Non-technical summary
Reduced atmospheric O2 availability (hypoxia) impairs muscle oxidative energy production and exercise tolerance. We show that dietary supplementation with inorganic nitrate reduces markers of muscle fatigue and improves high-intensity exercise tolerance in healthy adults inhaling air containing 14.5% O2. In the body, nitrate can be converted to nitrite and nitric oxide. These molecules can improve muscle efficiency and also dilate blood vessels allowing more O2 to be delivered to active muscle. These results suggest that dietary nitrate could be beneficial during exercise at moderate to high altitude and in conditions where O2 delivery to muscle is reduced such as in pulmonary, cardiovascular and sleep disorders.
Abstract
Abstract
Exercise in hypoxia is associated with reduced muscle oxidative function and impaired exercise tolerance. We hypothesised that dietary nitrate supplementation (which increases plasma [nitrite] and thus NO bioavailability) would ameliorate the adverse effects of hypoxia on muscle metabolism and oxidative function. In a double-blind, randomised crossover study, nine healthy subjects completed knee-extension exercise to the limit of tolerance (Tlim), once in normoxia (20.9% O2; CON) and twice in hypoxia (14.5% O2). During 24 h prior to the hypoxia trials, subjects consumed 0.75 L of nitrate-rich beetroot juice (9.3 mmol nitrate; H-BR) or 0.75 L of nitrate-depleted beetroot juice as a placebo (0.006 mmol nitrate; H-PL). Muscle metabolism was assessed using calibrated 31P-MRS. Plasma [nitrite] was elevated (P < 0.01) following BR (194 ± 51 nm) compared to PL (129 ± 23 nm) and CON (142 ± 37 nM). Tlim was reduced in H-PL compared to CON (393 ± 169 vs. 471 ± 200 s; P < 0.05) but was not different between CON and H-BR (477 ± 200 s). The muscle [PCr], [Pi] and pH changed at a faster rate in H-PL compared to CON and H-BR. The [PCr] recovery time constant was greater (P < 0.01) in H-PL (29 ± 5 s) compared to CON (23 ± 5 s) and H-BR (24 ± 5 s). Nitrate supplementation reduced muscle metabolic perturbation during exercise in hypoxia and restored exercise tolerance and oxidative function to values observed in normoxia. The results suggest that augmenting the nitrate–nitrite–NO pathway may have important therapeutic applications for improving muscle energetics and functional capacity in hypoxia.
Introduction
Hypoxia has far-reaching consequences for skeletal muscle energy metabolism and fatigue development during exercise. Breathing a gas mixture with a reduced fraction of O2 (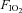 ) results in a reduced O2 partial pressure (
) results in a reduced O2 partial pressure (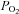 ) gradient between the microcirculatory and intracellular compartments, a reduction in intracellular
) gradient between the microcirculatory and intracellular compartments, a reduction in intracellular 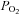 (Richardson et al. 1995), and a compensatory increase in blood flow (Heinonen et al. 2010). A fixed submaximal work rate is associated with the same O2 uptake (
(Richardson et al. 1995), and a compensatory increase in blood flow (Heinonen et al. 2010). A fixed submaximal work rate is associated with the same O2 uptake (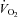 ) but a greater muscle metabolic perturbation in hypoxia compared to normoxia (Linnarsson et al. 1974; Adams & Welch 1980; Hogan et al. 1999; Wilkins et al. 2006). Reduced microvascular and intracellular
) but a greater muscle metabolic perturbation in hypoxia compared to normoxia (Linnarsson et al. 1974; Adams & Welch 1980; Hogan et al. 1999; Wilkins et al. 2006). Reduced microvascular and intracellular  mandates greater concentrations of other regulators of mitochondrial respiration to maintain the required rate of oxidative ATP turnover, namely, ADP, Pi and NADH, which are derived through elevated rates of phosphocreatine (PCr) hydrolysis and glycolysis (Hogan et al. 1983, 1999). The net result of hypoxia relative to normoxia at work rates >50% of maximum is accelerated depletion of muscle PCr and glycogen and a more rapid accumulation of fatigue-related metabolites (ADP, Pi, H+) which contribute to impaired exercise tolerance (Hogan et al. 1999; Richardson et al. 1999; Allen et al. 2008). Depending on the severity of hypoxia and the training status of the subjects, hypoxia also attenuates the maximal oxidative metabolic rate, which is reflected in a slowing of [PCr] recovery kinetics following cessation of exercise (Blei et al. 1993; Paganini et al. 1997; Haseler et al. 1999).
mandates greater concentrations of other regulators of mitochondrial respiration to maintain the required rate of oxidative ATP turnover, namely, ADP, Pi and NADH, which are derived through elevated rates of phosphocreatine (PCr) hydrolysis and glycolysis (Hogan et al. 1983, 1999). The net result of hypoxia relative to normoxia at work rates >50% of maximum is accelerated depletion of muscle PCr and glycogen and a more rapid accumulation of fatigue-related metabolites (ADP, Pi, H+) which contribute to impaired exercise tolerance (Hogan et al. 1999; Richardson et al. 1999; Allen et al. 2008). Depending on the severity of hypoxia and the training status of the subjects, hypoxia also attenuates the maximal oxidative metabolic rate, which is reflected in a slowing of [PCr] recovery kinetics following cessation of exercise (Blei et al. 1993; Paganini et al. 1997; Haseler et al. 1999).
The compensatory vasodilatation during hypoxic exercise is likely to be mediated by several synergistic factors including β-adrenergic and adenosine receptor activation, prostaglandin synthesis and the release of nitric oxide (NO) (MacLean et al. 1998; Casey et al. 2010, 2011; Crecelius et al. 2011). There is evidence to suggest that NO plays an increasingly important vasodilatory role at higher metabolic rates (Wilkins et al. 2008; Casey et al. 2010, 2011). NO is produced through the oxidation of l-arginine in a reaction catalysed by endothelial nitric oxide synthase (eNOS), but may also be produced through the reduction of nitrite (NO2−). NO2− is produced by the oxidation of endogenous NO but is also derived through the reduction of dietary inorganic nitrate (Govoni et al. 2008). Approximately 25% of the ingested nitrate enters the enterosalivary system and may subsequently be reduced to NO2− in the mouth by bacteria residing on the surface of the tongue (Duncan et al. 1995; Lundberg & Govoni 2004). The swallowed NO2− elevates plasma [NO2−] and may be reduced further to NO by various pathways (such as deoxyhaemoglobin and xanthine oxidoreductase) which are potentiated in hypoxic and acidic conditions (Millar et al. 1998; Zhang et al. 1998; Modin et al. 2001; Maher et al. 2008). It has been suggested that elevated NO availability may improve the diffusion of O2 to tissues further away from capillaries, resulting in a more precise local matching of O2 delivery to metabolic rate (Thomas et al. 2001).
The elevated plasma [NO2−] following dietary nitrate intake is associated with reduced blood pressure in normotensive humans (Larsen et al. 2007; Webb et al. 2008; Bailey et al. 2009, Kapil et al. 2010; Vanhatalo et al. 2010a). Nitrate supplementation has also been shown to reduce the O2 (and ATP) cost of steady-state low-intensity exercise, to reduce the rate of PCr degradation during high-intensity exercise, and to increase exercise tolerance (Larsen et al. 2007; Bailey et al. 2009; 2010;). While increased NO bioavailability appears to be beneficial to cardiovascular health, reduced NO synthesis is characteristic of a number of pathologies. Ageing and poor cardiovascular health are associated with uncoupling of eNOS resulting in reduced capacity for endogenous NO production (Sindler et al. 2009; Yang et al. 2009). The exogenous nitrate–nitrite–NO reduction pathway is enhanced by acidic and hypoxic conditions extant during repeated muscle contractions and in poorly perfused muscle (Bryan 2006; van Faassen et al. 2009). Dietary nitrate supplementation may therefore represent a potential therapeutic intervention to alleviate the negative effects of hypoxia on skeletal muscle metabolism and performance. This is important because tissue hypoxia contributes to exercise intolerance in several disease conditions including chronic heart failure, peripheral arterial disease and diabetes (Bulmer & Coombes 2004; Ellis et al. 2010; Kenjale et al. 2011) as well as on exposure to moderate and high altitude (Amann & Calbet, 2008).
We reasoned that the greater muscle metabolic perturbation and reduction in exercise tolerance that is typically observed in hypoxia compared to normoxia (Haseler et al. 1998; Hogan et al. 1999) may be attenuated when hypoxic exercise is preceded by dietary nitrate intake. To study this, we used nitrate-rich beetroot juice (BR; Webb et al. 2008; Bailey et al. 2009; Kapil et al. 2010; Lansley et al. 2011) to elevate NO bioavailability prior to exercise testing in moderate normobaric hypoxia (14.5% O2 in balance N2). We tested the hypotheses that: (1) the rates of change in muscle [PCr], [Pi], [ADP] and pH will be greater during exercise in hypoxia with placebo supplementation (H-PL) compared to normoxia (control; CON), but will be restored to CON values following nitrate supplementation in hypoxia (H-BR); (2) the time-to-exhaustion (Tlim) during high-intensity exercise will be shorter in H-PL compared to CON but not different between CON and H-BR; and (3) the time constant of PCr recovery following exercise in H-PL will be greater than that measured in CON but not different between CON and H-BR.
Methods
Ethical approval
All procedures were approved by the University of Exeter research ethics committee and were in accordance with the standards set by the Declaration of Helsinki. Subjects gave written informed consent to participate after the experimental procedures, associated risks, and potential benefits of participation had been explained.
Subjects
Nine healthy subjects, who were moderately trained in recreational sport, volunteered to participate in this study (7 males, mean ± SD: age 28 ± 7 years, body mass 78.1 ± 9.8 kg, height 1.78 ± 0.05 m; 2 females: age 28 ± 7 years, body mass 57.0 ± 2.8 kg, height 1.73 ± 0.04 m). Subjects were instructed to arrive at the laboratory in a rested and fully hydrated state, at least 3 h post-prandial, and to avoid strenuous exercise in the 24 h preceding each testing session. Participants were asked to refrain from consuming caffeine for 6 h and alcohol for 24 h before each test. Subjects also abstained from using antibacterial mouthwash throughout the study in order to preserve commensal oral bacteria which reduce nitrate to nitrite (Govoni et al. 2008). Subjects were instructed to avoid foods rich in nitrate (such as leafy green vegetables, beetroot and processed meats) during the study period.
Experimental procedures
Subjects were familiarized with the test protocol prior to data collection. During this initial visit, a high-intensity work rate which would result in exhaustion in approximately 5–8 min was determined for each subject. The following three visits (CON, H-PL, and H-BR) were allocated in a double-blind, counter-balanced, randomized order.
Exercise tests were performed in a prone position within the bore of a 1.5 T superconducting magnet (Gyroscan Clinical Intera, Philips, The Netherlands) using a custom-built non-ferrous ergometer. The feet were fastened securely to padded foot braces using Velcro straps and connected to the ergometer load baskets via a rope and pulley system. Two-legged knee-extensions over a distance of ∼0.22 m were performed continuously at a constant frequency which was set in unison with the magnetic pulse sequence (40 pulses min−1) to ensure the quadriceps muscle was in the same phase of contraction during each MR pulse acquisition. To prevent displacement of the quadriceps relative to the MRS coil, Velcro straps were fastened over the subject's thighs, hips and lower back. The exercise protocol consisted of 4 min of low-intensity exercise and, following 6 min of passive rest, two 24 s bouts of high-intensity exercise which were separated by 4 min of rest. These brief high-intensity exercise bouts were used for the assessment of [PCr] recovery kinetics in the absence of substantial alteration in muscle pH. After a further 6 min of rest, subjects completed one high-intensity exercise bout which was continued until the Tlim. Subjects received strong verbal encouragement to continue for as long as possible but no feedback was given on the elapsed time. Tlim was recorded to the nearest second. Knee extensor displacement was measured using a calibrated optical shaft encoder (Type BDK.06.05A 100-5-4; Baumer Electric, Swindon, UK) connected to the weight basket pulley, and load was measured using an aluminium load cell (Type F250EBR0HN, Novatech Measurements Ltd, St Leonards-on-Sea, UK). Work done was calculated as the product of force and displacement. The work rates were 28 ± 2 W for the 4 min low-intensity bout, 56 ± 3 W for the 24 s bouts, and 48 ± 4 W for the high-intensity bout which was continued to Tlim.
Subjects wore a facemask throughout all exercise tests and breathed the normoxic or hypoxic inspirate for 15 min prior to the start of the exercise protocol while resting in a prone position in the bore of the magnet. Blood pressure of the brachial artery was measured at the end of this 15 min period (Schiller Maglife Light, Siemens, Germany) and the mean value of three consecutive measurements was recorded. Heart rate and arterial O2 saturation (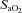 ) were monitored continuously throughout each testing session with a finger probe oximeter (Nonin 7500FO, Nonin Medical Inc., Plymouth, MN, USA). The inspirate was generated using a Hypoxico HYP-100 filtration system (Sporting Edge UK Ltd, Basingstoke, UK). The generator fed via an extension tube to a 150 L Douglas Bag (Cranlea & Co., Birmingham, UK) placed within the scanner room. This acted as a reservoir and mixing chamber, and had a separate output pipe feeding into a two-way breathing valve system (Hans Rudolf, Cranlea & Co.), which was connected to the facemask. Thus, the flow rate was maintained constant, and no re-breathing of expired air occurred.
) were monitored continuously throughout each testing session with a finger probe oximeter (Nonin 7500FO, Nonin Medical Inc., Plymouth, MN, USA). The inspirate was generated using a Hypoxico HYP-100 filtration system (Sporting Edge UK Ltd, Basingstoke, UK). The generator fed via an extension tube to a 150 L Douglas Bag (Cranlea & Co., Birmingham, UK) placed within the scanner room. This acted as a reservoir and mixing chamber, and had a separate output pipe feeding into a two-way breathing valve system (Hans Rudolf, Cranlea & Co.), which was connected to the facemask. Thus, the flow rate was maintained constant, and no re-breathing of expired air occurred.
The O2 and CO2 concentration of the inspirate was monitored during each test using a Servomex 5200 High Accuracy Paramagnetic O2 and CO2 Analyzer (Servomex, Crowborough, UK). The gas analyser was calibrated prior to each test with a 16.0% O2, 8.0% CO2 and 76.0% N2 gas mix (BOC Special Gases, Guildford, UK). For the normoxic CON trial, the Hypoxico HYP-100 was switched to normobaric mode (i.e. all O2 filters were inactivated such that no O2 was removed from ambient air), whereas during hypoxic tests, the generator was set to maximum O2 filtration, which yielded an 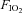 of 14.45 ± 0.05%, and an
of 14.45 ± 0.05%, and an 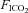 of 0.04 ± 0.00%. The subject and the researcher running the exercise test within the MR scanner room were blinded to the inspirate being used.
of 0.04 ± 0.00%. The subject and the researcher running the exercise test within the MR scanner room were blinded to the inspirate being used.
Supplementation and nitrite analyses
During 24 h prior to the hypoxic trials, subjects consumed 0.75 L of nitrate-rich beetroot juice containing 9.3 mmol nitrate (H-BR) or 0.75 L of nitrate-depleted beetroot juice containing 0.006 mmol nitrate (H-PL; Beet It, James White Drinks Ltd, Ipswich). Nitrate was removed from the placebo product before pasteurization by passing beetroot juice through a column containing Purolite A520E ion-exchange resin, which is specific for nitrate (Lansley et al. 2011). The supplement was taken in three equal doses approximately 24 h, 12 h and 2.5 h prior to the start of the exercise test. Upon arrival at the laboratory, a venous blood sample (6 mL) was drawn from the antecubital vein into a lithium-heparin tube (Vacutainer, Becton Dickinson, New Jersey, USA). Samples were centrifuged at 2700g and 4°C for 10 min, within 3 min of collection. Plasma was subsequently extracted and immediately frozen at –80°C, for later analysis of [NO2−] using a modification of the chemiluminescence technique which we have used previously (Bailey et al. 2010; Vanhatalo et al. 2010a). Equipment and surfaces were regularly rinsed with ionised water to minimise contamination of samples by extraneous sources of nitrite and nitrate. Before samples were analysed for NO2− content, they were thawed at room temperature and deproteinised using zinc sulfate precipitation. The deproteinised samples were then refluxed in 0.3 m sodium iodide and glacial acetic acid at room temperature and analysed for [NO2−] using a Sievers nitric oxide analyser (Sievers NOA 280i, Analytix Ltd, Durham, UK). The nitrate concentrations of diluted beetroot supplements (100- and 1000-fold) were determined by the reduction to NO in a solution of vanadium (III) chloride in hydrochloric acid. The gas-phase chemiluminescent reaction between NO and ozone was detected from the spectral emission of the electronically excited nitrogen dioxide product, by a thermoelectrically cooled, red-sensitive photomultiplier tube housed in the nitric oxide analyser (Sievers NOA 280i, Analytix Ltd, Durham, UK).
MRS measurements
Absolute concentrations of muscle metabolites were established using a calibrated 31P-MRS technique. Spatially localized spectroscopy was undertaken prior to the exercise protocol to determine the relative signal intensities obtained from a phosphoric acid source placed within the scanner bed and Pi in the muscle tissue. A subsequent scan was performed comparing the signals obtained from the phosphoric acid standard and an external Pi solution of known concentration. The voxel sampled within the external Pi solution was defined such that it was of the same dimensions and distance from the coil as the muscle in the previous scan. The muscle Pi concentration was calculated following corrections for relative coil loading. PCr and ATP concentrations were then calculated using the ratio of Pi/PCr and Pi/ATP for each individual. For the exercise protocol, once the phosphoric acid source had been removed, fast field echo images were acquired to determine whether the muscle was positioned correctly relative to the coil. Matching and tuning of the coil was performed and an automatic shimming protocol was then undertaken within a volume that defined the quadriceps muscle. Before and during exercise, data were acquired every 1.5 s, with a spectral width of 1500 Hz. Phase cycling with four phase cycles was employed, leading to a spectrum being acquired every 6 s. The subsequent spectra were quantified via peak fitting, assuming prior knowledge, using the jMRUI (v. 3) software package employing the AMARES fitting algorithm (Vanhamme et al. 1997). Spectra were fitted assuming the presence of the following peaks: Pi, phosphodiester, PCr, α-ATP (2 peaks, amplitude ratio 1:1), γ-ATP (2 peaks, amplitude ratio 1:1), and β-ATP (3 peaks, amplitude ratio 1:2:1). Intracellular pH was calculated using the chemical shift of the Pi spectral peak relative to the PCr peak. [ADP] was calculated via knowledge of [Pi], [PCr], and pH values, taking into account the dependency of rate constants on pH (Kemp et al. 2001).
The PCr recovery time constant (τ) was determined by fitting a single exponential function to the [PCr] recorded over 150 s following the two 24 s exercise bouts (GraphPad Prism, GraphPad Software, La Jolla, CA, USA). Each transition was fitted separately and the mean of the two time constants was calculated for each subject. The signal intensities representing the metabolite concentrations (PCr, ADP, Pi and also pH) at resting baseline were calculated as the mean over the final 120 s preceding the first exercise bout and the end-exercise values were taken as the mean values measured over the final 12 s of exercise. The rates of change during high-intensity exercise were calculated by dividing the change in metabolite concentrations ([PCr], [Pi], [ADP] or pH) between given time points by the time separating those points. The overall rate of change was calculated as the end-exercise value change in metabolite concentration relative to baseline divided by Tlim.
Statistical analyses
One-way repeated measures analyses of variance were used to assess differences across the treatments (CON, H-BR and H-PL trials) with follow-up LSD pair-wise comparisons as appropriate (SPSS v15.0, SPSS Inc., Chicago, IL, USA). Statistical significance was accepted at the P < 0.05 level and data are presented as means ± SD unless stated otherwise.
Results
Plasma [NO2−] and blood pressure
Plasma [NO2−] was elevated following supplementation with nitrate-rich beetroot juice (194 ± 51 nM) compared to placebo (129 ± 23 nM; P < 0.05) and control (142 ± 37 nM; P < 0.05). The blood pressure data are summarised in Table 1. The systolic BP was lower in H-BR than in H-PL (P < 0.05) and tended to be lower in H-BR than in CON (P = 0.07). The diastolic BP was reduced in the H-BR condition compared to H-PL and CON (both P < 0.05). Similarly, the mean arterial pressure (MAP) was lower in H-BR compared to H-PL and tended to be lower in H-BR than in CON (P = 0.08). The mean 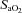 was lower in H-PL (91 ± 2 %) and H-BR (92 ± 1 %) compared to CON (98 ± 1 %) (both P < 0.05). Heart rate at rest was not significantly different between conditions (64 ± 5 b min−1 in CON, 72 ± 9 b min−1 in H-PL, and 68 ± 6 b min−1 in H-BR).
was lower in H-PL (91 ± 2 %) and H-BR (92 ± 1 %) compared to CON (98 ± 1 %) (both P < 0.05). Heart rate at rest was not significantly different between conditions (64 ± 5 b min−1 in CON, 72 ± 9 b min−1 in H-PL, and 68 ± 6 b min−1 in H-BR).
Table 1.
Blood pressure at rest and muscle metabolic responses (means ± SD) during low-intensity exercise in normoxic control and in hypoxia with placebo (H-PL) and nitrate supplementation (H-BR)
| H-PL | H-BR | CON | |
|---|---|---|---|
| BP systolic (mmHg) | 123 ± 4 | 114 ± 6* | 120 ± 6 |
| BP diastolic (mmHg) | 74 ± 7 | 67 ± 7*† | 71 ± 7 |
| MAP (mmHg) | 90 ± 5 | 83 ± 5* | 86 ± 5 |
| [PCr] (mm) | |||
| Baseline | 33.6 ± 2.8 | 30.9 ± 3.9 | 31.5 ± 2.6 |
| End-exercise | 27.4 ± 3.0 | 25.2 ± 3.6 | 25.6 ± 3.1 |
| Amplitude | −6.2 ± 1.3 | −5.7 ± 1.9 | −5.9 ± 1.0 |
| [Pi] (mm) | |||
| Baseline | 4.4 ± 0.6 | 4.1 ± 0.8 | 4.3 ± 0.8 |
| End-exercise | 9.2 ± 2.0 | 8.5 ± 2.6 | 8.6 ± 1.3 |
| Amplitude | 4.8 ± 2.1 | 4.4 ± 2.6 | 4.3 ± 1.4 |
| [ADP] (μm) | |||
| Baseline | 7.1 ± 0.9 | 6.7 ± 1.2 | 7.0 ± 1.3 |
| End-exercise | 20.0 ± 3.4 | 18.8 ± 4.2 | 19.8 ± 4.3 |
| Amplitude | 12.9 ± 3.5 | 12.1 ± 5.0 | 12.8 ± 4.0 |
| pH | |||
| Baseline | 7.06 ± 0.03 | 7.05 ± 0.03 | 7.03 ± 0.04 |
| End-exercise | 7.03 ± 0.03 | 7.03 ± 0.04 | 7.01 ± 0.04 |
| Amplitude | −0.03 ± 0.03 | −0.02 ± 0.04 | −0.02 ± 0.04 |
Amplitude indicates the change from baseline to end-of-exercise. (MAP: mean arterial pressure.)
Different from H-PL, P < 0.05
different from CON P < 0.05.
[PCr] recovery kinetics
The reduction in muscle [PCr] from resting baseline during the 24 s high-intensity bout was not different between conditions (9.8 ± 2.1 mm in CON, 9.6 ± 2.1 mm in H-PL and 9.3 ± 2.9 mm in H-BR). The end-exercise pH was not different from resting baseline (7.06 ± 0.03 in CON, 7.09 ± 0.04 in H-PL and 7.08 ± 0.04 in H-BR). The [PCr]τ measured during recovery was significantly greater in H-PL (29 ± 5 s) than H-BR (24 ± 5 s; P < 0.01) and CON (23 ± 5 s; P < 0.01). The [PCr]τ was not different between H-BR and CON.
Low-intensity exercise
The muscle metabolic responses to low-intensity exercise are presented in Table 1 and illustrated in Fig. 1. The HR measured at the end of exercise was lower in CON (76 ± 5 b min−1) than in H-PL (86 ± 6 b min−1) and H-BR (84 ± 9 b min−1) (both P < 0.05), but was not different between H-PL and H-BR. The ANOVA revealed no significant differences in the baseline or end-exercise [PCr], [Pi], [ADP] or pH between conditions. However, a direct comparison (one-tailed t test) between the [PCr] amplitude in H-PL and H-BR indicated a significant difference (P < 0.05) (Fig. 1).
Figure 1.
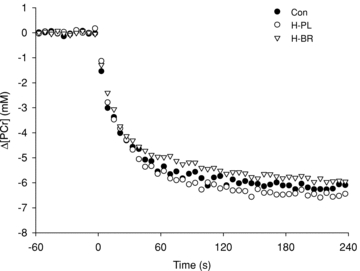
Intramuscular [PCr] relative to resting baseline illustrated as group mean (error bars excluded for clarity), during low-intensity exercise in normoxia (CON), hypoxia following placebo supplementation (H-PL) and hypoxia following nitrate supplementation (H-BR)
High-intensity exercise
During high-intensity exercise, the Tlim was reduced in H-PL (393 ± 169 s) compared to H-BR (477 ± 200 s; P < 0.05) and CON (471 ± 200 s; P < 0.05). The Tlim was not different between CON and H-BR. The [PCr] and [Pi] profiles are illustrated in Fig. 2 and the [ADP] and pH responses are shown in Fig. 3. There were no significant differences in muscle metabolite concentrations or pH measured at Tlim (Table 2). However, PCr had fallen to a greater extent in the H-PL trial compared to H-BR and CON after 60 s (both P < 0.05), and compared to H-BR after 120 s (P < 0.05; Table 2). The overall rates of [PCr] degradation, [Pi] accumulation and pH reduction during the entire exhaustive exercise bout were greater in H-PL than in H-BR and CON (all P < 0.05; Table 2). The increase in [ADP] was greater in H-PL compared to H-BR and CON after 60 s and compared to H-BR after 120 s (all P < 0.05; Table 2).
Figure 2. Group mean intramuscular [PCr] (A) and [Pi] (B) during high-intensity exercise.
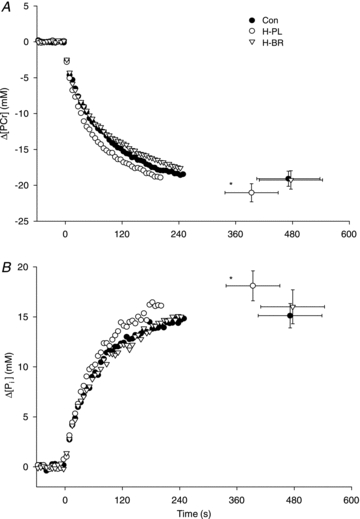
The Tlim was significantly reduced in H-PL compared to (*P < 0.05). Error bars indicate SEM at task failure.
Figure 3. Group mean intramuscular [ADP] and pH during high-intensity exercise.
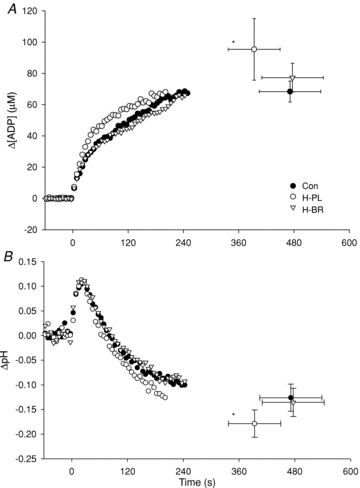
Error bars indicate SEM at task failure. *Tlim less in the H-PL trial compared to H-BR and CON (P < 0.05).
Table 2.
Muscle metabolic responses (means ± SD) during high-intensity exercise in normoxic control and in hypoxia after placebo (H-PL) and nitrate supplementation (H-BR)
| H-PL | H-BR | CON | |
|---|---|---|---|
| [PCr] (mm) | |||
| Baseline | 33.1 ± 2.8 | 30.6 ± 3.9 | 31.2 ± 2.8 |
| Δ 60 s | −13.2 ± 2.7 | −11.1 ± 2.9* | −11.4 ± 2.7* |
| Δ 120 s | −16.6 ± 2.2 | −14.1 ± 3.0* | −15.1 ± 3.1 |
| Δ 180 s | −18.4 ± 2.4 | −16.1 ± 3.2* | −17.3 ± 3.3 |
| At task failure | 12.0 ± 3.6 | 11.3 ± 3.3 | 12.1 ± 2.4 |
| Δ (μm s−1) | −63 ± 28 | −48 ± 21* | −48 ± 24* |
| [Pi] (mm) | |||
| Baseline | 3.3 ± 0.7 | 3.1 ± 0.7 | 3.2 ± 0.9 |
| Δ 60 s | 10.4 ± 2.5 | 8.5 ± 2.1* | 8.5 ± 2.5* |
| Δ 120 s | 15.0 ± 3.3 | 12.2 ± 3.0* | 11.7 ± 3.9* |
| Δ 180 s | 17.1 ± 4.4 | 13.8 ± 4.3* | 13.4 ± 4.0* |
| At task failure | 21.4 ± 4.7 | 19.1 ± 5.4 | 18.3 ± 3.3 |
| Δ (μM s−1) | 54 ± 26 | 40 ± 21* | 39 ± 24* |
| [ADP] (μM) | |||
| Baseline | 7.6 ± 1.1 | 6.9 ± 1.6 | 7.2 ± 1.4 |
| Δ 60 s | 48.1 ± 17.4 | 33.6 ± 11.3* | 34.4 ± 13.0* |
| Δ 120 s | 59.7 ± 18.5 | 44.0 ± 13.6* | 46.7 ± 15.0 |
| At task failure | 102.9 ± 58.6 | 84.2 ± 28.6 | 75.5 ± 19.9 |
| Δ (nm s−1) | 286 ± 177 | 191 ± 104 | 177 ± 107 |
| pH | |||
| Baseline | 7.03 ± 0.03 | 7.02 ± 0.03 | 7.01 ± 0.03 |
| At task failure | 6.85 ± 0.07 | 6.89 ± 0.07 | 6.88 ± 0.08 |
| Rate of change (ks−1) | −0.52 ± 0.24 | −0.34 ± 0.30* | −0.32 ± 0.29* |
Note that there were no significant differences in any of the variables between H-BR and CON. Amplitude indicates the change from baseline to task failure. ks, kiloseconds.
Different from H-PL, P < 0.05.
Discussion
The principal novel finding of this study was that dietary nitrate supplementation reduced muscle metabolic perturbation during high-intensity exercise in hypoxia and restored exercise tolerance to that observed in normoxia. Nitrate supplementation also abolished the reduction in the rate of PCr recovery in hypoxia, possibly due to better NO-mediated matching of tissue O2 supply to local metabolic rate. Essentially, with nitrate supplementation it was possible to attain the same maximal oxidative rate under mild hypoxia as was possible in normoxia. We also showed a trend towards reduced net PCr utilisation during low-intensity steady-state exercise in hypoxia following nitrate intake. The role of NO and nitrite in hypoxic signalling is well recognised. However, this is the first study to demonstrate that the deleterious effects of systemic hypoxia on muscle energetics and exercise tolerance can be ameliorated by increasing NO and nitrite availability by dietary means in humans.
PCr recovery kinetics
An important finding of this study was the speeding of the PCr recovery kinetics by ∼16% in the nitrate supplemented condition relative to placebo in hypoxia. The rate of recovery of intramuscular [PCr] immediately following exercise is considered to reflect the maximal rate of oxidative ATP reconstitution alone, with minimal or no contribution from glycolysis (Arnold et al. 1984; Kemp et al. 1993). Provided that the pH has not declined markedly, the τ of the mono-exponential [PCr] recovery profile is independent of the level of PCr depletion at the cessation of exercise (Thompson et al. 1995). The recovery [PCr]τ in this study was similar to values reported for moderately to well-trained subjects in normoxia (Haseler et al. 1999). O2 delivery would not be considered limiting to maximal oxidative rate in this population during small muscle mass exercise performed in normoxia. A speeding of the [PCr] recovery kinetics reflects an increase in maximal oxidative rate, and can be subsequent to factors such as increased mitochondrial mass, increased oxidative enzyme activity and/or hyperoxia (Arnold et al. 1984; Haseler et al. 1999). Possible mechanisms underlying the observed speeding of [PCr] recovery kinetics in hypoxia following nitrate supplementation therefore include increased mitochondrial efficiency (Larsen et al. 2011), increased bulk O2 delivery and/or a better matching of local perfusion to metabolic rate (Thomas et al. 2001; Victor et al. 2009).
An improved mitochondrial efficiency following nitrate intake, reported in a recent study by Larsen et al. (2011), may have allowed the same maximal ATP re-synthesis rate to be attained in hypoxia as was observed in the normoxic control condition. The mitochondrial P/O ratio was elevated by 19% in human biopsy samples after 3 days of nitrate supplementation (0.1 mmol kg−1 day−1; Larsen et al. 2011), which may be sufficient to account for the 16% reduction in the in vivo[PCr] recovery τ in the present study (using a dose of 0.13 ± 0.02 mmol kg−1 over 24 h). However, we have previously shown that 3–6 days of nitrate supplementation does not speed [PCr] recovery kinetics in normoxia in healthy humans (Bailey et al. 2010 unpublished observation; Lansley et al. 2011). Therefore, while an improved P/O ratio may contribute to the reduced [PCr] recovery τ in hypoxia to some extent, other factors related to altered muscle perfusion and O2 delivery must also be considered.
The combination of systemic hypoxia and muscle contraction creates a powerful stimulus for compensatory vasodilatation to ensure sufficient O2 delivery to active muscle (Calbet et al. 2009; Casey et al. 2010). The complex interactions of numerous vasodilatory mechanisms remain under investigation. However, it is clear that NO and nitrite represent key agents in this signalling cascade (Modin et al. 2001; Maher et al. 2008; Casey et al. 2010, 2011; Heinonen et al. 2011). Elevated NO availability, consistent with the reduced blood pressure after nitrate intake (Cosby et al. 2003), may alter O2 distribution in the active muscle, assuming that there is no simultaneous reduction in cardiac output. The mean plasma [nitrite] was elevated by 50% in H-BR compared to H-PL. In addition to liberating bioactive NO, nitrite itself is recognised as a potent vasodilator especially in hypoxia (Maher et al. 2008). Furthermore, NO may modulate the distribution of tissue and intracellular O2 via inhibition of cytochrome c oxidase, such that fibres situated further away from capillaries are better oxygenated (Thomas et al. 2001; Hagen et al. 2003; Victor et al. 2009). When O2 availability in the mitochondrion is low, cytochrome c oxidase is predominantly in a reduced state and NO competes with O2 for binding at its haem a3 site (Brown & Cooper, 1994). As a result, the available O2 is redistributed away from the mitochondrion causing an attenuation of hypoxic signalling (Hagen et al. 2003; Victor et al. 2009). NO may reduce the heterogeneity of perfusion relative to metabolic activity in skeletal muscle by quenching the metabolic activity of fibres in the close proximity of blood supply, and facilitating improved oxygenation of the more distant fibres by increasing the O2 gradient (Thomas et al. 2001). The level of hypoxia induced in this study was relatively mild such that the elevated NO availability in H-BR may have sufficiently increased the blood flow and improved O2 distribution within the active muscle to compensate for the reduced arterial PO2 and enable the same maximal oxidative rate to be achieved as in normoxia. It should be noted that while nitrate supplementation appears to increase blood volume in skeletal muscle as estimated using near infra-red spectroscopy (Bailey et al. 2009; Kenjale et al. 2011), effects on HR, cardiac output, and the distribution of perfusion to active and inactive tissue remain to be investigated.
High-intensity exercise tolerance
High-intensity exercise tolerance was increased by ∼21% following nitrate supplementation compared to placebo at a fixed work rate in hypoxia. The mechanisms responsible for this effect may include the restoration of the maximal oxidative rate and reduced metabolic perturbation, both of which may be afforded by increased O2 delivery especially to regions that may be relatively more ‘hypoxic’ (as discussed above). Effectively, the fixed work rate demanded a greater proportion of the maximal oxidative rate in H-PL than in H-BR. The rates of PCr degradation and Pi accumulation and the fall in pH were all attenuated following nitrate supplementation. The attenuation of metabolic perturbation allowed high-intensity exercise to be continued for longer before the same (presumably limiting) intramuscular environment was attained as in the placebo and control conditions (Hogan et al. 1999; Vanhatalo et al. 2010b). The effect of nitrate supplementation in hypoxia resembles the effect of hyperoxia (compared to normoxia) on [PCr] and exercise tolerance (Vanhatalo et al. 2010b), suggesting that changes in the microvascular and/or intracellular 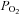 contributed to these effects.
contributed to these effects.
We have previously reported a 25% increase in exercise tolerance and attenuated PCr degradation during high-intensity exercise in normoxia following nitrate supplementation (Bailey et al. 2010). The reduction in the O2 cost and the estimated ATP cost of force production during high-intensity exercise afforded by dietary nitrate (Bailey et al. 2009; 2010;) point to a possibility that the O2 requirement of the active muscle for the same work rate may also have been lower in H-BR compared to H-PL. In the present study, however, the potential synergistic effects of improved O2 delivery, reduced ATP cost of cross-bridge cycling or Ca2+ resequestration by the sarcoplasmic reticulum (contractile efficiency) and/or improved mitochondrial efficiency in hypoxia did not result in an improvement in exercise tolerance beyond what has been observed in normoxia (∼25%; Bailey et al. 2010). It is important to note that the calculation of the ATP turnover rate using 31P-MRS data relies on the [PCr] recovery τ (Lanza et al. 2006; Kemp et al. 2007; Bailey et al. 2010). Because changes in O2 delivery are known to alter the [PCr]τ (Haseler et al. 1999, 2004, 2007), this method cannot differentiate between changes in muscle efficiency and changes in convective or diffusive O2 supply. The effect of nitrate supplementation on muscle perfusion and the resolution of the relative contribution of possible changes in O2 delivery, mitochondrial and/or contractile efficiency on exercise tolerance in hypoxia await further study.
Low-intensity exercise
The capacity of the respiratory and cardiovascular systems to compensate for the reduced 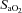 was unlikely to be exceeded in this study given the relatively small active muscle mass (Calbet et al. 2009). Although acute hypoxia does not alter muscle O2 consumption at a fixed low-intensity work rate, net PCr utilisation typically increases compared to normoxia (Haseler et al. 1998; Fig. 1). In the present study, with relatively mild hypoxia, the fall in [PCr] was not significantly greater in H-PL compared to CON during low-intensity exercise, but the fall in [PCr] was smaller in H-BR compared to H-PL (Fig. 1). We have previously shown that the amplitude of [PCr] fall is reduced during low-intensity exercise in normoxia following nitrate supplementation (Bailey et al. 2010). The linear relationship between pulmonary O2 uptake (
was unlikely to be exceeded in this study given the relatively small active muscle mass (Calbet et al. 2009). Although acute hypoxia does not alter muscle O2 consumption at a fixed low-intensity work rate, net PCr utilisation typically increases compared to normoxia (Haseler et al. 1998; Fig. 1). In the present study, with relatively mild hypoxia, the fall in [PCr] was not significantly greater in H-PL compared to CON during low-intensity exercise, but the fall in [PCr] was smaller in H-BR compared to H-PL (Fig. 1). We have previously shown that the amplitude of [PCr] fall is reduced during low-intensity exercise in normoxia following nitrate supplementation (Bailey et al. 2010). The linear relationship between pulmonary O2 uptake (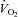 ) and intramuscular [PCr] both before and after nitrate supplementation (Bailey et al. 2010) implies that the reduced O2 cost of exercise largely derives from the contractile apparatus. Additionally, increased O2 delivery in H-BR may have reduced the reliance on substrate-level phosphorylation, thereby sparing muscle [PCr] and resulting in a lower steady-state [PCr] amplitude. It should be noted that the importance of NO as a hypoxic vasodilator may increase with exercise intensity (Casey et al. 2010), which could explain in part why the effects of dietary nitrate were greater during high-intensity exercise compared to low-intensity exercise in this study. It may also be considered that the interplay between the redox state of cytochrome c oxidase and mitochondrial O2 availability may be more sensitive to the manipulation of NO availability at higher exercise intensities. This is because the inhibition of cytochrome c oxidase by NO in competition with O2 requires the cytochrome c oxidase to be in the reduced state, which is increasingly the case when the metabolic rate is high (Wilson et al. 1979; Taylor & Moncada, 2010).
) and intramuscular [PCr] both before and after nitrate supplementation (Bailey et al. 2010) implies that the reduced O2 cost of exercise largely derives from the contractile apparatus. Additionally, increased O2 delivery in H-BR may have reduced the reliance on substrate-level phosphorylation, thereby sparing muscle [PCr] and resulting in a lower steady-state [PCr] amplitude. It should be noted that the importance of NO as a hypoxic vasodilator may increase with exercise intensity (Casey et al. 2010), which could explain in part why the effects of dietary nitrate were greater during high-intensity exercise compared to low-intensity exercise in this study. It may also be considered that the interplay between the redox state of cytochrome c oxidase and mitochondrial O2 availability may be more sensitive to the manipulation of NO availability at higher exercise intensities. This is because the inhibition of cytochrome c oxidase by NO in competition with O2 requires the cytochrome c oxidase to be in the reduced state, which is increasingly the case when the metabolic rate is high (Wilson et al. 1979; Taylor & Moncada, 2010).
Implications
The present findings suggest that dietary nitrate may have important therapeutic applications for improving skeletal muscle energetics and functional capacity when muscle O2 delivery is compromised. Skeletal muscle may face a hypoxic challenge in conditions such as exercise and exposure to moderate-to-high altitude, as well as in cardiovascular, pulmonary and sleep disorders. For instance, it appears that chronic exposure to altitude upregulates endogenous NO production, as evidenced by a 10-fold increase in concentrations of NO products in native high-altitude compared to sea-level dwellers (Erzurum et al. 2007). The reduction in the rates of substrate utilisation and fatigue development during exercise and the greater maximal oxidative rate in hypoxia afforded by nitrate supplementation in this study illustrate the therapeutic potential of dietary nitrate in this environment. With regard to clinical applications, our results are in agreement with a recent study by Kenjale et al. (2011) who showed that dietary nitrate intake improved exercise tolerance by 17% in peripheral arterial disease patients. Dietary nitrate may therefore represent a powerful therapeutic intervention which can alleviate the reduction in maximal oxidative rate in hypoxia by improving mitochondrial and contractile efficiency (Bailey et al. 2010; Larsen et al. 2011) and/or enhancing O2 delivery and distribution within the active muscle.
In the present study, subjects consumed 0.25 L of beetroot juice on three occasions (24 h, 12 h and 2.5 h) prior to completing the hypoxic exercise protocol. While this supplementation regimen was clearly successful in ameliorating the deleterious effects of hypoxia on muscle metabolism, it is unclear whether a single nitrate ‘bolus’ consumed prior to exercise might have been equally effective. Various nitrate supplementation regimens ranging from a single acute bolus (1–2.5 h; Larsen et al. 2010; Vanhatalo et al. 2010a) to continued supplementation over 3–6 days (Larsen et al. 2007; Bailey et al. 2009, 2010; Vanhatalo et al. 2010a) and up to 15 days (Vanhatalo et al. 2010a) have been shown to result in significant alterations in plasma [NO2−], blood pressure and the O2 cost of exercise.
Conclusions
Dietary nitrate intake resulted in a 50% increase in the mean plasma [nitrite] and a significant reduction in the mean arterial pressure, indicating greater NO bioavailability compared to placebo. A key finding of this study was that nitrate supplementation restored high-intensity exercise tolerance in hypoxia to a level which was not different from that measured during the same exercise in normoxia. This effect was accompanied by a reduction in the rate of muscle metabolic perturbation (as indicated by PCr degradation and Pi accumulation) during hypoxic exercise. We also showed that the [PCr] recovery time constant, which reflects the maximal oxidative rate and is normally slowed under hypoxia, was not different in the nitrate supplemented hypoxic condition compared to normoxic control. The restoration of the maximal oxidative rate may be attributed to NO- and nitrite-mediated enhancements to O2 delivery and distribution within the active muscle, with some contribution from enhanced mitochondrial efficiency. These findings have implications for the development of dietary interventions to alleviate the deleterious effects of systemic hypoxia on skeletal muscle energetics and exercise tolerance. Further research is warranted to identify the relative contribution of putative changes in O2 delivery, mitochondrial P/O ratio and muscle contractile efficiency on hypoxic exercise tolerance following nitrate supplementation.
Glossary
Abbreviations
- BP
blood pressure
- MAP
mean arterial pressure
- MRS
magnetic resonance spectroscopy

arterial O2 saturation
- Tlim
limit of tolerance
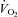
O2 uptake
Author contributions
Experiments were performed at the Peninsula MR Research Unit, University of Exeter. A.V. contributed to the conception and design of the experiment, collection, analysis and interpretation of the data, and writing of this article. J.F. contributed to the design of the experiment, collection and analysis of the NMR data, and critical revision of this article. S.J.B. contributed to the critical interpretation of data and revision of this article. J.R.B. contributed to the collection and analysis of data and critical revision of this article. P.G.W. contributed to the design of the experiment and critical revision of this article. A.M.J. contributed to the conception and design of the experiment and writing of this article. All authors approved the final version of the manuscript.
References
- Adams RP, Welch HG. Oxygen uptake, acid-base status, and performance with varied inspired oxygen fractions. J Appl Physiol. 1980;49:863–868. doi: 10.1152/jappl.1980.49.5.863. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1:307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna F, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of sub-maximal exercise and enhances exercise tolerance in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med. 2006;41:691–701. doi: 10.1016/j.freeradbiomed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med. 2004;34:983–1003. doi: 10.2165/00007256-200434140-00004. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Rådegran G, Boushel R, Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol. 2009;587:477–490. doi: 10.1113/jphysiol.2008.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of β-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol. 2011;110:687–694. doi: 10.1152/japplphysiol.00787.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol. 2010;588:373–385. doi: 10.1113/jphysiol.2009.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011;589:3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C, Dougall H, Johnston P, Green S, Brogan R, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- Ellis CG, Goldman D, Hanson M, Stephenson AH, Milkovich S, Benlamri A, Ellsworth ML, Sprague RS. Defects in oxygen supply to skeletal muscle of prediabetic ZDF rats. Am J Physiol Heart Circ Physiol. 2010;298:H1661–H1670. doi: 10.1152/ajpheart.01239.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci U S A. 2007;104:17593–17598. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Lin A, Hoff J, Richardson RS. Oxygen availability and PCr recovery rate in untrained human calf muscle: evidence of metabolic limitation in normoxia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2046–R2051. doi: 10.1152/ajpregu.00039.2007. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97:1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol. 1998;85:1457–1463. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;299:R72–R79. doi: 10.1152/ajpregu.00056.2010. [DOI] [PubMed] [Google Scholar]
- Heinonen IH, Saltin B, Kemppainen J, Sipilä HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol. 2011;300:H1510–H1517. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Cox RH, Welch HG. Lactate accumulation during incremental exercise with varied inspired oxygen fractions. J Appl Physiol. 1983;55:1134–1140. doi: 10.1152/jappl.1983.55.4.1134. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PY. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol. 2001;535:901–928. doi: 10.1111/j.1469-7793.2001.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6:66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36:399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation. 1998;98:1990–1992. doi: 10.1161/01.cir.98.19.1990. [DOI] [PubMed] [Google Scholar]
- Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol. 1997;272:C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Moncada S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler Thromb Vasc Biol. 2010;30:643–647. doi: 10.1161/ATVBAHA.108.181628. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CH, Kemp GJ, Sanderson AL, Radda GK. Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J Appl Physiol. 1995;78:2131–2139. doi: 10.1152/jappl.1995.78.6.2131. [DOI] [PubMed] [Google Scholar]
- van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010a;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol. 2010b;95:528–540. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- Victor VM, Nuñez C, D'Ocón P, Taylor CT, Esplugues JV, Moncada S. Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ Res. 2009;104:1178–1183. doi: 10.1161/CIRCRESAHA.109.197228. [DOI] [PubMed] [Google Scholar]
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of β-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol. 2006;101:1343–1350. doi: 10.1152/japplphysiol.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DF, Erecińska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys. 1979;195:485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]
- Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MC. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]


