–41. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 25.Prado M, Gusman H, Gomes BP, Sanao RA. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J Endod. 2011;37:255–8. doi: 10.1016/j.joen.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement for zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent Mater. 2010;26:1059–1067. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, et al. Critical review of methacrylates resin-based root canal sealers. J Endod. 2010;36:383–399. doi: 10.1016/j.joen.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjäderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrilho MB, Carvalho RM, Sousa EN, Nicolau J, Breschi L, Mazzoni A, Tjäderhane L, Tay FR, Agee KA, Pashley DH. Substantivity of chlorhexidine to human dentin. Dent Mater. 2010;26:779–85. doi: 10.1016/j.dental.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]