SUMMARY
Ribonucleotide reductase (RNR) is an essential enzyme required for DNA synthesis and repair. Although iron is necessary for class Ia RNR activity, little is known about the mechanisms that control RNR in response to iron deficiency. In this work, we demonstrate that yeast cells control RNR function during iron deficiency by redistributing the Rnr2–Rnr4 small subunit from the nucleus to the cytoplasm. Our data support a Mec1/Rad53-independent mechanism in which the iron-regulated Cth1/Cth2 mRNA-binding proteins specifically interact with the WTM1 mRNA in response to iron scarcity, and promote its degradation. The resulting decrease in the nuclear-anchoring Wtm1 protein levels leads to the redistribution of the Rnr2–Rnr4 heterodimer to the cytoplasm, where it assembles as an active RNR complex and increases deoxyribonucleoside triphosphate levels. When iron is scarce, yeast selectively optimizes RNR function at the expense of other non-essential iron-dependent processes that are repressed, to allow DNA synthesis and repair.
Keywords: ribonucleotide reductase, iron, Saccharomyces cerevisiae, yeast, Cth2
INTRODUCTION
Ribonucleotide reductase (RNR) is an essential enzyme that catalyzes the reduction of ribonucleoside diphosphates to the corresponding deoxy forms. RNR constitutes the rate-limiting step in the de novo synthesis of deoxyribonucleoside triphosphates (dNTPs), which are the precursors for DNA synthesis and repair. Of the three major classes of RNRs, class Ia are conserved from yeasts to mammals (Cotruvo and Stubbe, 2011a). They are composed of a large R1 subunit, where the catalytic site and two allosteric effector binding sites reside, and a small R2 subunit, which harbors a di-iron (di-Fe) center that is responsible for generating and maintaining a tyrosyl radical required for catalysis. In the budding yeast Saccharomyces cerevisiae, the large R1 subunit is composed of an Rnr1 homodimer, whereas the active small R2 subunit is formed by an Rnr2–Rnr4 heterodimer (Huang and Elledge, 1997; Perlstein et al., 2005). Only Rnr2 contains the essential di-Fe tyrosyl radical cofactor (Perlstein et al., 2005). Rnr4, which shares sequence and structural homology with Rnr2, lacks key ligands for Fe-binding but contributes to the correct folding and assembly of the di-Fe center in Rnr2 (Huang and Elledge, 1997; Ortigosa et al., 2006; Sommerhalter et al., 2004; Voegtli et al., 2001; Wang et al., 1997).
The activity of RNR is tightly regulated by the cell cycle and environmental cues in order to generate and maintain proper dNTP pools that ensure the fidelity of DNA synthesis and repair. In addition to allosteric regulation, yeast cells possess three characterized mechanisms that modulate RNR activity, all of which are regulated by the Mec1/Rad53/Dun1 checkpoint kinase pathway. First, in response to DNA damage and replication blockage, a checkpoint-dependent phosphorylation and release of the Crt1-Ssn6-Tup1 repressor complex from the RNR gene promoters leads to an increase in transcription of genes including RNR2, RNR3 and RNR4 (Huang et al., 1998). Second, in response to genotoxic stress and during S phase, the yeast R1 inhibitor protein Sml1 undergoes a checkpoint-dependent phosphorylation and degradation that relieves RNR inhibition (Chabes et al., 1999; Zhao et al., 2001; Zhao et al., 1998). A third mechanism regulates the subcellular distribution of the RNR subunits. Under normal conditions, the Rnr1 homodimer is predominantly localized to the cytoplasm, whereas the Rnr2–Rnr4 heterodimer localizes to the nucleus of the cell, except during the S phase of the cell cycle (Yao et al., 2003). The nuclear localization of the Rnr2–Rnr4 complex is achieved by a dual mechanism. The nuclear WD40 protein Wtm1 binds to Rnr2–Rnr4 and anchors the complex to the nucleus limiting its export (Lee and Elledge, 2006; Zhang et al., 2006), whereas Dif1 facilitates the nuclear import of the Rnr2–Rnr4 heterodimer by directly interacting with the complex (Lee et al., 2008; Wu and Huang, 2008). In response to genotoxic stress, the Rnr2–Rnr4 heterodimer redistributes in a checkpoint-dependent manner from the nucleus to the cytoplasm, where it presumably assembles with the large subunit to form the active RNR holoenzyme (Yao et al., 2003). DNA-damage induced R2 redistribution involves the checkpoint-dependent regulation of both Wtm1 and Dif1. When cells encounter DNA damage, the association between Rnr2–Rnr4 and Wtm1 in the nucleus is disrupted leading to release of Rnr2–Rnr4 from the nucleus, while Dif1 is phosphorylated and degraded, thereby diminishing nuclear import. DNA damage-induced redistribution of RNR subunits has also been reported in fission yeast, plant and mammalian cells, although the underlying mechanisms have not been elucidated.
Fe is an essential cofactor in the class Ia RNRs and an indispensable micronutrient for all eukaryotic organisms. However, the low solubility of Fe3+ at physiological pH highly restricts the availability of Fe for living organisms. Indeed, Fe deficiency is the most common and widespread nutritional disorder in the world, predominantly affecting women and children. Studies in the model organism S. cerevisiae have importantly contributed to advance in the characterization of the molecular strategies that eukaryotic cells use to adapt to Fe limitation. Under Fe-sufficient conditions, Fe enters yeast cells through low-affinity transporters such as the plasma membrane protein Fet4 (Dix et al., 1994). In response to Fe depletion, yeast cells activate the transcription of a group of genes, denoted as the Fe regulon, which increase Fe acquisition (such as the high-affinity Fe uptake complex Ftr1-Fet3), mobilize and recycle intracellular Fe, and promote a coordinated genome-wide remodeling of Fe-dependent pathways (Kaplan et al., 2006). The metabolic adaptation to Fe depletion is, in part, mediated by two Fe-deficiency induced proteins, Cth1 and Cth2, characterized by an RNA-binding motif consisting of two tandem zinc-fingers (TZFs) of the Cx8Cx5Cx3H-type, which are conserved in the mammalian tristetraprolin (TTP) family of mRNA destabilizing proteins (Puig et al., 2005; Puig et al., 2008). Cth1 and Cth2 interact through their TZFs with AU-rich elements (AREs) within the 3’-untranslated region (3’-UTR) of select groups of mRNAs, promoting specific destabilization of the bound transcripts (Pedro-Segura et al., 2008). Upon Fe depletion, Cth1 and Cth2 promote the coordinated decay of many mRNAs encoding proteins that participate in Fe-storage and in processes with elevated Fe demands including the mitochondrial electron transport chain, the tricarboxylic acid (TCA) cycle, synthesis of multiple amino acids, and heme biosynthesis (Ihrig et al., 2010; Puig et al., 2005; Puig et al., 2008). As cth1Δcth2Δ mutants exhibit a growth defect under Fe deficiency (Puig et al., 2005), it has been proposed that Fe depletion-mediated metabolic remodeling may serve to optimize Fe utilization by prioritizing essential Fe-requiring processes such as Fe-S cluster synthesis or RNR function over other non-essential pathways including mitochondrial respiration (Kaplan et al., 2006; Massé et al., 2007; Vergara and Thiele, 2008).
Although class Ia RNRs utilize Fe as an essential cofactor for catalysis, little is known about the mechanisms that control RNR function upon Fe deficiency. In this report, we uncover a mechanism that regulates RNR function during Fe starvation. The R2 subunit is redistributed from the nucleus to the cytoplasm upon Fe depletion in a manner that is independent of the checkpoint kinases Mec1 and Rad53. Instead, a strategy involving Cth1 and Cth2-mediated turnover of the WTM1 mRNA promotes Rnr2–Rnr4 relocalization to the cytoplasm. Furthermore, Cth1 and Cth2 induce the down-regulation of RNR2 and RNR4 mRNAs leading to a multilayered mechanism of RNR regulation that optimizes RNR function when Fe bioavailability is limited.
RESULTS
Ribonucleotide reductase function under iron-deficient conditions
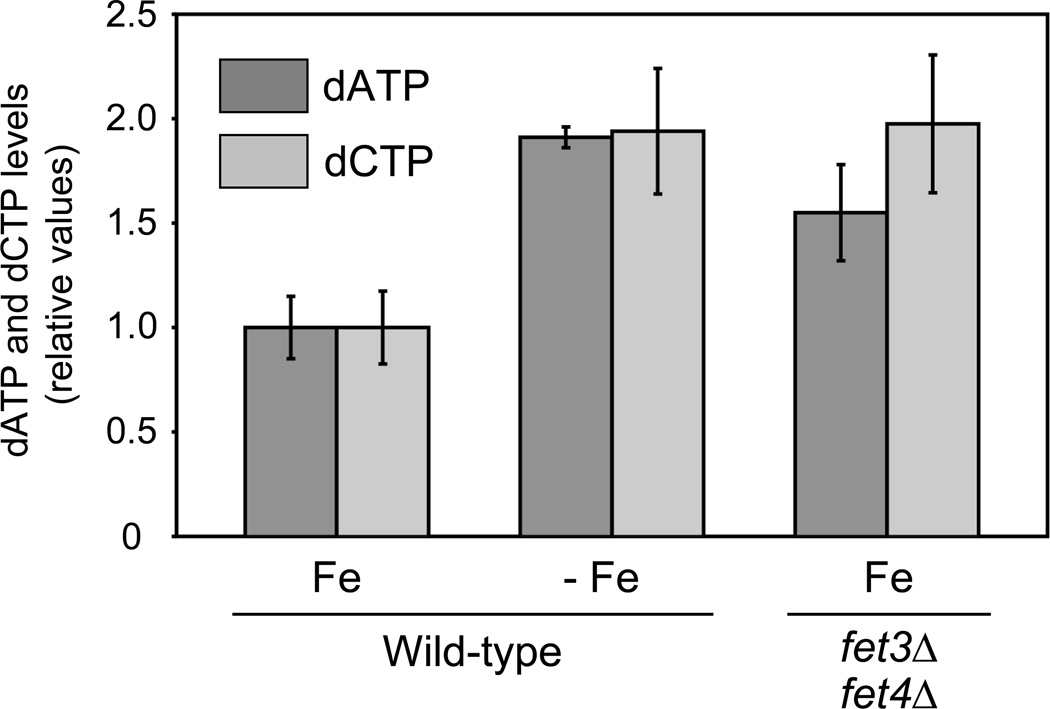
Multiple metabolic pathways that depend on Fe as an essential cofactor, such as respiration, synthesis of some amino acids and heme synthesis, are down-regulated in response to Fe deficiency in S. cerevisiae (Ihrig et al., 2010; Puig et al., 2005; Shakoury-Elizeh et al., 2004). Given that the essential enzyme RNR requires a di-Fe cofactor for catalysis, we investigated RNR function under different conditions of Fe bioavailability. For this purpose, we grew wild-type cells under Fe-sufficient conditions (Fe) as well as in Fe-deficient conditions (−Fe) induced by the addition of 100 µM of the membrane-impermeable Fe2+-specific chelator bathophenanthroline disulfonic acid (BPS), and determined intracellular deoxyadenosine triphosphate (dATP) and deoxycytidine triphosphate (dCTP) concentrations. Our previous data indicate that these conditions of Fe deficiency induce the down-regulation of multiple Fe-using pathways including respiration (Puig et al., 2005; Puig et al., 2008). Interestingly, we observed that, after seven hours of growth on Fe-deficient conditions, the yeast intracellular dATP and dCTP levels did not diminish but displayed a 2-fold increase (Figure 1). To further ascertain whether the increase in dATP and dCTP levels was due to Fe-deficiency and not to a secondary effect of BPS, we deleted FET3 and FET4 genes, which are required for high- and low-affinity Fe transport respectively. As shown in Figure 1, fet3Δfet4Δ cells grown under Fe-sufficient conditions display an increase in dATP and dCTP levels when compared to wild-type cells grown under the same conditions. The increase in dATP and dCTP levels is unlikely caused by perturbation of cell cycle by Fe chelation as no significant difference in cell cycle distribution was observed between cells grown in the absence and presence of BPS for the initial eight hours (data not shown). These results strongly suggest that RNR function, which depends on Fe as an essential cofactor, is sustained under nutritional and genetic Fe deficiencies.
Figure 1. Yeast dATP and dCTP levels increase upon nutritional or genetic iron deficiency.
Wild-type BY4741 and fet3Δfet4Δ cells were grown for 7 hours in SC (Fe) or SC with 100 µM BPS (−Fe), and dATP and dCTP levels determined by the DNA polymerase-based enzymatic assay. The values of dATP and dCTP are represented as relative to those of the wild-type strain grown under Fe-sufficient conditions. The average and standard deviation of at least 3 independent experiments is represented.
Subcellular redistribution of RNR small subunits in response to Fe deficiency
An important mechanism for RNR activation consists of the subcellular redistribution of the Rnr2–Rnr4 subunits from the nucleus to the cytoplasm, where the RNR holoenzyme assembles (Yao et al., 2003). In order to investigate whether changes in the cellular availability of Fe had an effect on the subcellular distribution of Rnr2–Rnr4, we grew wild-type cells in Fe-sufficient (Fe) and Fe-deficient conditions (−Fe) induced by the addition of 100 µM BPS, and examined Rnr2 and Rnr4 subcellular localization by indirect immunofluorescence (IMF) (Yao et al., 2003). As shown in Figure 2 (panels A and B), both Rnr2 and Rnr4 relocalize from the nucleus to the cytoplasm upon incubation with BPS. Similar results were obtained for genomically integrated carboxy-terminal GFP-tagged versions of Rnr2 and Rnr4 proteins (Supplemental Figure S1A; and data not shown). To quantitatively analyze the RNR subcellular pattern, we classified cells according to Rnr2 and Rnr4 distribution (Supplemental Figure S1B). Thus, under Fe-sufficient conditions (Fe), >80% of the cells exhibit Rnr2 and Rnr4 IMF signals that are predominantly nuclear (Figure 2C,D). However, under Fe-deficient conditions (−Fe), only 10% of the cells display a predominantly Rnr2 and Rnr4 IMF nuclear signal and 60–80% of the cells show a cytoplasmic pattern (Figure 2C,D). A similar redistribution was observed for the GFP-tagged versions of Rnr2 and Rnr4 proteins (Supplemental Figure S1C,D). At least six hours were necessary to complete the R2 nuclear exit when cells grown overnight under an Fe-sufficient medium were reinoculated in the BPS-containing medium (Supplemental Figure S1E). To further investigate the Fe-deficiency specificity of Rnr2–Rnr4 redistribution, we examined the localization of both proteins in the fet3Δfet4Δ mutant, which is genetically defective in Fe uptake. As shown in Figure 2E and 2F, >80% of the fet3Δfet4Δ cells display a predominantly cytoplasmic localization pattern for both Rnr2 and Rnr4 even under Fe-sufficient conditions (Fe), whereas the wild-type cells display a predominantly nuclear pattern for both proteins under these conditions (Figure 2A,B). Supplementation of 100 µM ferrous ammonium sulfate (FAS) (+Fe) led to the redistribution of both Rnr2 and Rnr4 to the nucleus of fet3Δfet4Δ cells (Figure 2E,F). Taken together, these results demonstrate that the small R2 subunit of yeast RNR redistributes from the nucleus to the cytoplasm in response to nutritional or genetic Fe deficiency, perhaps to facilitate the assembly with the large R1 subunit and activate RNR function.
Figure 2. Yeast Rnr2 and Rnr4 redistribute to the cytoplasm in response to nutritional or genetic Fe deficiencies.
(A,B) Wild-type BY4741 cells were grown for 6 hours in SC (Fe) or SC with 100 µM BPS (−Fe). Then DNA was stained with 1 µg/mL DAPI for 3 min, and Rnr2 and Rnr4 localization determined by IMF with anti-Rnr2 (A) and anti-Rnr4 (B) antibodies, respectively. An overlap of DAPI and IMF signals is also shown (Merge). (C,D) Quantitative analysis of Rnr2 (C) and Rnr4 (D) subcellular localization patterns in wild-type cells grown as indicated in panels A and B. Percentages of cells with distinct localization patterns were represented as follows: black bars, cells with a predominantly nuclear IMF signal; grey bars, cells with both nuclear and cytoplasmic IMF signal; and white bars, cells with a predominantly cytoplasmic IMF signal. (E,F) Quantitation of Rnr2 (E) and Rnr4 (F) subcellular localization patterns in fet3Δfet4Δ cells grown for 6 hours in SC (Fe) or SC with 100 µM FAS (+Fe). Cells were processed and the results represented as indicated in panels C and D. In all cases, at least 200 cells were counted for each independent experiment performed by triplicate. The average and the standard deviation are represented. See also Supplemental Figure S1.
The redistribution of RNR small subunits in response to Fe deficiency is independent of the Mec1 and Rad53 checkpoint kinases
Previous studies have demonstrated that the subcellular redistribution of the yeast RNR small subunits in response to genotoxic stress is regulated by the DNA damage checkpoint pathway. To ascertain whether the DNA damage checkpoint pathway is activated in response to Fe deprivation, we analyzed Rad53 and Dun1 proteins by immunoblotting. As reported previously, upon treatment with the replication-blocking agent hydroxyurea (HU) or the DNA-damaging agent methyl methanesulfonate (MMS), the DNA damage checkpoint pathway is activated leading to an electrophoretic mobility shift due to phosphorylation of both Rad53 and Dun1 proteins (Bashkirov et al., 2003; Lee et al., 2003). By contrast, no obvious modification is observed when cells are grown on the nutritional Fe-deficient conditions that promote R2 relocalization (Figure 3A,B). Activation of the DNA damage checkpoint pathway by genotoxic stresses also promotes the derepression of RNR2 and RNR4 transcription (Huang et al., 1998). However, Fe deficiency does not induce RNR2 and RNR4 transcription (Supplemental Figure S2). Taken together, these results strongly suggest that the DNA damage checkpoint pathway is not activated when the availability of Fe is low and, furthermore, they predict that the relocalization of the RNR subunits in response to Fe depletion should occur in mutants defective in this signaling cascade. To test this notion, we examined the Rnr2 and Rnr4 localization patterns in cells deficient for the checkpoint kinases Mec1 and Rad53. As previously reported, treatment of wild-type cells with HU or MMS leads to the nuclear to cytoplasmic redistribution of Rnr2 and Rnr4 in a Mec1- and Rad53-dependent manner ((Yao et al., 2003), Figure 3C–H). In contrast, redistribution of Rnr2–Rnr4 upon low Fe availability occurs in the mec1Δsml1Δ and rad53Δsml1Δ mutants to the same extent as in the wild-type cells (Figure 3C–H). Taken together, these results demonstrate that the redistribution of Rnr2 and Rnr4 in response to Fe deficiency is independent of the Mec1 and Rad53 kinases, and they suggest that it may depend on an uncharacterized regulatory mechanism.
Figure 3. The Mec1 and Rad53 kinases do not participate in the redistribution of Rnr2 and Rnr4 to the cytoplasm in response to Fe deficiency.
The phosphorylation stage of Rad53 (A) and Dun1 (B) proteins was determined. Wild-type BY4741 (A) and Dun1-MYC13 (B) cells were grown on SC without (Fe) or with 100 µM BPS (−Fe) for 6 hours, SC with 0.04 % MMS for 2 hours, or SC with 0.2 M HU for 2 hours. Proteins were extracted and analyzed by Western blotting with anti-Rad53 (A) and anti-c-Myc (B) antibodies. Pgk1 was used as a loading control. Wild-type Y300 (C,D), mec1Δsml1Δ (E,F), and rad53Δsml1Δ cells (G,H) were grown as described in panel A, and cells were processed and the data analyzed as described in Figure 2. See also Supplemental Figure S2.
The Fe-regulated Cth1 and Cth2 proteins promote the redistribution of the RNR small subunits to the cytoplasm in response to Fe deficiency
Two mRNA-binding proteins, Cth1 and Cth2, play a crucial role in the adaptation of yeast cells to low Fe conditions by post-transcriptionally inducing the coordinated degradation of many transcripts encoding proteins that participate in Fe-dependent processes (Ihrig et al., 2010; Puig et al., 2005; Puig et al., 2008). To gain insight into the mechanisms that lead to the RNR subunit redistribution as a consequence of Fe depletion, we ascertained whether CTH1 and CTH2 had an effect on the Rnr2 and Rnr4 distribution pattern. Toward this end, we determined the subcellular distribution of both Rnr2 and Rnr4 in cth1Δcth2Δ cells grown under Fe-sufficient (Fe) and Fe-deficient (−Fe) conditions. Similar to wild-type cells, the cth1Δcth2Δ mutants displayed a predominantly nuclear localization pattern for Rnr2 and Rnr4 when grown under Fe sufficiency (Supplemental Figure S3A). Interestingly, upon Fe starvation, which activates CTH1 and CTH2 expression, cth1Δcth2Δ mutants exhibit a significant defect in RNR small subunit redistribution to the cytoplasm as compared to wild-type cells (Supplemental Figure S3A). While less than 20% of the wild-type cells retain R2 in the nucleus upon Fe depletion, 50–70% of the cth1Δcth2Δ mutant cells still display a predominantly nuclear pattern for Rnr2 and Rnr4. It is important to highlight that the residual R2 redistribution displayed by cth1Δcth2Δ mutants upon Fe deficiency is not a consequence of activation of the DNA damage checkpoint kinase pathway as indicated by the lack of Rad53 phosphorylation and RNR2/RNR4 transcriptional activation (Supplemental Figure S3B,C). Taken together, these results strongly suggest that Cth1 and Cth2 participate in the mechanisms that regulate the RNR small subunit redistribution occurring upon Fe limitation.
The role of Cth1 and Cth2 in the adaptation of S. cerevisiae to Fe deficiency is known to be mediated by targeted mRNA binding and degradation. Two Cx8Cx5Cx3H-type TZFs within the carboxy-terminal region of both proteins are essential for the interaction between Cth1 and Cth2 proteins with ARE-containing mRNAs that leads to a coordinated transcript turnover. Indeed, Cth1 and Cth2 proteins harboring mutations at a specific cysteine residue within their TZFs (specifically Cth1-C225R and Cth2-C190R mutants) are not functional because they have lost the ability to bind and promote the destabilization of their target transcripts (Pedro-Segura et al., 2008; Puig et al., 2005; Puig et al., 2008). To test a potential role played by the targeted mRNA degradation activity of Cth1 and Cth2 proteins on the regulation of the RNR small subunit relocalization upon Fe starvation, we investigated the subcellular distribution pattern of Rnr2 and Rnr4 in cells expressing two TZF-mutant alleles of CTH1 and CTH2. For this purpose, cth1Δcth2Δ cells were co-transformed with plasmids expressing CTH1 and CTH2, CTH1-C225R and CTH2-C190R, or empty vectors. We observed that whereas cth1Δcth2Δ cells expressing wild-type CTH1 and CTH2 export Rnr2 and Rnr4 to the cytoplasm at an identical extent to wild-type cells, cth1Δcth2Δ cells expressing the CTH1-C225R and CTH2-C190R mutant alleles display a defect in RNR small subunit redistribution to the cytoplasm similar to that of the cth1Δcth2Δ mutants with (or without) the empty vectors (Figure 4). These results strongly suggest that Cth1 and Cth2 proteins promote the redistribution of Rnr2 and Rnr4 to the cytoplasm in response to Fe starvation through their mRNA binding and decay activity.
Figure 4. Cells defective in CTH1 and CTH2 exhibit a defect in the redistribution of Rnr2 and Rnr4 to the cytoplasm in response to Fe deficiency.
cth1Δcth2Δ cells co-transformed with pRS416-CTH1 and pRS415-CTH2 (CTH1 + CTH2), pRS416 and pRS415 (cth1Δ + cth2Δ), or pRS416-CTH1-C225R and pRS415-CTH2-C190R (CTH1-C225R + CTH2-C190R), were grown for 6 hours in SC (Fe) or SC with 100 µM BPS (−Fe), and then processed and the data analyzed as described in Figure 2. See also Supplemental Figure S3.
Cth1 and Cth2 promote the decrease of Wtm1 levels in response to Fe deficiency
To gain insight into the mechanism by which Cth1 and Cth2 promote the subcellular redistribution of RNR subunits during Fe depletion, we analyzed the genome-wide expression data under Fe-deficient conditions reported previously for cth1Δcth2Δ cells containing an empty vector, or expressing either CTH1 or CTH2. Interestingly, we observed that cth1Δcth2Δ cells expressing CTH2 display a 2.3–3.0 down-regulation of WTM1 mRNA levels when compared to cth1Δcth2Δ cells grown under Fe scarcity (Puig et al., 2005; Puig et al., 2008). Under non-stressful conditions the nuclear WD40 protein Wtm1 interacts with Rnr2–Rnr4 and anchors the heterodimer to the nucleus limiting its export. To further test whether Cth1 and Cth2 regulate WTM1 mRNA in response to Fe deficiency, we analyzed the WTM1 expression pattern of cth1Δcth2Δ cells co-transformed with wild-type CTH1 and CTH2, empty vectors, or the TZF-mutant alleles of CTH1 and CTH2 grown under both Fe-sufficient and Fe-deficient conditions. As shown in Figure 5A, cells expressing wild-type CTH1 and CTH2 display a decrease in WTM1 mRNA levels upon Fe deficiency. Notably, WTM1 down-regulation by Fe depletion does not occur in cells lacking CTH1 and CTH2 or expressing the TZF mutant alleles (Figure 5A). These data indicate that WTM1 mRNA levels are regulated by Fe availability in a Cth1/Cth2-dependent manner.
Figure 5. Cth1 and Cth2 promote the down-regulation of Wtm1 levels in response to Fe deficiency.
cth1Δcth2Δ cells co-transformed with pRS416 and pRS415 (vector + vector), pRS416-CTH1 and pRS415-CTH2 (CTH1 + CTH2), or pRS416-CTH1-C225R and pRS415-CTH2-C190R (C225R + C190R), were grown for 8 hours in SC-ura-leu (+) or SC-ura-leu with 100 µM BPS (−). (A) Total RNA was extracted and analyzed by Northern blotting with a WTM1 specific probe. ACT1 was used as a loading control. (B) Proteins were extracted and analyzed by Western blotting with anti-c-Myc. Ponceau staining was used as a loading control.
Given that Wtm1 protein is responsible for Rnr2 and Rnr4 anchoring to the nucleus under Fe-sufficient conditions, it is tempting to speculate that the Cth1/Cth2-mediated down-regulation of WTM1 mRNA could lead to a decrease in Wtm1 protein levels that promotes the release of Rnr2 and Rnr4 proteins from the nucleus. To test this hypothesis, we tagged the genomic copy of WTM1 with a 3MYC epitope at its amino-terminus, without altering its endogenous promoter and 3’-UTR, and determined 3MYC-Wtm1 protein levels under Fe-sufficient and Fe-deficient conditions. As shown in Figure 5B, cells expressing both CTH1 and CTH2 exhibit a decrease in 3MYC-Wtm1 protein levels in response to Fe deficiency. In contrast, Wtm1 protein levels do not significantly change upon Fe limitation in cells lacking CTH1 and CTH2 or expressing the CTH1-C225R and CTH2-C190R mutant alleles. Together, our data strongly suggest that Cth1 and Cth2 stimulate the decrease of Wtm1 protein levels in response to Fe deficiency.
Cth1 and Cth2 proteins specifically interact with WTM1 mRNA
Cth1 and Cth2 proteins interact with and recruit the Dhh1 RNA helicase to specific ARE-containing mRNAs, promoting the 5’ to 3’ degradation of the bound transcript (Pedro-Segura et al., 2008). To ascertain whether the down-regulation of WTM1 mRNA is a direct consequence of Cth2-binding to the WTM1 transcript or an indirect effect of Cth2-mediated gene expression remodeling, we examined the potential interaction between Cth2 protein and the WTM1 mRNA using the yeast three-hybrid system (Y3H) (Supplemental Figure S4A; SenGupta et al., 1996). A visual inspection of WTM1 3’-UTR shows that it contains two potential ARE patches (Supplemental Figure S4B). Therefore, 141 base pairs from the WTM1 3’ region containing both putative AREs were fused to the bacteriophage MS2 RNA, and co-expressed in a HIS3 and LacZ reporter yeast strain with a Cth1- or Cth2-Gal4 transactivation domain fusion protein. The interactions between Cth1/Cth2 and the WTM1 fusion RNA were monitored by growth on media lacking histidine (−His) or by measuring reporter gene-driven β-galactosidase activity. As a control, the 3’-UTR of the succinate dehydrogenase SDH4 mRNA, a well-known target of Cth2, was included in the assay (Pedro-Segura et al., 2008; Puig et al., 2005). As shown in Figure 6A, cells co-expressing the wild-type CTH1 or CTH2 with either the SDH4 or the WTM1 3’-UTR fusion RNAs grew in media without histidine, indicative of an interaction between Cth1/Cth2 and the 3’-UTR of both SDH4 and WTM1 mRNAs. The Cth1/Cth2-WTM1 3’UTR interaction was abolished in the CTH1-C225R and CTH2-C190R mutants suggesting that the integrity of the Cth1/Cth2 TZFs is required for its interaction with the WTM1 3’-UTR (Figure 6A). To further evaluate the specificity of the Cth2 interaction with WTM1 mRNA, we assayed Cth1/Cth2-binding to WTM1 mutant alleles in which the first (mt1), the second (mt2), or both putative AREs (mt3) within the WTM1 3’-UTR mRNA were mutated (Supplemental Figure S4B). Mutation of both AREs within WTM1 3’-UTR (WTM1-mt3 allele) is necessary to completely abrogate growth on media lacking histidine without altering growth on the synthetic complete medium (Figure 6A). These results were quantitatively corroborated for Cth2-WTM1 interaction by determining β-galactosidase activity of the LacZ reporter (Supplemental Figure S4C). Taken together, these results indicate that Cth1 and Cth2 specifically bind in vivo to the 3’-UTR of the WTM1 mRNA in a manner that is dependent upon the functional integrity of the Cth2 TZF RNA-binding motif and both AREs within the WTM1 mRNA.
Figure 6. Cth1 and Cth2 proteins specifically interact with WTM1 mRNA to promote Rnr2 and Rnr4 redistribution to the cytoplasm and sustain RNR function in response to Fe deficiency.
(A) The Y3H assay was used to monitor in vivo interactions between Cth1 or Cth2 proteins and the ARE-containing fragment of the WTM1 3’-UTR mRNA. L40-coat cells were co-transformed with (1) pIIIA/MS2-1 containing the 3’-UTR of WTM1, WTM1-mt1, WTM1-mt2, WTM1-mt3, SDH4 (as a positive control), or vector alone (as a negative control); and (2) pACT2 vector alone or fused to CTH1, CTH1-C225R, CTH2 or CTH2-C190R. Cells were grown on SC-ura-leu (+His), and SC-ura-leu-his (−His) containing 250 or 750 µM 3-aminotriazol (3-AT) plates for 2–6 days at 30°C. (B) Yeast cells mutagenized in both WTM1 ARE patches exhibit a defect in the redistribution of Rnr2 and Rnr4 to the cytoplasm in response to Fe deficiency similar to CTH1- and CTH2-defective cells. WTM1-mt3 cells were grown for six hours in SC (Fe) or SC with 100 µM BPS (−Fe), and then processed and the data analyzed as described in Figure 2. (C) Determination of dATP and dCTP levels in cth1Δcth2Δ and WTM1-mt3 cells. Yeast cells were grown for seven hours in SC (Fe) or SC with 100 µM BPS (−Fe), and analyzed as described in Figure 1. The values of dATP and dCTP from the wild-type strain, previously shown in Figure 1, have been included for clarity. See also Supplemental Figure S4.
WTM1 AREs are essential for the Cth1/Cth2-mediated redistribution of RNR small subunits to the cytoplasm in response to Fe deficiency
Multiple lines of evidence shown here indicate that Cth2 promotes both the down-regulation of Wtm1 protein levels and the redistribution of Rnr2 and Rnr4 to the cytoplasm in response to Fe deficiency. However, these results do not exclude the possibility that the Cth1 and Cth2 regulation of RNR small subunit localization also functions through WTM1-independent mechanisms. To ascertain whether the down-regulation of WTM1 mRNA promoted by Cth1 and Cth2 is the reason for the Rnr2 and Rnr4 redistribution to the cytoplasm occurring under low Fe conditions, we constructed a yeast strain in which both WTM1 AREs have been mutated (WTM1-mt3 mutant cells). Mutagenesis of both AREs abrogates the WTM1 mRNA down-regulation observed in wild-type cells or a strain containing the KanMX6 cassette (WTM1-Kan cells) integrated at the same position than in WTM1-mt3 mutants (Supplemental Figure S4D). Importantly, cells expressing the WTM1-mt3 allele display a significant defect in RNR small subunit redistribution to the cytoplasm upon Fe deficiency (Figure 6B), similar to cth1Δcth2Δ cells and cells expressing the TZF mutant alleles (Figure 4). These data strongly suggest that the binding of Cth1 and Cth2 proteins to the AREs within WTM1 transcript promote a decrease in Wtm1 protein levels that leads to the redistribution of RNR small subunits to the cytoplasm in response to Fe deficiency.
Cth1 and Cth2 proteins promote RNR function in response to Fe deficiency
To ascertain the effect of Cth1- and Cth2-mediated regulation of Rnr2–Rnr4 redistribution via WTM1 mRNA on RNR function, we determined dATP and dCTP levels in the WTM1-mt3 mutant, which contains mutations on both AREs of its 3’-UTR, and compared to wild-type cells grown under both Fe-sufficient and Fe-deficient conditions. Under Fe-sufficient conditions WTM1-mt3 mutants exhibit dATP and dCTP levels similar to those observed in wild-type cells (Figure 6C). However, while wild-type cells display a 2-fold increase in their dATP and dCTP levels upon Fe deficiency, dATP and dCTP concentrations remain relatively unchanged in WTM1-mt3 mutants under Fe-deficient conditions (Figure 6C). These results indicate that the ARE-mediated down-regulation of WTM1 mRNA contributes to the optimization of RNR function under Fe-deficient conditions. We then determined the contribution of Cth1 and Cth2 to the RNR function by measuring dATP and dCTP levels in the cth1Δcth2Δ mutant. Similar dATP and dCTP concentrations were observed for wild-type and cth1Δcth2Δ cells under Fe-sufficient conditions, with a slight decrease in dATP levels observed for cth1Δcth2Δ mutants (Figure 6C). Interestingly, in response to Fe deficiency the dATP and dCTP levels in cth1Δcth2Δ mutants decrease ~2.5-fold, whereas they increase ~2-fold in wild-type cells (Figure 6C). Collectively, these results indicate that, in addition to the down-regulation of WTM1 mRNA that leads to R2 subunit redistribution upon Fe-deficiency, Cth1 and Cth2 further contribute to the sustained RNR activity observed upon Fe depletion, likely by promoting the degradation of additional mRNAs that perhaps lead to an increase in Fe bioavailability for RNR instead of other non-essential and Fe-using pathways such as respiration.
Cth1 and Cth2 regulate the expression of Rnr2 and Rnr4 during Fe deficiency
We have shown that upon Fe scarcity yeast cells redistribute most R2 subunits to the cytoplasm, whereas dATP and dCTP levels only increase by 2-fold (Figures 1 and 2). Multiple factors including R2 protein levels could contribute to RNR activity during low Fe conditions. To further investigate this, we have determined mRNA and protein levels for both Rnr2 and Rnr4 under low Fe conditions. As shown in Figure 7 (panels A and B), both Rnr2/Rnr4 mRNA and protein levels decrease to around 30% and 65% of their respective original values in response to Fe deficiency. Interestingly, previous global gene expression studies under low Fe conditions have shown that cth1Δcth2Δ cells display a 1.4–1.8 increase in RNR2 and RNR4 mRNA levels when compared to cells expressing CTH1 and CTH2 (Puig et al., 2005; Puig et al., 2008), suggesting that Cth1 and Cth2 may regulate RNR2 and RNR4 mRNAs. Furthermore, the 3’-UTR of RNR2 and RNR4 transcripts contains putative AREs (Supplemental Figure S5). Therefore, we have used the Y3H assay to test a potential interaction between Cth1/Cth2 proteins and RNR2/RNR4 mRNAs. As shown in Figure 7C, both Cth1 and Cth2 proteins interact in vivo with the 3’-UTR of RNR2 and RNR4 mRNAs in a TZF-dependent manner. Consistent with these results, we observe a significant defect in the down-regulation of Rnr2 and Rnr4 mRNAs and protein levels upon Fe deficiency in cth1Δcth2Δ cells or cells expressing TZF mutant alleles of CTH1 and CTH2 (Figure 7A, B). These results demonstrate that Cth1 and Cth2 proteins control RNR function at multiple levels. In addition to promoting Rnr2/Rnr4 redistribution to the cytoplasm, Cth1 and Cth2 proteins tightly control the levels of Rnr2 and Rnr4 subunits in response to Fe deficiency.
Figure 7. Cth1 and Cth2 control the levels of Rnr2 and Rnr4 subunits in response to iron deficiency.
The levels of Rnr2 and Rnr4 mRNAs (A) and proteins (B) were determined in cth1Δcth2Δ cells co-transformed, grown, and processed as detailed in Figure 5. The levels of RNA and protein were quantified and normalized to SCR1 (RNA loading control) and Pgk1 (protein loading control), respectively. The Y3H assay was used to monitor in vivo interactions between Cth1 and Cth2 (C) proteins and the ARE-containing fragment of the RNR1 and RNR2 3’-UTR mRNAs. L40-coat cells were co-transformed with (1) pIIIA/MS2-1 containing the 3’-UTR of RNR2, RNR4, SDH4, and vector alone; and (2) pACT2 vector alone or fused to CTH1, CTH1-C225R, CTH2 or CTH2-C190R. Cells were grown as described in Figure 6. See also Supplemental Figure S5.
DISCUSSION
The low solubility of Fe at physiological pH has led to the development of sophisticated strategies to optimize iron acquisition and utilization in both prokaryotes and eukaryotes. The budding yeast S. cerevisiae promotes the replacement of Fe-dependent processes including glutamate synthase, biotin synthesis and respiration with Fe-independent alternatives when Fe becomes scarce (Puig et al., 2008; Shakoury-Elizeh et al., 2004). Similarly, the bacteria Escherichia coli responds to Fe deficiency by down-regulating multiple transcripts encoding dispensable Fe-using proteins thereby reducing cellular demands for Fe (Massé et al., 2007). Interestingly, E. coli also activates a manganese-dependent class Ib RNR isoenzyme, which is responsible for DNA synthesis so long as the Fe restriction persists (Cotruvo and Stubbe, 2011b; Martin and Imlay, 2011). Such a substitution is not possible in eukaryotes because the only RNR enzyme available fully depends on Fe as a cofactor. In mammals, a severe reduction in Fe bioavailability leads to significant decreases in RNR activity, dNTP pools, DNA synthesis, and consequently cell proliferation (Cavanaugh et al., 1985; Furukawa et al., 1992). Interestingly, in some cases RNR activity seems to be maintained or increased during the early stages of Fe deficiency (Furukawa et al., 1992), suggesting that cells may possess strategies to optimize the essential function of RNR over other less important Fe-using pathways when Fe becomes limiting.
In this work, we have shown that in response to Fe deficiency S. cerevisiae activates specific mechanisms to preferentially sustain RNR function over other non-essential Fe-using processes. A decrease in the intracellular Fe availability, achieved by either limiting the environmental Fe with specific chelators or abolishing the ability of yeast cells to efficiently acquire Fe (fet3Δfet4Δ mutant), activates the translocation of the R2 subunits to the cytoplasm where R1 subunits constitutively reside. This subcellular redistribution of R2 subunits seems crucial to sustain RNR function under Fe deficient conditions.
Our data indicate that the redistribution of yeast R2 subunits to the cytosol in response to Fe deficiency is independent of the Mec1 and Rad53 checkpoint kinases. First, Rad53 and Dun1 proteins are not phosphorylated in response to low Fe. Second, transcription of RNR2 and RNR4 is not induced by low Fe conditions. And third, mec1Δsml1Δ and rad53Δsml1Δ cells do not exhibit any defect in R2 redistribution to the cytoplasm in response to Fe deficiency. We and others have previously demonstrated that, in response to Fe depletion, Cth1 and Cth2 proteins promote the degradation of many ARE-containing mRNAs encoding for proteins that participate in non-essential Fe-dependent metabolic pathways that are probably repressed under these conditions (Ihrig et al., 2010; Puig et al., 2005). Here we show that the Cth1 and Cth2-mediated degradation of WTM1 mRNA in response to low Fe leads to a decrease in Wtm1 protein levels that favors Rnr2–Rnr4 redistribution to the cytoplasm. The conserved TZF domains in Cth1 and Cth2, and the AREs within the 3’-UTR of WTM1 mRNA, are both required for R2 redistribution strongly suggesting that Cth1 and Cth2 control R2 localization primarily by targeting WTM1 transcript for degradation. Interestingly, we observe that cth1Δcth2Δ mutants display a more severe decrease in dATP and dCTP levels than that of the WTM1-mt3 cells under low Fe suggesting that Cth1 and Cth2 may promote RNR function via other mechanisms in addition to R2 redistribution. It is possible that the Cth1/Cth2-dependent down-regulation of dispensable Fe-using processes increases Fe bioavailability for RNR utilization. Consistent with this hypothesis, Cth1 and Cth2 specifically promote the down-regulation of both RNR2 and RNR4 transcripts while promoting redistribution of existing Rnr2 and Rnr4 proteins, probably to optimize the utilization of the scarce available Fe. Thus, our study uncovers how the RNA-binding Cth1 and Cth2 proteins control yeast RNR function at multiple levels in response to Fe deficiency. Further studies are necessary to ascertain additional mechanisms that regulate yeast and human RNRs in response to changes in Fe availability including intracellular Fe-cofactor delivery and loading into RNR.
EXPERIMENTAL PROCEDURES
Yeast strains, plasmids and growth conditions
Genotypes for the yeast strains used in this study are listed in Supplemental Experimental Procedures. For tagging the genomic copy of WTM1 with the 3MYC epitope at the amino-terminus without altering its endogenous promoter (strain SPY374), we used the pMPY-3xMYC plasmid. The pFA6a-13MYC-KanMX6 plasmid was used to tag DUN1 with the 13MYC epitope at the carboxy-terminus. The CTH1- and CTH2-containing plasmids, and the pIIIA/MS2-1-SDH4 plasmid used in this work have been described previously (Puig et al., 2005; Puig et al., 2008). The WTM1 3’-UTR was cloned into SmaI-digested pIIIA/MS2-1 vector (SenGupta et al., 1996). The mutagenesis of WTM1 AREs (mt1, mt2 and mt3) was generated by the overlap extension method (Puig et al., 2005). All PCR amplifications were performed with the Expand High Fidelity PCR System (Roche), and inserts were sequenced.
To determine the Rnr2 and Rnr4 distribution pattern and dNTP pools, and β-galactosidase assays, a yeast preculture was incubated overnight at 30°C in synthetic complete SC medium lacking specific requirements when necessary, reinoculated at A600nm = 0.2–0.4, and incubated between six and seven hours in SC (Fe-sufficient conditions: Fe), SC supplemented with 100 µM BPS (Fe-deficient conditions: −Fe), or SC supplemented with 100 µM FAS (Fe-excess conditions: +Fe) before processing. Treatment with 0.04% MMS and 0.2 M HU was performed during the last 2 hours of SC incubation. For RNA and protein analyses, cells were grown in SC lacking specific requirements (Fe +) or supplemented with 100 µM BPS (Fe −) for six to nine hours before processing. Y3H and β-galactosidase assays were performed as described previously (Puig et al., 2005; SenGupta et al., 1996).
Fluorescence microscopy
Indirect immunofluorescence (IMF) was performed as described previously (Yao et al., 2003). For Rnr2-GFP and Rnr4-GFP subcellular localization, living cells were analyzed in an Axioskop 2 microscope (Zeiss) and images captured with a SPOT camera (Diagnostic Instruments). In all cases, more than 200 cells from at least 3 independent experiments were scored as cells with a predominantly nuclear signal, localization in both the nucleus and the cytoplasm, or a predominantly cytoplasmic signal. The average and the standard deviation were represented.
dATP and dCTP measurements
Approximately 2 × 108 cells were harvested, washed with water, resuspended in 200 µL of cold 60% methanol, and extracts obtained by vigorous shacking in the presence of glass beads. Then, cell debris were pelleted at 27000 g for 1 min, and the supernatant was held at −20°C for 2 h. The samples were boiled for 3 min, and followed by centrifugation at 13400 g for 10 min at 4°C. The supernatant was taken to dryness under vacuum and resuspended in 40 µL of water. The dATP and dCTP levels were determined by the DNA polymerase-based enzymatic assay (Mathews and Wheeler, 2009). Briefly, the incorporation of dATP and dCTP into specific oligonucleotides, containing poly(AAAT) and poly(AAAG) sequences respectively, by the Klenow DNA polymerase was determined in the presence of excess [3H]-labeled dTTP.
RNA and protein analyses
Total yeast RNA isolation and RNA blotting was performed as described previously (Pedro-Segura et al., 2008). Yeast proteins were extracted using the alkali method. Approximately 50 µg of total protein were resolved in an SDS-PAGE gel and transferred onto a nitrocellulose membrane. The primary antibodies used in this study include anti-Rnr2 and anti-Rnr4 (Yao et al., 2003), anti-Rad53 (Santa Cruz Biotechnology), anti-c-Myc (9E10, Roche), and anti-Pgk1 (Invitrogen). Blots were developed with HRP-labeled secondary antibodies using the ECL Advance Western Blotting Detection Kit (GE Healthcare Life Sciences).
HIGHLIGHTS.
Cth1 and Cth2 proteins control Rnr2 and Rnr4 (R2) levels in response to low iron.
Cth1/Cth2 promote R2 redistribution in response to low iron by degrading WTM1 mRNA.
Mec1 and Rad53 do not participate in the R2 redistribution observed in low iron.
Regulation of RNR by Cth1 and Cth2 in response to low iron increases dNTP pools.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to A. Francone, B. Natalucci, the members of the Copper-Iron Homeostasis and Cell Cycle laboratories at the University of Valencia, and the members of the Huang’s group at the University of Colorado, for their help in some experiments. We also thank Drs. L. Peñarrubia, J. C. Igual, R. de Llanos and D. J. Thiele for critically reading the manuscript; Drs. J. Stubbe, S. J. Elledge, J. Kaplan, B. L. Schneider, M. Wickens and R. S. Zitomer for plasmids and antibodies used in this work, Dr. X. Wu and Ms. X. An for technical help with immunofluorescence imaging, and Dr. L. J. Wheeler for details on the protocol of dNTPs extraction and measurement. N. S. is recipient of a predoctoral fellowship from the Spanish “Conselleria d’Educació de la Generalitat Valenciana”. This work has been supported by the BIO2008-02835 grant from the Spanish Ministry of Science and Innovation, the ACOMP2011-268 grant from the “Generalitat Valenciana” and FEDER funds from the European Union to S. P.; BFU2010-20927 grant from the Spanish Ministry of Science and Innovation to M. C. B.; and National Institute of Health grants CA125574 and GM081393 to M. H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bashkirov VI, Bashkirova EV, Haghnazari E, Heyer WD. Direct kinase-to-kinase signaling mediated by the FHA phosphoprotein recognition domain of the Dun1 DNA damage checkpoint kinase. Mol Cell Biol. 2003;23:1441–1452. doi: 10.1128/MCB.23.4.1441-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh PF, Jr, Porter CW, Tukalo D, Frankfurt OS, Pavelic ZP, Bergeron RJ. Characterization of L1210 cell growth inhibition by the bacterial iron chelators parabactin and compound II. Cancer Res. 1985;45:4754–4759. [PubMed] [Google Scholar]
- Chabes A, Domkin V, Thelander L. Yeast Sml1, a protein inhibitor of ribonucleotide reductase. J Biol Chem. 1999;274:36679–36683. doi: 10.1074/jbc.274.51.36679. [DOI] [PubMed] [Google Scholar]
- Cotruvo JA, Stubbe J. Class I ribonucleotide reductases: metallocofactor assembly and repair in vitro and in vivo. Annu Rev Biochem. 2011a;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, Stubbe J. Escherichia coli class Ib ribonucleotide reductase contains a dimanganese(III)-tyrosyl radical cofactor in vivo. Biochemistry. 2011b;50:1672–1681. doi: 10.1021/bi101881d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994;269:26092–26099. [PubMed] [Google Scholar]
- Furukawa T, Naitoh Y, Kohno H, Tokunaga R, Taketani S. Iron deprivation decreases ribonucleotide reductase activity and DNA synthesis. Life Sci. 1992;50:2059–2065. doi: 10.1016/0024-3205(92)90572-7. [DOI] [PubMed] [Google Scholar]
- Huang M, Elledge SJ. Identification of RNR4, encoding a second essential small subunit of ribonucleotide reductase in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6105–6113. doi: 10.1128/mcb.17.10.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Zhou Z, Elledge SJ. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell. 1998;94:595–605. doi: 10.1016/s0092-8674(00)81601-3. [DOI] [PubMed] [Google Scholar]
- Ihrig J, Hausmann A, Hain A, Richter N, Hamza I, Lill R, Muhlenhoff U. Iron regulation through the back door: iron-dependent metabolite levels contribute to transcriptional adaptation to iron deprivation in Saccharomyces cerevisiae. Eukaryot Cell. 2010;9:460–471. doi: 10.1128/EC.00213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, McVey Ward D, Crisp RJ, Philpott CC. Iron-dependent metabolic remodeling in S. cerevisiae. Biochim Biophys Acta. 2006;1763:646–651. doi: 10.1016/j.bbamcr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schwartz MF, Duong JK, Stern DF. Rad53 phosphorylation site clusters are important for Rad53 regulation and signaling. Mol Cell Biol. 2003;23:6300–6314. doi: 10.1128/MCB.23.17.6300-6314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YD, Elledge SJ. Control of ribonucleotide reductase localization through an anchoring mechanism involving Wtm1. Genes Dev. 2006;20:334–344. doi: 10.1101/gad.1380506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YD, Wang J, Stubbe J, Elledge SJ. Dif1 is a DNA-damage-regulated facilitator of nuclear import for ribonucleotide reductase. Mol Cell. 2008;32:70–80. doi: 10.1016/j.molcel.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Imlay JA. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol. 2011;80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Curr Opin Microbiol. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Mathews CK, Wheeler LJ. Measuring DNA precursor pools in mitochondria. Methods Mol Biol. 2009;554:371–381. doi: 10.1007/978-1-59745-521-3_22. [DOI] [PubMed] [Google Scholar]
- Ortigosa AD, Hristova D, Perlstein DL, Zhang Z, Huang M, Stubbe J. Determination of the in vivo stoichiometry of tyrosyl radical peRbetabeta' in Saccharomyces cerevisiae ribonucleotide reductase. Biochemistry. 2006;45:12282–12294. doi: 10.1021/bi0610404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro-Segura E, Vergara SV, Rodriguez-Navarro S, Parker R, Thiele DJ, Puig S. The Cth2 ARE-binding protein recruits the Dhh1 helicase to promote the decay of succinate dehydrogenase SDH4 mRNA in response to iron deficiency. J Biol Chem. 2008;283:28527–28535. doi: 10.1074/jbc.M804910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein DL, Ge J, Ortigosa AD, Robblee JH, Zhang Z, Huang M, Stubbe J. The active form of the Saccharomyces cerevisiae ribonucleotide reductase small subunit is a heterodimer in vitro and in vivo. Biochemistry. 2005;44:15366–15377. doi: 10.1021/bi051616+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Askeland E, Thiele DJ. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Puig S, Vergara SV, Thiele DJ. Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 2008;7:555–564. doi: 10.1016/j.cmet.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta DJ, Zhang B, Kraemer B, Pochart P, Fields S, Wickens M. A three-hybrid system to detect RNA-protein interactions in vivo. Proc Natl Acad Sci USA. 1996;93:8496–8501. doi: 10.1073/pnas.93.16.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoury-Elizeh M, Tiedeman J, Rashford J, Ferea T, Demeter J, Garcia E, Rolfes R, Brown PO, Botstein D, Philpott CC. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:1233–1243. doi: 10.1091/mbc.E03-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerhalter M, Voegtly WC, Perlstein DL, Ge J, Stubee J, Rosenzweig AC. Structures of the yeast ribonucleotide reductase Rnr2 and Rnr4 homodimers. Biochemistry. 2004;43:7736–7742. doi: 10.1021/bi049510m. [DOI] [PubMed] [Google Scholar]
- Vergara SV, Thiele DJ. Post-transcriptional regulation of gene expression in response to iron deficiency: coordinated metabolic reprogramming by yeast mRNA-binding proteins. Biochem Soc Trans. 2008;36:1088–1090. doi: 10.1042/BST0361088. [DOI] [PubMed] [Google Scholar]
- Voegtly WC, Ge J, Perlstein DL, Pochart P, Stubee J, Rosenzweig AC. Structure of yeast ribonucleotide reductase Y2Y4 heterodimer. Proc Natl Acad Sci USA. 2001;98:10073–10078. doi: 10.1073/pnas.181336398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Chabes A, Casagrande R, Tian XC, Thelander L, Huffaker TC. Rnr4p, a novel ribonucleotide reductase small-subunit protein. Mol Cell Biol. 1997;17:6114–6121. doi: 10.1128/mcb.17.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Huang M. Dif1 controls subcellular localization of ribonucleotide reductase by mediating nuclear import of the R2 subunit. Mol Cell Biol. 2008;28:7156–7167. doi: 10.1128/MCB.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Zhang Z, An X, Bucci B, Perlstein DL, Stubbe J, Huang M. Subcellular localization of yeast ribonucleotide reductase regulated by the DNA replication and damage checkpoint pathways. Proc Natl Acad Sci USA. 2003;100:6628–6633. doi: 10.1073/pnas.1131932100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, An X, Yang K, Perlstein DL, Hicks L, Kelleher N, Stubbe J, Huang M. Nuclear localization of the Saccharomyces cerevisiae ribonucleotide reductase small subunit requires a karyopherin and a WD40 repeat protein. Proc Natl Acad Sci USA. 2006;103:1422–1427. doi: 10.1073/pnas.0510516103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chabes A, Domkin V, Thelander L, Rothstein R. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 2001;20:3544–3553. doi: 10.1093/emboj/20.13.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.