Non-technical summary
This is the first study, to our knowledge, to use cardiac MRI before and after intensive and closely supervised resistance and endurance exercise training in humans. There is a long held belief that these different forms of training induce ‘concentric’ and ‘eccentric’ adaptation of the heart, but this concept is based on echocardiographic assessments and cross-sectional comparison of different types of elite athletes. Our findings, using highly sensitive MRI methodology, suggest that concept may need to be reconsidered. This study is of fundamental importance to the understanding of the impact of exercise on human cardiac morphology and physiology.
Abstract
Abstract
The principle that ‘concentric’ cardiac hypertrophy occurs in response to strength training, whilst ‘eccentric’ hypertrophy results from endurance exercise has been a fundamental tenet of exercise science. This notion is largely based on cross-sectional comparisons of athletes using echocardiography. In this study, young (27.4 ± 1.1 years) untrained subjects were randomly assigned to supervised, intensive, endurance (END, n = 10) or resistance (RES, n = 13) exercise and cardiac MRI scans and myocardial speckle tracking echocardiography were performed at baseline, after 6 months of training and after a subsequent 6 weeks of detraining. Aerobic fitness increased significantly in END (3.5 to 3.8 l min−1, P < 0.05) but was unchanged in RES. Muscular strength significantly improved compared to baseline in both RES and END (Δ = 53.0 ± 1.1 versus 36.4 ± 4.5 kg, both P < 0.001) as did lean body mass (2.3 ± 0.4 kg, P < 0.001 versus 1.4 ± 0.6 kg P < 0.05). MRI derived left ventricular (LV) mass increased significantly following END (112.5 ± 7.3 to 121.8 ± 6.6 g, P < 0.01) but not RES, whilst training increased end-diastolic volume (ΔLVEDV, END: +9.0 ± 5.0 versus RES +3.1 ± 3.6 ml, P = 0.05). Interventricular wall thickness significantly increased with training in END (1.06 ± 0.0 to 1.14 ± 0.06, P < 0.05) but not RES. Longitudinal strain and strain rates did not change following exercise training. Detraining reduced aerobic fitness, LV mass and wall thickness in END (P < 0.05), whereas LVEDV remained elevated. This study is the first to use MRI to compare LV adaptation in response to intensive supervised endurance and resistance training. Our findings provide some support for the ‘Morganroth hypothesis’, as it pertains to LV remodelling in response to endurance training, but cast some doubt over the proposal that remodelling occurs in response to resistance training.
Introduction
Left ventricular (LV) adaptation to prolonged exercise training in humans has been dominated by the sport-specificity concept embedded in the ‘Morganroth hypothesis’ (1975). Using non-guided M-mode echocardiography, Morganroth et al. (1975) observed that endurance athletes (swimmers, long-distance runners) possessed increased LV end-diastolic volume (LVEDV), normal LV wall thickness and increased LV mass compared to sedentary subjects. In contrast, resistance-trained athletes (wrestlers) exhibited increased LV wall thickness and LV mass with no change in LVEDV compared to sedentary controls. These divergent patterns were later described as ‘eccentric’ and ‘concentric’ left ventricular hypertrophy, respectively, and were postulated to result from episodic elevations in preload during endurance exercise and afterload during resistance exercise.
Subsequent studies have largely reinforced the ‘Morganroth hypothesis’, to the extent that it has become textbook dogma (McArdle et al. 2010). However, these studies have a number of important limitations (Naylor et al. 2008). Most utilised cross-sectional designs, which limit the ability to discriminate between changes induced by exercise per se, versus those associated with other differences between individuals. Longitudinal studies are less affected by this limitation, but few have been completed. Secondly, most data have been derived using M-mode or 2D echocardiography which relies upon geometric assumptions to estimate left ventricular (LV) mass and volumes. Indeed differences between cardiac dimensions of athletes and non-athletes lie within the methodological error range for 2D echocardiographic assessments, estimated to be ∼60 g (at 95% confidence) (Jenkins et al. 2007). In contrast, magnetic resonance imaging (MRI) is considered the ‘gold-standard’ for cardiac morphological assessment, is highly accurate and reproducible and possesses enhanced sensitivity to detect LV mass changes compared to echocardiography (Myerson et al. 2002).
Whilst the ‘Morganroth hypothesis’ has been widely adopted in scientific and clinical environments, some concerns have been expressed, particularly as to whether concentric hypertrophy occurs in resistance-trained athletes due to the limited impact of resistance exercise involving a Valsalva manoeuvre on LV wall stress (Haykowsky et al. 2001). Given the lack of previous training studies and recent advances in the sensitivity of LV assessment tools, we aimed to examine the ‘Morganroth hypothesis’ using a prospective randomised trial of distinct and controlled training interventions using MRI. Our hypothesis was that endurance training would result in an eccentric hypertrophy of the LV but resistance training would not alter LV morphology.
Methods
Ethical approval
All study procedures were approved by the Human Research and Ethics Committee of the University of Western Australia. Written, informed consent was obtained from all subjects in writing, and the studies conformed to the Declaration of Helsinki.
Subject characteristics
Twenty-three young healthy male subjects (27.4 ± 5.5 years) were recruited to this study after undergoing a thorough pre-screening involving a detailed medical history, physical examination, standard blood panels and a physical activity questionnaire. Those taking medication or at moderate (or higher) cardiovascular risk according to the ACSM guidelines (American College of Sports Medicine, 2009) were excluded from the study. Subjects were then randomly assigned (http://www.randomization.com) to either an endurance- (END, n = 10) or resistance-training group (RES, n = 13). Based on previous methodological assessments, we determined that a sample size of nine subjects would be required to observe a 10 g change in LV mass using MRI at 90% power level (Bellenger et al. 2000).
Study design
A mixed-model design was employed with randomised assignment of participants to a 24 week resistance or endurance-training programme followed by 6 weeks of detraining. Measures were taken at three time points: baseline, following 24 weeks of training and after a further 6 weeks of detraining. Experimental measures included cardiac MRI, echocardiography, body composition assessment using dual-energy X-ray absorptiometry (DXA), aerobic fitness evaluated using a graded exercise test and muscular strength using one-repetition maximum (1RM) tests.
Experimental procedures
Left ventricular morphology
Measures of LV morphology were assessed with cardiac MRI (Siemens 1.5 T Magnetom Espree with Tim open bore magnet). The subjects were scanned supine with a posterior phased array spine coil and an anterior flexible phased array body surface coil. Multi-plane breath-hold (BH) trueFISP localizers were acquired to obtain the standard cardiac imaging planes. For all sequences the BH times were between 5 and 20 s, dependant upon the subject's heart rate. To evaluate functional parameters BH trueFISP cine images were acquired using a retrogated ECG trigger covering the whole R-R interval. Images of the LV were done in short axis plane, perpendicular to the ventricular septum. Between 10 and 12 slices were acquired (6 mm slices/4 mm gap, FoV 320–350 mm, TR = 37.68, TE = 1.29 flip angle 70–80 deg, resolution 256 × 166, BW 930). Cine images of the four-chamber and LV outflow tract were also acquired (6 mm slices, FoV 300–330 mm, TR = 38.28, TE = 1.32, flip angle 70–80 deg, resolution 224 × 224, BW 930).
All cardiac MRI analysis was performed by a blinded observer using specialized software (ARGUS, Siemens). Analyses were independently repeated and confirmed by an experienced cardiologist, who was also blinded. To assess LV mass, short axis cine loops were inspected to define end-systole as the frame with the smallest ventricular cavity. The basal LV slice was taken as the first slice below the level of the mitral valve, and thus volumes above the aortic valve and those surrounding the thin myocardial wall in the mitral valve plane were excluded. Endocardial and epicardial borders were then manually traced, including the septum but excluding the papillary muscles which were added to LVEDV and LV end-systolic volume (LVESV) in accordance with the methods described by Scharhag et al. (2002). Left ventricular mass was calculated by summing the LVEDV within the epi- and endocardial borders of the short-axis slices, multiplying the myocardial tissue volume by its specific density (1.05 g cm-3). The LVEDV and LVESV were utilised to ascertain stroke volume (SV), ejection fraction (EF) and cardiac output. To facilitate valid comparison to previously obtained echocardiographic data, a representative measure of left ventricular internal cavity dimension during diastole (LVIDd) and systole (LVIDs), as well as interventricular septum (IVS) and posterior wall (PW) thickness were determined from the long-axis three-chamber cine view. Any error of greater than 2% resulted in both measurements being re-measured at a later date by a blinded reviewer.
Left ventricular global function
All echocardiographic images were acquired using a commercially available ultrasound system (Vivid I, GE Medical, Horton, Norway) by a single experienced sonographer. Segmental and global longitudinal strain (ɛ) and strain rate (SR) data were obtained from an apical four-chamber apical view. Two-dimensional image optimisation was performed and care was taken to reproduce frame rates for all scans within individuals (between 40 and 90 fps). Cine loops of LV motion were recorded to DVD in a raw DICOM format and analysed off-line by a single experienced technician with no knowledge of group allocation. Data reflect the average of three to five continuous cardiac cycles. Using specific speckle tracking software (Echopac, GE Healthcare, Norway) that tracks natural acoustic markers or ‘kernals’, the estimation of longitudinal ɛ and strain rate (SR) in six wall segments (basal, mid-wall and apical segments for the septum and lateral wall) were analysed. These were averaged to provide global measurements of ɛ and SR recorded in systole (SRS), early diastole (SRE) and late diastole (SRA). The presentation of longitudinal strain and strain rate data reflects its greater reliability, feasibility and clinical uptake/utility (Mor-Avi et al. 2011).
Body composition
Prior to the fitness test, subjects underwent a whole body DXA assessment (Lunar Prodigy, GE Medical Systems, Madison, WI, USA) to determine body composition, specifically total fat mass, total lean body mass and body fat percentage.
Aerobic fitness
Graded exercise tests were performed on a treadmill, with the gradient set at 1%, and consisted of 3 min exercise and 1 min rest periods, increasing by 1 km h−1 for each workload until volitional exhaustion. Expired air was analysed for O2 and CO2 concentrations (Ametek Gas Analysers, Applied Electrochemistry, SOC S-3A/1 and COV CD-3A, Pittsburgh, PA, USA) and ventilation (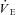 ) was recorded at 15 s intervals using a turbine ventilometer (P.K. Morgan Ltd, Rainham, Kent, UK). Prior to and following each test, the ventilometer and gas analysers were calibrated according to the manufacturer's instructions using a 1 litre syringe and gases of known concentration (BOC Gases, Chatswood, Australia). Peak oxygen consumption (
) was recorded at 15 s intervals using a turbine ventilometer (P.K. Morgan Ltd, Rainham, Kent, UK). Prior to and following each test, the ventilometer and gas analysers were calibrated according to the manufacturer's instructions using a 1 litre syringe and gases of known concentration (BOC Gases, Chatswood, Australia). Peak oxygen consumption (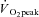 ) was determined by summing the four highest consecutive 15 s
) was determined by summing the four highest consecutive 15 s 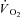 values in each workload. Heart rate was continually monitored using a Polar Heart Rate Monitor (Polar F1, Kempele Finland) throughout the test and recorded in the last 10 s of each workload.
values in each workload. Heart rate was continually monitored using a Polar Heart Rate Monitor (Polar F1, Kempele Finland) throughout the test and recorded in the last 10 s of each workload.
Muscular strength
Maximal upper and lower body strength were determined using bench press and squat exercises, respectively, according to a 1RM protocol. Participants were briefed on correct form for each exercise and performed familiarisation lifts. A warm-up of 10 repetitions at 50% of their predicted 1RM was given followed by five repetitions at 70%, three repetitions at 80% and one repetition at 90% of predicted 1RM. Subjects were then given three attempts to determine their actual 1RM to the nearest 2.5 kg. A recovery of 5 min was given between efforts. Combined muscular strength is expressed as the summation of 1RM scores for bench press and squat exercises.
Exercise interventions
Subjects attended three 1 h exercise-training sessions per week for a period of 24 weeks. An experienced exercise physiologist (A.S.) closely supervised all exercise sessions to ensure compliance with the prescribed exercise programmes.
Endurance training
The 24 week training programme followed a periodised progressive design consisting of eight 3 week mesocycles with the programme divided into three training phases. The ‘general preparatory phase’ (weeks 1–12; mesocycles 1–4) consisted of low–moderate intensity walking, jogging and stretching to condition the body for activity. Training volume was gradually and progressively increased during this phase. The second, ‘specific phase’ (weeks 13–18; mesocycles 5 and 6) increased running intensity and included elements of hill running and short intervals. In the final ‘competition phase’ (weeks 19–24; mesocycles 7 and 8), intensity was maintained but volume was somewhat reduced to prepare the subjects for a 12 km competitive road running event. A loading structure of 1:2 was used for the initial four mesocycles (i.e. 1 ‘hard’ load week following by 2 ‘easy’ weeks) and progressed to a 2:1 load structure for the remaining four mesocycles. During each training session, intensities were individualised by prescribing training paces based on 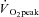 values and time trial performances (Daniels, 2005). Subjects wore heart rate monitors (Polar F1, Finland) during all training sessions to enable the exercise physiologist to monitor and adjust intensities accordingly. Regular stretching, core strengthening exercises and running drills were included in the sessions to minimise injury risk and improve running technique.
values and time trial performances (Daniels, 2005). Subjects wore heart rate monitors (Polar F1, Finland) during all training sessions to enable the exercise physiologist to monitor and adjust intensities accordingly. Regular stretching, core strengthening exercises and running drills were included in the sessions to minimise injury risk and improve running technique.
Resistance training
The 24 week resistance-training program consisted of six 4 week mesocycles with a focus on muscular strength, including elements of Olympic weightlifting. The ‘general preparatory phase’ (week 1–12, mesocycles 1–3) aimed to condition the body and develop correct lifting technique with low volume and load (e.g. 2–3 sets of 12–15 repetitions at 65–85% 1RM, resting 60–120 s between sets for each exercise). Each mesocyle was progressively overloaded so that volume and load peaked in the third week with the fourth week acting as a recovery week. The ‘specific phase’ (week 13–20; mesocycles 4 and 5) focused on furthering skill development of the main lifts (i.e. clean and jerk, snatch) while increasing strength of assistance lifts (e.g. front squat, back squat, overheard squat, deadlift and press). The ‘competition phase’ (week 21–24; mesocycle 6) emphasised skill development while keeping low volume and heavy load. As load was given as a percentage of 1RM, maximal lifts were periodically tested throughout the programme to ensure adequate load was maintained during training.
Statistical analysis
A two-way mixed model ANOVA was used to determine the effect of exercise training modalities on measures of cardiac morphology, aerobic fitness, body composition and strength. Statistical significance was assumed at P < 0.05. Student's t test was conducted post hoc and reported, as indicated by significant ANOVA results. Data are means ± SEM. Comparison of change scores was undertaken using t tests.
Results
No significant differences existed in any subject characteristics at baseline and the groups were therefore well matched at entry (Table 1).
Table 1.
Subject characteristics before (Pre) and after 6 months (Post) of supervised training and a 6 week detraining period
| Endurance trained (n = 10) | Resistance trained (n = 13) | |||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | Detraining | Pre | Post | Detraining |
| Age | 28.4 ± 1.9 | — | — | 26.6 ± 1.3 | — | — |
| Height (m) | 1.79 ± 0.02 | — | — | 1.81 ± 0.02 | — | — |
| Weight (kg) | 78.0 ± 5.4 | 78.3 ± 5.5 | 78.0 ± 5.5 | 81.7 ± 4.2 | 83.3 ± 4.4* | 83.4 ± 4.4* |
| BSA (m2) | 1.96 ± 0.07 | 1.97 ± 0.08 | 1.97 ± 0.07 | 2.02 ± 0.06 | 2.04 ± 0.06 | 2.05 ± 0.06* |
| BMI | 24.2 ± 1.3 | 24.3 ± 1.4 | 24.2 ± 1.4 | 24.7 ± 1.0 | 25.2 ± 1.0* | 25.2 ± 1.0* |
| SBP (mmHg)† | 122 ± 2 | 119 ± 2 | 119 ± 1 | 125 ± 1 | 119 ± 2** | 117 ± 2** |
| DBP (mmHg) | 69 ± 3 | 68 ± 2 | 67 ± 2 | 71 ± 2 | 70 ± 2 | 69 ± 3 |
| MAP (mmHg)† | 87 ± 3 | 85 ± 2 | 84 ± 1 | 89 ± 1 | 86 ± 2* | 85 ± 2 |
| Resting HR (bpm)‡ | 65 ± 3 | 58 ± 2** | 52 ± 9 | 66 ± 3 | 65 ± 2 | 64 ± 2 |
| Maximum HR (bpm)† | 197 ± 3 | 193 ± 2* | 190 ± 4* | 200 ± 2 | 196 ± 2* | 195 ± 3* |
| Aerobic fitness | ||||||
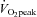 (l min−1)† (l min−1)†
|
3.5 ± 0.2 | 3.8 ± 1.8* | 3.6 ± .17 | 3.6 ± 0.3 | 3.6 ± 0.2 | 3.6 ± 0.2 |
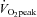 (ml kg−1 min−1) (ml kg−1 min−1) |
45.8 ± 1.6 | 49.3 ± 2.2* | 46.7 ± 2.1 | 44.0 ± 2.5 | 44.0 ± 2.2 | 42.9 ± 1.8 |
| Strength measures | ||||||
| Bench press (kg)†‡ | 57.9 ± 4.5 | 61.0 ± 4.5 | 61.8 ± 4.2 | 57.8 ± 4.9 | 69.0 ± 5.4*** | 68.3 ± 4.9*** |
| Squat (kg)† | 89.0 ± 6.7 | 122.3 ± 4.6*** | 133.5 ± 4.0*** | 96.7 ± 6.3 | 138.5 ± 4.4*** | 147.3 ± 3.9*** |
| Total strength (kg)†‡ | 146.9 ± 10.4 | 183.3 ± 8.6*** | 195.3 ± 7.5*** | 154.5 ± 10.4 | 207.5 ± 8.6*** | 215.6 ± 8.1*** |
| Body composition | ||||||
| Total body fat (%)† | 22.7 ± 2.4 | 21.1 ± 2.5 | 21.8 ± 2.7 | 23.1 ± 2.0 | 21.4 ± 2.1* | 22.8 ± 2.1 |
| Total fat mass (kg)§ | 17.7 ± 2.9 | 16.7 ± 3.1 | 17.3 ± 3.3 | 18.6 ± 2.3 | 17.8 ± 2.5 | 18.9 ± 2.6 |
| Total lean mass (kg)† | 56.9 ± 2.9 | 58.3 ± 3.0* | 57.5 ± 2.8 | 59.7 ± 2.3 | 62.0 ± 2.2*** | 60.9 ± 2.1** |
P < 0.05 training effect by ANOVA
P = 0.05 training effect by ANOVA
P < 0.05 training × group interaction effect by ANOVA
significantly different from pre-training values at P < 0.05
significantly different from pre-training values at P < 0.005
significantly different from pre-training values at P < 0.001.
BSA, body surface area; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Impact of exercise training on subject characteristics
Aerobic fitness increased significantly in END (P < 0.05), but was unchanged in RES (Table 1). Combined upper and lower limb strength significantly increased as a result of training (P < 0.05) and training-by-group interactions were evident for combined strength and bench press 1RM measures (P < 0.05). Post hoc analysis revealed that the combined strength increased by 53.0 kg in the RES group (P < 0.001) and by 36.4 kg in the END group (P < 0.001). RES training induced increases in both squat (41.8 kg, P < 0.001) and bench press (11.2 kg, P < 0.001) measures, whereas significant changes in the END group occurred only in squat strength (33.3 kg, P < 0.001).
ANOVA revealed a significant effect of training on total body fat (%) (P < 0.005). Post hoc t tests revealed a significant decrease in the RES subjects (P < 0.02, Table 1), whereas the decrease in END did not achieve statistical significance. Despite these changes in percentage body fat, there were no significant changes in fat mass (kg) due to a significant increase in lean mass in both the RES (2.3 kg, P < 0.001) and END (1.4 kg, P < 0.05) groups.
Impact of exercise training on cardiac measures
A significant impact of training on LV mass (P < 0.05) was evident and post hoc tests revealed a significant increase of 9.3 g following END (P < 0.05), whereas the change observed in the RES group (4.6 g) was not significant (Fig. 1, Table 2). The effect of training on LVEDV approached significance (P = 0.05) with an increase of 9 ml in END, compared to 3 ml in RES and the ratio of LV mass:LVEDV was unchanged following training in both groups (Table 2). A significant increase in IVS (P < 0.05) was also apparent in END subjects following training. Training-induced differences were also apparent in stroke volume (P < 0.05), with an average increase in END of 7.2 ml and RES of 2.6 ml. Indices of longitudinal ɛ and SR were not significantly altered with exercise training (Table 3).
Figure 1. Impact of exercise training and detraining on MRI derived measures of cardiac mass, volume and wall thickness.
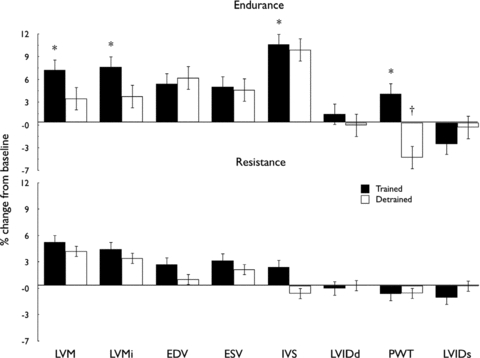
Bars represent percentage change from baseline after training (filled bars) and detraining (open bars) values in the endurance (upper panel) and resistance-trained (lower panel) groups. *P < 0.05 post-training vs. baseline, †P < 0.05 post-detraining vs. baseline. For measures of IVS, PWT, LVIDd and LVIDs, n = 7 for endurance group.
Table 2.
Cardiac MRI left ventricular measures before (pre) and after (post) 6 months of training
| Endurance trained (n = 10) | Resistance trained (n = 13) | |||
|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post |
| LVM (g)† | 112.5 ± 7.3 | 121.8 ± 6.6** | 125.7 ± 7.6 | 130.3 ± 6.4 |
| LVMi (g m−2)† | 57.1 ± 2.6 | 61.9 ± 2.1* | 61.7 ± 2.8 | 63.7 ± 2.3 |
| LVEDV (ml)‡ | 134.1 ± 7.9 | 143.1 ± 7.8 | 147.1 ± 7.0 | 150.2 ± 7.1 |
| LVESV (ml) | 56.9 ± 4.5 | 58.4 ± 3.5 | 59.3 ± 3.2 | 59.8 ± 2.7 |
| LVM/LVEDV | 0.84 ± 0.03 | 0.86 ± 0.03 | 0.86 ± 0.03 | 0.88 ± 0.03 |
| EF (%) | 57.9 ± 1.4 | 59.1 ± 1.4 | 59.6 ± 1.2 | 59.8 ± 1.6 |
| Stroke volume (ml)† | 77.4 ± 4.2 | 84.7 ± 5.2 | 87.8 ± 4.8 | 90.4 ± 5.7 |
| Cardiac output (l min−1) | 5.1 ± 0.4 | 4.8 ± 0.3 | 5.7 ± 0.3 | 5.9 ± 0.4 |
| LVIDd (cm) | 4.98 ± 0.12 | 4.98 ± 0.15 | 5.10 ± 0.10 | 5.07 ± 0.09 |
| LVIDs (cm) | 3.45 ± 0.12 | 3.37 ± 0.08 | 3.52 ± 0.11 | 3.44 ± 0.08 |
| IVST (cm) | 1.02 ± 0.06 | 1.12 ± 0.05* | 1.07 ± 0.02 | 1.09 ± 0.03 |
| PWT (cm) | 0.94 ± 0.02 | 0.95 ± 0.03 | 0.95 ± 0.05 | 0.94 ± 0.05 |
P < 0.05 training effect by ANOVA
P = 0.05 training effect by ANOVA
significantly different from pre-training P < 0.05
significantly different from pre-training P < 0.005
EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; LVM, left ventricular mass; LVMi, left ventricular mass index; LVIDd, left ventricular internal diameter during diastole; LVIDs, left ventricular internal diameter during systole; IVS, interventricular septal thickness; PWT, posterior wall thickness.
Table 3.
Global longitudinal strain (ɛ) and strain rate (SR) using two-dimensional myocardial speckle tracking echocardiography taken before (Pre), after 6 months (Post) of supervised training and a 6-week detraining period
| Endurance Trained (n = 10) | Resistance Trained (n = 12) | |||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | Detraining | Pre | Post | Detraining |
| Peak ɛ (%) | −16.80 ± 0.69 | −17.37 ± 0.72 | −17.25 ± 0.62 | −16.59 ± 0.64 | −17.06 ± 0.39 | −18.71 ± 0.44 |
| SRS (s−1) | −1.24 ± 0.05 | −1.12 ± 0.05 | −1.15 ± 0.04 | −1.14 ± 0.03 | −1.10 ± 0.03 | −1.17 ± 0.03 |
| SRE (s−1) | 1.73 ± 0.08 | 1.73 ± 0.09 | 1.57 ± 0.11 | 1.57 ± 0.08 | 1.56 ± 0.06 | 1.71 ± 0.08 |
| SRA (s−1) | 0.78 ± 0.07 | 0.74 ± 0.08 | 0.70 ± 0.08 | 0.80 ± 0.05 | 0.77 ± 0.04 | 0.84 ± 0.06 |
E/A, ratio of peak early to peak late diastolic strain rate; SRS, peak systolic strain rate; SRE, peak early diastolic strain rate; SRA, peak late diastolic strain rate.
Impact of detraining
Following detraining, fitness returned to baseline in the END group, whereas no differences were evident between time points in RES (Table 1). In the RES group, strength remained elevated for both bench press and squat measures (P < 0.05), whereas in the END group only squat strength remained significantly elevated compared to pre training values.
In general terms, measures of body composition returned toward baseline values during the detraining phase (Table 1). The significant effects of training on body fat % (RES) and lean body mass (END) were reversed in both groups, whereas the increase in lead body mass in the RES group remained elevated compared to pre-training levels.
MRI data for two subjects in the END group were unavailable following the detraining phase, so analysis of cardiac variables was performed on n = 8 END versus n = 13 RES. Detraining was associated with a decrease in LV mass in the END group such that the significant impact of training was no longer apparent (Fig. 1). The RES group, in whom training did not significantly elevate LV mass, possessed similar detraining data to those at baseline and post-training. End-diastolic volume did not decrease in response to detraining in the END group, who also exhibited a significant decrease in posterior wall thickness (PWT) with detraining (Fig. 1). Detraining did not significantly modify wall thicknesses or LVEDV in the RES group. All echocardiography-derived parameters of LV function showed no significant changes over the detraining phase (Table 3).
Discussion
Our principal finding was that LV mass and wall thickness significantly increased following END training, whereas no significant change was evident in RES trained subjects. A greater increase in LVEDV was apparent in END compared to RES trained subjects. The ratio of LV mass:LVEDV was unchanged following exercise training, which is indicative of an ‘eccentric’ hypertrophy pattern in the END group as a result of proportionate increases in both LV mass and LVEDV. Our training programmes significantly enhanced aerobic fitness in the END group and substantially improved both upper and lower body muscular strength in the RES group. These data suggest that ‘eccentric’ cardiac hypertrophy, broadly consistent with that proposed by the ‘Morganroth hypothesis’, is evident in response to END, whereas RES training was not associated with any substantive LV remodelling despite large gains in muscular strength and a significant increase in lean body mass.
To our knowledge, no previous longitudinal study has directly compared the impacts of intensive RES and END training on cardiac morphology using MRI. However, cross-sectional comparisons have been published. Scharhag et al. (2002) compared endurance athletes to matched non-athletic controls and observed enlarged ventricular mass (200 vs. 148 g) and volumes (167 vs. 125 ml) in the athletic population. Wernstedt et al. (2002) compared endurance athletes, strength athletes and controls (all n = 10) and reported higher LV mass (210 g) and volume in the endurance group, compared to both the resistance (163 g) and control groups (144 g). Our within-subject changes in LV mass were substantially smaller in END trained subjects (9 g) than those between endurance athletes and controls reported by Sharhag et al. (52 g) and Wernstedt et al. (66 g). Whilst it is possible that longer or more frequent training may have induced larger effects in the present study, the modest changes we observed compared to athlete/control differences raises the possibility that putative impacts of training, based on cross-sectional studies, may have been exaggerated. Indeed another recent within-subjects MRI observational study of the impact of military training reported similar changes in LV mass to those observed in our controlled trial (Batterham et al. 2011).
The mechanistic underpinning of changes observed in the END group may be explained by haemodynamic stress and transduction of that stress to increase protein synthesis, which could involve a number of signalling pathways including growth factors, Akt and P13K (Ruwhof & van der Laarse, 2000). Our finding that RES was not associated with marked morphological change are in agreement with some recent studies in untrained subjects. For example, Haykowsky et al. (2000) studied the impact of 16 weeks of resistance training in older (∼68 years) healthy subjects on wall thickness and cavity dimensions, neither of which were affected. Similarly strength training or a combination of aerobic and strength training both failed to induced cardiac morphological adaptations in older women over a 12 week training period (Haykowsky et al. 2005). A common belief in sport cardiology is that increased arterial pressure during lifting is the key stimulus for increasing LV mass. However, it has been shown using invasive haemodynamic and transthoracic echocardiography, that submaximal and maximal resistance efforts performed with a brief Valsalva manoeuvre (natural response to repetitive submaximal and maximal exercise) do not increase wall stress (Haykowsky et al. 2001). This apparent lack of wall stress during resistive efforts may explain why concentric left ventricular hypertrophy is not an obligatory adaptation to strength training. Conversely, Baggish et al. (2008a) reported training effects which were entirely consistent with the ‘Morganroth hypothesis’. This study was observational and assessed rowers and American-footballers, sports which may be described as ‘mixed’, involving a combination of aerobic and resistive components (duManoir et al. 2007) which generate unique hemodynamic loads (Naylor et al. 2008). Subjects were also trained at baseline and possessed large LV mass values (∼260 g) compared to studies of healthy young subjects using MRI (∼120–190 g) (Scharhag et al. 2002). Nonetheless, the average change in LV mass in response to 90 days of rowing was ∼30 g, substantially larger than the ∼9 g change we and others (Batterham et al. 2011) have observed as a result of intensive endurance training using MRI. Furthermore, American-footballers exhibited an increase of ∼35 g in LV mass, compared to essentially no change in the present study (∼4 g), which involved intensive supervised ‘Olympic’ weight lifting. Some of the disparity between the above findings may be attributed to differences in measurement technology or training frequency. Previous estimates suggest that the smallest change in LV mass detectable using 2D echocardiography may be as large as 60 g (Jenkins et al. 2007) and it has also been estimated that to detect an LV mass difference of 30 g using echocardiography requires a sample size of several hundreds of subjects (Bellenger et al. 2000). MRI decreases sample size requirements for detection of LV mass change by >95% (Bellenger et al. 2000), strongly suggesting that, when performing repeated measurements in training studies, MRI is a preferable technology (Myerson et al. 2002).
We observed that following END training, longitudinal ɛ, although slightly elevated, was not significantly different from pre-training levels, despite LV morphological adaptations. Similarly, Nottin et al. (2008) showed no differences in longitudinal ɛ and SR between elite cyclists and sedentary controls, suggesting athletes maintain normal LV function at rest. These observations are in contrast with those of Baggish et al. (2008b) who noted significantly improved LV systolic strain following 90 days of rowing exercise. This cohort were athletes with prior training experience and not untrained subjects, as in the current study.
Measures collected 6 weeks following the cessation of training in our study provide some basis for assessment of detraining effects. In the END subjects, LV mass decreased following training, such that it was no longer significantly elevated from pre-training levels. In contrast, LVEDV did not decrease across the detraining period in END. These findings can be rationalised by the significant decrease in wall thickness we observed 6 weeks post END training. Although confirmation will be required in further studies, these observations raise the hypothesis that changes in wall thickness occur before those in cavity dimension after the cessation of training. This suggestion is broadly in agreement with the echocardiographic findings of Pelliccia et al. (2002), who observed persistent cavity dilatation, but normalisation of wall thickness, following long-term deconditioning in athletes.
There are several limitations of the present study. Our sample size was relatively small, but this is offset by our observation of significant changes in LV mass in the END group and our within-subjects design, which minimised important sources of error. It is also notable that MRI is considered to be substantially more precise in terms of cardiac morphological assessment than echocardiography (Myerson et al. 2002; Jenkins et al. 2007) and that previous studies employing MRI in cross-sectional comparisons have employed smaller sample sizes than ours (Wernstedt et al. 2002; Vogelsang et al. 2008). Our sample was also larger than that estimated as required to demonstrate significant differences using MRI in detailed repeatability analysis (Bellenger et al. 2000). We therefore think our study was appropriately powered a priori and we observed a significant difference in our primary outcome variable. A second limitation relates to our training regime, which was based on three sessions of exercise per week for a 6 month period. Whilst we acknowledge that a longer training duration or more frequent bouts of training may have induced larger cardiac adaptations, we based our regimen on typical training regimes used in previously untrained individuals (Vogelsang et al. 2008). Our training approaches were in fact consistent with, or exceeded, those recommended by various peak bodies for use in healthy subjects and the magnitude of aerobic fitness improvement in the END group substantiates this (Garber et al. 2011). Previous studies have also suggested that 3 h of training per week is adequate to induce observable changes in cardiac morphology (Fagard, 2003). Whilst we acknowledge that a 6 month training study does not approach the volume of exercise exposure in elite athletes, the rationale of this study was to evaluate the haemodynamic mechanisms purported to underpin the ‘Morganroth hypothesis’ and this is best achieved in a randomised control trial. Compliance with training sessions was >80% in our study. Finally, we did not include women in this study and acknowledge that the impact of training on the heart may be sex dependent (Wernstedt et al. 2002; Baggish et al. 2008a).
Implications from the current study largely relate to our fundamental understanding of the cardiac response to training of divergent modalities. The lack of concentric hypertrophy of the LV with resistance training may raise questions regarding the validity of this aspect of the ‘Morganroth’ hypothesis, but should also allay concerns about negative remodelling in clinical groups when resistance exercise is used (Meyer, 2001). Further research should examine the relationship between other cardiac structural and functional measures, such as adaptation of the right ventricle and atria, in response to exercise training in healthy subjects and also subjects with cardiovascular disease. The issue of appropriate scaling approaches (George et al. 2009) for cardiac morphological measures also needs to be addressed using MRI derived parameters, particularly in longitudinal studies.
In summary, we undertook a randomised trial of the impact of 6 months resistance and endurance exercise training on LV morphology in previously untrained subjects using highly sensitive MRI. Our findings provide some support for the ‘Morganroth hypothesis’, particularly as it pertains to remodelling of the LV in response to endurance exercise training. Detraining changes in the endurance-trained group endorse this conclusion. However, our findings in the cohort who undertook 6 months of intensive resistance training cast some doubt over this aspect of the ‘Morganroth’ hypothesis, which deserve further investigation. Finally, our study has defined the magnitude of change in MRI-assessed left ventricular mass, which occurs in response to exercise training in previously untrained young healthy humans as a result of intensive, supervised and controlled exercise training.
Acknowledgments
DJG received funding from the Australian Research Council.
Glossary
Abbreviations
- BSA
body surface area
- DXA
dual-energy X-ray absorptiometry
- EF
ejection fraction
- IVS
interventricular septum
- LV
left ventricular
- LVIDd
left ventricular internal cavity dimension during diastole
- LVIDs
left ventricular internal cavity dimension during systole
- LVEDV
LV end-diastolic volume
- MRI
magnetic resonance imaging
- 1RM
one-repetition maximum
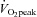
peak oxygen consumption
- PWT
posterior wall thickness
- ɛ
strain
- SR
strain rate
- SV
stroke volume
Author contributions
All authors contributed to writing the paper and approved the final draft of the revised manuscript. D.G. devised the experiment, A.S., L.N., H.C. and K.P. contributed to study design, these authors and D.O. contributed to data analysis and acquisition, A.S. supervised the study data collection with assistance from H.C. and C.B. L.D., C.M. and P.W. contributed to MRI data collection and analysis.
References
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th edition. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008a;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Yared K, Wang F, Weiner RB, Hutter AM, Picard MH, Wood MJ. The impact of endurance exercise training on left ventricular systolic mechanics. Am J Physiol Heart Circ Physiol. 2008b;295:H1109–H1116. doi: 10.1152/ajpheart.00395.2008. [DOI] [PubMed] [Google Scholar]
- Batterham AM, George KP, Birch KM, Pennell DJ, Myerson SG. Growth of left ventricular mass with military basic training in army recruits. Med Sci Sports Exerc. 2011;43:1295–1300. doi: 10.1249/MSS.0b013e3182093300. [DOI] [PubMed] [Google Scholar]
- Bellenger N, Davies L, Francis J, Coats A, Pennell D. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- Daniels J. Daniels’ Running Formula. Champaign: Human Kinetics; 2005. [Google Scholar]
- duManoir G, Haykowsky M, Syrotuik D, Taylor D, Bell G. The effect of high-intensity rowing and combined strength and endurance training on left ventricular systolic function and morphology. Int J Sports Med. 2007;28:488–494. doi: 10.1055/s-2006-955897. [DOI] [PubMed] [Google Scholar]
- Fagard R. Athlete's heart. Heart. 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, Nieman DC, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- George KP, Birch KM, Pennell DJ, Myerson SG. Magnetic-resonance-imaging-derived indices for the normalization of left ventricular morphology by body size. Magn Reson Imaging. 2009;27:207–213. doi: 10.1016/j.mri.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Humen D, Teo K, Quinney A, Souster M, Bell G, Taylor D. Effect of 16 weeks of resistance training on left ventricular morphology and systolic function in healthy men> 60 years of age. Am J Cardiol. 2000;85:1002–1006. doi: 10.1016/s0002-9149(99)00918-2. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, McGavock J, Vonder Muhll I, Koller M, Mandic S, Welshe R, Taylor D. Effect of exercise training on peak aerobic power, left ventricular morphology, and muscle strength in healthy older women. J Gerontol A Biol Sci Med Sci. 2005;60A:307–311. doi: 10.1093/gerona/60.3.307. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Taylor D, Teo K, Quinney A, Humen D. Left ventricular wall stress during leg-press exercise performed with a brief Valsalva maneuver. Chest. 2001;119:150–154. doi: 10.1378/chest.119.1.150. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Bricknell K, Chan J, Hanekom L, Marwick TH. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol. 2007;99:300–306. doi: 10.1016/j.amjcard.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FL, Katch VL, editors. Exercise Physiology: Nutrition, Energy and Human Performance. Baltimore: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- Meyer K. Exercise training in heart failure: recommendations based on current research. Med Sci Sports Exerc. 2001;33:525–531. doi: 10.1097/00005768-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Mor-Avi V, Lang R, Badano L, Belohlavek M, Cardim N, Derumeaux G, Galderisi M, Marwick T, Nagueh S, Sengupta P, Sicari R, Smiseth O, Smulevitz B, Takeuchi M, Thomas J, Vannan M, Voigt J, Zamorano J. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–524. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- Myerson SG, Montgomery HE, World M, Pennell D. Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension. 2002;40:673–678. doi: 10.1161/01.hyp.0000036401.99908.db. [DOI] [PubMed] [Google Scholar]
- Naylor LH, George K, O'Driscoll G, Green DJ. The athlete's heart: a contemporary appriasal of the ‘Morganroth hypothesis’. Sports Med. 2008;38:69–90. doi: 10.2165/00007256-200838010-00006. [DOI] [PubMed] [Google Scholar]
- Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol. 2008;586:4721–4733. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliccia A, Maron BJ, De Luca R, Di Paolo FM, Spataro A, Culasso F. Remodelling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation. 2002;105:944–949. doi: 10.1161/hc0802.104534. [DOI] [PubMed] [Google Scholar]
- Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete's heart: right and left ventricular mass and function in male endurance athletes and untraied individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- Vogelsang TW, Hanel B, Kristoffersen US, Petersen CL, Mehlsen J, Holmquist N, Larsson B, Kjaer A. Effect of eight weeks of endurance training on right and left ventricular volume and mass in untrained obese subjects: a longitudinal MRI study. Scand J Med Sci Sports. 2008;18:354–359. doi: 10.1111/j.1600-0838.2007.00706.x. [DOI] [PubMed] [Google Scholar]
- Wernstedt P, Sjöstedt C, Ekman I, Du H, Thuomas KA, Areskog NH, Nylander E. Adaptation of cardiac morphology and function to endurance and strength training: A comparative study using MR imaging and echocardiography in males and females. Scand J Med Sci Sports. 2002;12:17–25. doi: 10.1034/j.1600-0838.2002.120104.x. [DOI] [PubMed] [Google Scholar]


