Abstract
Treatment strategies, whether as follow-up or “total incisional biopsy” for gastric noninvasive intraepithelial neoplasia diagnosed by examination of an endoscopic forceps biopsy specimen, are controversial due to problems associated with the diagnostic accuracy of endoscopic forceps biopsy and questions about the safety and efficacy of endoscopic treatment. Based on the histological findings of the biopsy specimen, it is difficult to differentiate between reactive or regenerative changes, inflammation and neoplastic changes, intraepithelial and invasive tumors. Therefore, gastric neoplasia diagnosed as noninvasive intraepithelial often develop into invasive carcinoma during follow-up. Recent advances in endoscopic modalities and treatment devices, such as image-enhanced endoscopy and high-frequency generators, may make endoscopic treatment, such as endoscopic submucosal dissection (ESD), a therapeutic option for gastric intraepithelial neoplasia, including low-grade neoplasms. Future studies are required to evaluate whether ESD is a valid strategy for gastric intraepithelial neoplasm with regard to safety and cost effectiveness.
Keywords: Gastric intraepithelial neoplasia, Adenoma, Dysplasia, Endoscopic submucosal dissection, Endoscopic mucosal resection, Endoscopic resection, Adenocarcinoma
INTRODUCTION
Gastric cancer is the second most common cause of death from cancer worldwide[1,2] and more than half of the world’s gastric cancer cases arise in eastern Asia. Early gastric cancer (EGC) is typically small, asymptomatic and has a good prognosis[3,4], but advanced gastric cancer has higher mortality rate[5]. Therefore, early detection and treatment is important for reducing the gastric cancer mortality rate. In particular, early detection of EGC is important to improve the prognosis of patients with gastric cancer. Surveillance with endoscopy and biopsy sampling is important in patients with premalignant lesions and may lead to the early detection of cancer[6].
Gastric intraepithelial dysplasia/adenomas are considered to be precancerous lesions with a variable clinical course[7,8]. The term intraepithelial dysplasia/adenoma, however, is complex and confusing because of the lack of a uniform classification regarding the features that differentiate between dysplasia/adenoma and EGC. Moreover, it is difficult to differentiate gastric epithelial dysplasia/adenoma and ECG using biopsy specimens because of the inaccuracy of obtaining a biopsy specimen from a malignant region of cancer in an adenoma. This diagnostic inconsistency leads to inappropriate treatment and often results in over- or under-treatment of gastric intraepithelial neoplasias.
In this editorial, we discuss clinical problems in making a diagnosis and treating gastric intraepithelial neoplasia lesions as premalignant.
CHANGES IN CLASSIFICATION FOR GASTRIC INTRAEPITHELIAL NEOPLASIA
In the early 1980s, guidelines for the diagnosis and grading of gastric epithelial neoplasia were developed and a three-stage classification (mild, moderate and severe dysplasia) was proposed. The term “dys” means abnormal and “plasia” means growth; thus, dysplasia is the term for abnormal growth of epithelial cells. Dysplasia is generally defined as unequivocally neoplastic epithelium that may be associated with or develop into invasive adenocarcinoma[9-11]. On the other hand, lesions that most European and American pathologists identify as dysplasia are often considered adenocarcinoma in Japan because, according to the Japanese viewpoint, gastric carcinoma is diagnosed based on nuclear and structural atypia, even when invasion is absent. Therefore, the reports of many Japanese and western pathologists show considerable differences. Schlemper et al[12,13], however, reported that diagnoses based on nuclear and structural atypia are somewhat discrepant between biopsy and resection specimens. They concluded that this may be the reason for the relatively high incidence and good prognosis of gastric carcinoma in Japan compared to western countries. In addition, the term adenoma is applied mostly to macroscopically protruding or superficially elevated lesions in Europe and America but in Japan the term applies to all gross types of the lesions: flat, elevated and depressed.
This confusion has led to several classifications for the terminology between non-neoplastic changes and early invasive cancer[9,11,14-16]. In September 1998, approximately 30 pathologists from 12 countries met in Vienna just before the World Congress of Gastroenterology and reached a consensus on the terminology for gastrointestinal epithelial neoplasia, termed the Vienna classification[17]. In this classification, “high-grade adenoma/dysplasia”, “non-invasive carcinoma (carcinoma in situ)” and “suspected invasive carcinoma” were clustered into a single category (category 4), termed “noninvasive high-grade neoplasia” to eliminate the diagnostic discrepancies between western and Japanese pathologists. Because these three diagnoses cannot be reproducibly distinguished and the treatment recommendation would be the same for each diagnosis, these lesions are considered to be premalignant lesions[18-20]. At the beginning of 2000, the Vienna classification was revised[21] and, for a similar reason, intramucosal carcinoma was added as a fourth subcategory of category 4, because it is often hard to determine whether there is invasion into the lamina propria and because from a therapeutic viewpoint, the distinction between any of the four subcategories is irrelevant. After the revised Vienna classification was introduced, agreement on the diagnosis improved to 80% for gastric lesions[22]. The practical difficulty for diagnosing gastric epithelial dysplasia, however, remains the interpretation by clinicians of the terminology used by pathologists. The use of the term dysplasia confuses clinicians because endoscopy and surgery are linked to legal and social problems and most western surgeons will not operate if pathologists do not clearly diagnose the dysplasia as cancer. Therefore, it is currently not feasible to eliminate the diagnoses of dysplasia in the gastric mucosa. The recently revised new World Health Organization classification of neoplasia of the gastrointestinal tract was published in 2010, in which the term dysplasia is described as “intraepithelial neoplasia (dysplasia)” with dysplasia in parentheses.
Description of intraepithelial neoplasia
Endoscopy is the most sensitive and specific diagnostic tool for gastric neoplasms[23]. It is possible to detect slight changes in color and architecture of the mucosal surface that suggest EGC, in particular, using high-resolution endoscopy[24] and narrow band imaging[25] with chromo endoscopy[26] such as indigo carmine solution. Not to miss a lesion of gastric intraepithelial neoplasia, we often use biopsy specimens as a golden standard for diagnosis. In some cases, we supplement by ultrasonographical assessments of the depth of invasion to judge the lesions that suggest EGC. Those biopsy specimens are diagnosed as below (Table 1).
Table 1.
Definition of intraepithelial neoplasia
| Definition | ||
| Japanese view | Western view | |
| Indefinite for intraepithelial neoplasia | A temporary term | A temporary term |
| It is difficult to distinguish whether a lesion is neoplastic or non-neoplastic, or reactive or regenerative | ||
| LGIN | Characterized by a slightly modified mucosal architecture, including the presence of tubular structures with budding and branching, papillary enfolding, crypt lengthening with serration and cystic changes | |
| HGIN | Characterized by an increasing architectural distortion with glandular crowding and prominent cellular atypia without stromal invasion | |
| Adenocarcinoma/carcinoma | Diagnosed on nuclear and structural atypia, even when invasion is absent[45] | Diagnosed when evident invasive growth of neoplastic epithelium into the lamina propria of the mucosa or beyond is observed[46] |
LGIN: Low-grade intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia.
Indefinite for intraepithelial neoplasia - category 2 in the Vienna classification: Depending on the condition of a biopsy sample, particularly small biopsy specimens, it is occasionally difficult to distinguish whether a lesion is neoplastic or non-neoplastic, reactive or regenerative. A diagnosis of “indefinite for dysplasia” is not a strict biological entity but rather a temporary term that is necessary to keep the patient in follow-up and to obtain more biopsies to make a definitive diagnosis.
Low-grade intraepithelial neoplasia - category 3 in the Vienna classification: Low-grade intraepithelial neoplasia (LGIN) belongs to this category. This lesion shows a slightly modified mucosal architecture, including the presence of tubular structures with budding and branching, papillary enfolding, crypt lengthening with serration and cystic changes.
High-grade intraepithelial neoplasia - category 4.1 in the Vienna classification: There is increasing architectural distortion with glandular crowding and prominent cellular atypia. Tubules can be irregular in shape, with frequent branching, budding and intra-luminal bridges, but there is no stromal invasion. The pleomorphic nuclei show prominent amphophilic nucleoli and a loss of polarity. Increased proliferative activity is present throughout the epithelium.
Difficulty of accurate diagnosis based on biopsy
Endoscopic forceps biopsy is the gold standard for histological diagnosis of gastric epithelial neoplasia. A pathological diagnosis established by biopsy specimen, however, sometimes results in an “under-diagnosis” when compared with diagnosis established by resected specimens. The frequency of discrepant diagnoses between biopsy specimens and the corresponding resected specimens of the same lesions ranges widely in published reports (Table 2). At least, high-grade intraepithelial neoplasia (HGIN) may already have a high probability of a carcinoma. We recently reported that the under-diagnosis rate of intraepithelial neoplasia proven by biopsy was 44% (95% CI: 39%-49%). Moreover, in that study, there were 2 lesions (0.42%) of adenocarcinoma with submucosal invasion of more than 500 μm, one of which involved the lymphatic system[27]. The reasons for the difficulty in making an accurate diagnosis based on a biopsy specimen are as follows: (1)the structural atypia of both adenoma and well-differentiated adenocarcinoma is too subtle to detect in small biopsy specimens; (2) cancer sometimes exists focally in the lesion and a sampling error might occur; and (3) regeneration of atypia induced by gastritis induces histological modification (Figure 1). Recent reports tend to show a higher under-diagnosis rate between biopsy and endoscopic resection samples than previous studies. Moreover, active inflammation of the gastric mucosa infected by Helicobacter pylori may conceal neoplastic architectural distortion and lead to false negative results. We speculate that recent optical advances in endoscopy such as high-resolution endoscopy could lead to “stricter” indication criteria.
Table 2.
Histological discrepancy rates between biopsy and endoscopic resection sample n (%)
| Reports (yr) | Endoscopic biopsy | Resected specimens | Overall | |
| Underdiagnosis1 | Overdiagnosis2 | Discrepancy3 | ||
| Yoon et al[47], 2006 | Tubular adenoma | 2/41 (4.9) | 2/41 (4.9) | 4/41 (9.8) |
| Jung et al[29], 2008 | LGIN | 31/74 (42) | - | - |
| HGIN | 36/40 (90) | 2/40 (5) | 38/40 (95) | |
| Lee et al[48], 2010 | IN | 114/311 (37) | 41/311 (13) | 155/311 (50) |
| Carcinoma | 7/86 (8.1) | 16/86 (19) | 23/86 (26) | |
| Total | 121/397 (30) | 57/397 (14) | 178/397 (45) | |
| Kato et al[27], 2010 | IN | 255/468 (44) | 4/468 (1.7) | 259/468 (46) |
Underdiagnosis was defined as if endoscopic biopsy showed tubular adenoma/ intraepithelial neoplasia but resected specimens finally led to the diagnosis of adenocarcinoma/carcinoma;
Overdiagnosis was defined as if endoscopic biopsy showed tubular adenoma/intraepithelial neoplasia or adenocarcinoma/carcinoma but resected specimens finally led to the diagnosis of non-neoplastic, reactive, regenerative or tubular adenoma, respectively;
Discrepancy was defined as if endoscopic biopsy does not correspond with resected specimen. This can be calculated using resected specimen as a golden standard. LGIN: Low-grade intraepithelial neoplasia; IN: Intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia.
Figure 1.
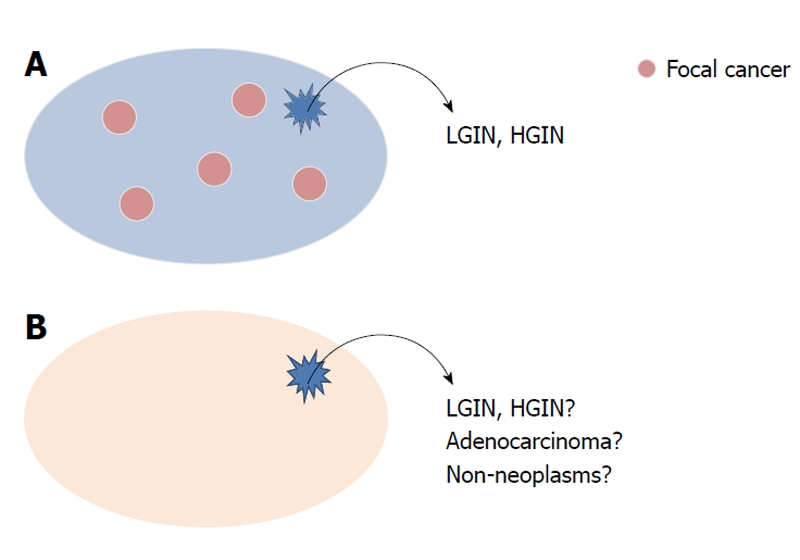
Reasons for the difficulty in making an accurate diagnosis based on a biopsy specimen. Cancer sometimes exists focally in the lesion and sampling error might occur (A); the structural atypia of both adenoma and well-differentiated adenocarcinoma is too subtle for small biopsy specimen. Moreover, regeneration of atypia induced by gastritis induces histological modification (B). LGIN: Low-grade intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia.
Advantage of endoscopic diagnosis
Some endoscopic findings have been reported to predict high-risk lesions for malignancy: (1) diameter > 20 mm; and (2) depressed macroscopic type[28]. Recently, Jung et al[29] reported that depressed type (OR, 4.1) and combined ulceration (OR, 5.6) were significant predictive factors correlated with cancer after endoscopic resection of gastric adenoma using multivariate analysis. For several decades, adenomatous polyps larger than 20 mm have been considered potentially malignant[30] and the rate of malignant transformations increased in accordance with an increase in the size. The depressed type is also reported to have more malignant potential. We, however, revealed that even patients with both smaller and elevated neoplasms had a greater than 40% under-diagnosis rate[27]. Similarly, cancer foci have been reported in hyperplastic polyps with a diameter of 5 mm[31]. Therefore, it is possible that a conventional endoscopic diagnosis based on size alone is not sufficient to make a precise pre-operative diagnosis. Moreover, the surface appearance of an adenoma may also be an important factor for the diagnosis of malignancy[32].
Recent novel diagnostic modalities, including image-enhanced endoscopy, are useful for the differentiation of intraepithelial neoplasia[33]. Yao[33] reported that finding a white opaque substance on magnified endoscope with narrow band imaging differentiates intraepithelial neoplasia with a sensitivity of 94% and a specificity of 96%. Moreover, confocal laser endomicroscopy is reported to identify gastric superficial cancer/HGIN lesions with high validity and reliability compared to conventional white-light endoscopy and histological analysis for the final diagnosis[34]. Although image-enhanced endoscopy and confocal laser endomicroscopy are promising methods and modalities to improve the pre-therapeutic diagnostic accuracy of intraepithelial neoplasia, it is not yet clear whether they are clinically useful because of expert bias.
Treatment strategy for gastric intraepithelial neoplasia: follow-up or endoscopic resection?
There are two therapeutic principles for gastric intraepithelial neoplasias; one is to observe the intraepithelial neoplasia as a benign lesion unless biopsy specimens reveal an unequivocal malignant finding in consideration of the risk of treatment, and the other is to treat the intraepithelial neoplasia actively as a “diagnostic therapy”. There are few guidelines to manage gastric intraepithelial neoplasia. Since 2000, the revised Vienna classification has helped to provide guidance for clinical management[17,21]. The category 4 lesions (high-grade dysplasia and intramucosal cancer) should be resected because they have a high potential for progression to adenocarcinoma[35]. On the other hand, there are no precise guidelines for the management of LGIN. Follow-up studies of HGIN reveal a striking high incidence, around 60%, of developing a carcinoma diagnosed within 1 year in the very short-term follow-up period (Table 3), supporting the validity of a treatment strategy for HGIN.
Table 3.
Histological follow-up studies of gastric intraepithelial neoplasia through mild to severe dysplasia
| Reports (yr) | LGIN (including mild to moderate dysplasia) | HGIN (including severe dysplasia) | ||
| Detection of carcinoma n (%) | Interval (mean) n (%) | Detection of carcinoma | Interval (mean) | |
| Saraga et al[49], 1987 | 1/64 (2) | 4 yr | 17/21 (81) | 4 mo |
| Lansdown et al[46], 1990 | 0/7 (0) | - | 11/13 (85) | 5 mo |
| Rugge et al[50], 1991 | 12/69 (17) | 1 yr | 6/8 (75) | 4 mo |
| Fertitta et al[51], 1993 | 7/30 (23) | 10 mo | 25/31 (81) | 5 mo |
| Farinati et al[52], 1993 | - | - | 16/49 (33)1 | - |
| Di Gregorio et al[53], 1993 | 6/89 (7) | 2 yr | 6/10 (60) | 11 mo |
| Bearzi et al[54], 1994 | 8/81 (9.9) | - | 27/44 (61) | - |
| Rugge et al[55], 1994 | 13/90 (14) | 2 yr | 14/18 (78) | 9 mo |
| Kolodziejczyk et al[56], 1994 | 2/351 (5.72) | - | 7/7 (100) | - |
| Kokkola et al[57], 1996 | 0/9 (0) | - | 2/3 (67) | 1.5 yr |
| Rugge et al[19], 2003 | 8/90 (8.9) | 4 yr | 11/16 (69) | 34 mo |
| Yamada et al[58], 2004 | 0/38 (0) | - | 1/10 (10) | 54 mo |
| Park et al[59], 2008 | 3/26 (12) | 58 mo3 | 1/1 (100) | 58 mo3 |
| Overall | 60/628 (9.5) | 145/231 (63) | ||
Proportion progressing to carcinoma and mean interval.
Moderate or severe;
Mild or moderate dysplasia;
Overall follow-up interval. LGIN: Low-grade intraepithelial neoplasia; IN: Intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia.
The natural course of gastric intraepithelial neoplasia remains unclear. In particular, previous prospective long-term follow-up studies indicated that the gastric cancer incidence in LGIN ranges around 10% (Table 3). This low risk of malignant transformation compared to HGIN may be due to the slowly progressive natural course of LGIN and supports the follow-up strategy. Our current knowledge based on initial intervention, not follow-up, indicates that over 40% of LGIN is diagnosed as adenocarcinoma after resection[27]. This under-diagnosis rate may be higher than that in previous reports. In addition to optical advances in endoscopy, we speculated that the reason for our result was that we used endoscopic submucosal dissection (ESD), not endoscopic mucosal resection (EMR), because an ESD sample has an adequate tumor-free margin; in other words, it is easier to evaluate the atypical structures even if there is less nuclear atypia. Furthermore, we found two cases with submucosal cancers that required radical gastrectomy. These facts mean that even LGIN might not only be a premalignant lesion, but also a lesion that already contains cancer foci and a follow-up strategy might miss the chance for endoscopic therapy. Most LGIN progression to carcinoma, however, is generally low. Repeated endoscopic examination with biopsies burdens the patient with physiological, psychological and financial strains, although few reports discuss these points. Taken together, we consider that initial interventional strategy might be one option, even for LGIN if it is safer with an acceptable range and higher cost effectiveness.
EMR or ESD
Table 4 lists the merit and demerit of both EMR and ESD. EMR with a snare allows for a more accurate histopathological diagnosis than forceps biopsy because the lesion can be resected as a large piece. Previous prospective studies indicate that EMR provides higher diagnostic accuracy than forceps biopsy and the histopathology and complications are within expected norms; based on these studies, EMR is recommended by several reports for diagnosis[36-39]. EMR is limited, however, in that it sometimes results in a multiple piecemeal resection. Multiple piecemeal resection is associated with a specimen burning effect that interferes with an accurate pathological diagnosis. Additionally, a local recurrence may occur, with a reported incidence of approximately 10%[37]. The ESD-related complication rate is relatively low, based on a multicenter study of more than 1000 cases with gastric neoplasm[40].
Table 4.
Endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer[60]
| EMR | ESD | |
| Merits | Minimally invasive technique which is safe, convenient and efficacious | The advantage of achieving large en-bloc resections, not necessarily limited by lesion size |
| Demerits | Insufficient when treating larger lesions, especially larger that 15 mm | Requiring significant additional technical skills and a longer procedure time |
| Prolonged learning curve | ||
| High risks of local recurrence, especially when resections are not performed en bloc or when the resection margins are involved by tumor | A higher complication rate compared to standard EMR |
ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection.
ESD allows for a more secure resection of larger lesions[41,42], resulting in a more accurate diagnosis because the margin of resected sample is larger than that for EMR. Although ESD requires greater skill, causes more complications, such as perforation, and has a longer procedure duration[41-44], in our recent study, the complication rate of ESD for gastric intraepithelial neoplasms was 5.4% for bleeding and 4.3% for perforation and the complete en bloc resection rate was 97%[27]. These rates are almost equivalent to those found in our multicenter study of more than 1000 cases with gastric neoplasm[40], although the perforation rate was only slightly more frequent than that reported for EMR. In detail, all patients with perforation were treated successfully with endoscopic clipping alone and the serious complication rate was only 0.45%. Therefore, the indication of ESD for LGIN, which is considered to be clinically less malignant, is controversial. Future use of ESD for LGIN requires further validation.
CONCLUSION
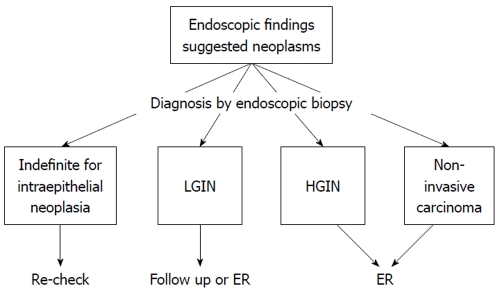
Endoscopic forceps biopsy is insufficient for a definitive diagnosis and therapeutic planning in patients with gastric intraepithelial neoplasia. Endoscopic resection should be considered as not only definitive treatment but also a procedure for a precise histological diagnosis for lesions initially assessed as gastric intraepithelial neoplasia by forceps biopsy specimens. ESD may be a therapeutic option for gastric intraepithelial neoplasia for the purpose of total incisional biopsy. Finally, we have shown the treatment strategy for gastric non-invasive intraepithelial neoplasia diagnosed by endoscopic biopsy as a treatment flowchart (our opinion) (Figure 2). However, we still need to clarify the issue of evaluating the validity as to whether or not to follow-up or ER for LGIN diagnosed by endoscopic biopsy. A prospective study to clarify this is now planned.
Figure 2.
Treatment strategy for gastric non-invasive intraepithelial neoplasia diagnosed by endoscopic biopsy as a treatment flowchart (our opinion). LGIN: Low-grade intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia; ER: Endoscopic resection.
Footnotes
Peer reviewers: Jennifer S Tirnauer, Assistant Professor, Center for Molecular Medicine, University of CT Health Center, Farmington, CT 06030-3101, United States; Ruben Hummelen, Dr., Department of Public Health, Erasmus University Medical Center, Rotterdam 3081HH, The Netherlands
S- Editor Wu X L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 3.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 4.Everett SM, Axon AT. Early gastric cancer in Europe. Gut. 1997;41:142–150. doi: 10.1136/gut.41.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervantes A, Roselló S, Roda D, Rodríguez-Braun E. The treatment of advanced gastric cancer: current strategies and future perspectives. Ann Oncol. 2008;19 Suppl 5:v103–v107. doi: 10.1093/annonc/mdn321. [DOI] [PubMed] [Google Scholar]
- 6.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 7.Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst. 1970;44:297–306. [PubMed] [Google Scholar]
- 8.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110–1113. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980;33:711–721. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 11.Ming SC, Bajtai A, Correa P, Elster K, Jarvi OH, Munoz N, Nagayo T, Stemmerman GN. Gastric dysplasia. Significance and pathologic criteria. Cancer. 1984;54:1794–1801. doi: 10.1002/1097-0142(19841101)54:9<1794::aid-cncr2820540907>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Schlemper RJ, Itabashi M, Kato Y, Lewin KJ, Riddell RH, Shimoda T, Sipponen P, Stolte M, Watanabe H, Takahashi H, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet. 1997;349:1725–1729. doi: 10.1016/S0140-6736(96)12249-2. [DOI] [PubMed] [Google Scholar]
- 13.Schlemper RJ, Kato Y, Stolte M. Well-differentiated adenocarcinoma or dysplasia of the gastric epithelium: rationale for a new classification system. Verh Dtsch Ges Pathol. 1999;83:62–70. [PubMed] [Google Scholar]
- 14.Takagi K, Kumakura K, Sugano H, Nakamura K. [Polypoid lesions of the stomach--with special reference to atypical epithelial lesions] Gan No Rinsho. 1967;13:809–817. [PubMed] [Google Scholar]
- 15.Grundmann E. Histologic types and possible initial stages in early gastric carcinoma. Beitr Pathol. 1975;154:256–280. doi: 10.1016/s0005-8165(75)80034-5. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127–133. doi: 10.1016/s0046-8177(97)90095-2. [DOI] [PubMed] [Google Scholar]
- 17.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Cassaro M, Di Mario F, Leo G, Leandro G, Russo VM, Pennelli G, Farinati F. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–1116. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378–381. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99–106. doi: 10.1007/s00428-002-0680-3. [DOI] [PubMed] [Google Scholar]
- 22.Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36:445–456. doi: 10.1007/s005350170067. [DOI] [PubMed] [Google Scholar]
- 23.Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, Hayashi H, Hinoshita T. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30:669–674. doi: 10.1055/s-2007-1001386. [DOI] [PubMed] [Google Scholar]
- 24.Lambert R, Saito H, Saito Y. High-resolution endoscopy and early gastrointestinal cancer...dawn in the East. Endoscopy. 2007;39:232–237. doi: 10.1055/s-2006-945109. [DOI] [PubMed] [Google Scholar]
- 25.Uedo N, Fujishiro M, Goda K, Hirasawa D, Kawahara Y, Lee JH, Miyahara R, Morita Y, Singh R, Takeuchi M, et al. Role of narrow band imaging for diagnosis of early-stage esophagogastric cancer: current consensus of experienced endoscopists in Asia-Pacific region. Dig Endosc. 2011;23 Suppl 1:58–71. doi: 10.1111/j.1443-1661.2011.01119.x. [DOI] [PubMed] [Google Scholar]
- 26.Kiesslich R, Neurath MF. Magnifying chromoendoscopy for the detection of premalignant gastrointestinal lesions. Best Pract Res Clin Gastroenterol. 2006;20:59–78. doi: 10.1016/j.bpg.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Nishida T, Tsutsui S, Komori M, Michida T, Yamamoto K, Kawai N, Kitamura S, Zushi S, Nishihara A, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46:325–331. doi: 10.1007/s00535-010-0350-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Sakaguchi H, Enjoji M. Depressed adenoma of the stomach. Cancer. 1988;62:2197–2202. doi: 10.1002/1097-0142(19881115)62:10<2197::aid-cncr2820621021>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Jung MK, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg GG, Al-Kawas FH, Fleischer DE, Reilly HF, Benjamin SB. Gastric polyps: relationship of size and histology to cancer risk. Am J Gastroenterol. 1996;91:714–717. [PubMed] [Google Scholar]
- 31.Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. Am J Gastroenterol. 1987;82:1016–1025. [PubMed] [Google Scholar]
- 32.Park DI, Rhee PL, Kim JE, Hyun JG, Kim YH, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506. doi: 10.1055/s-2001-15089. [DOI] [PubMed] [Google Scholar]
- 33.Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574–580. doi: 10.1016/j.gie.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T, Chu CL, Zhang TG, Li YQ. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut. 2011;60:299–306. doi: 10.1136/gut.2010.223586. [DOI] [PubMed] [Google Scholar]
- 35.Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut. 1999;45:784–790. doi: 10.1136/gut.45.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szalóki T, Tóth V, Tiszlavicz L, Czakó L. Flat gastric polyps: results of forceps biopsy, endoscopic mucosal resection, and long-term follow-up. Scand J Gastroenterol. 2006;41:1105–1109. doi: 10.1080/00365520600615880. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara Y, Arakawa T, Fukuda T, Kimura S, Uchida T, Obata A, Higuchi K, Wakasa K, Sakurai M, Kobayashi K. Diagnosis of borderline adenomas of the stomach by endoscopic mucosal resection. Endoscopy. 1996;28:425–430. doi: 10.1055/s-2007-1005505. [DOI] [PubMed] [Google Scholar]
- 38.Muehldorfer SM, Stolte M, Martus P, Hahn EG, Ell C. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465–470. doi: 10.1136/gut.50.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szalóki T, Tóth V, Németh I, Tiszlavicz L, Lonovics J, Czakó L. Endoscopic mucosal resection: not only therapeutic, but a diagnostic procedure for sessile gastric polyps. J Gastroenterol Hepatol. 2008;23:551–555. doi: 10.1111/j.1440-1746.2007.05247.x. [DOI] [PubMed] [Google Scholar]
- 40.Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73–77. doi: 10.1111/j.1443-1661.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 41.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Uedo N, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Higashino K, Ishihara R, Tatsuta M, Ishiguro S. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos) Gastrointest Endosc. 2007;66:186–193. doi: 10.1016/j.gie.2007.03.1059. [DOI] [PubMed] [Google Scholar]
- 43.Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30–36. doi: 10.1007/s00535-009-0137-4. [DOI] [PubMed] [Google Scholar]
- 44.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 45.Itabashi M, Hirota T, Unakami M, Ueno M, Oguro Y, Yamada T, Kitaoka H, Ichikawa H. The role of the biopsy in diagnosis of early gastric cancer. Jpn J Clin Oncol. 1984;14:253–270. [PubMed] [Google Scholar]
- 46.Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31:977–983. doi: 10.1136/gut.31.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon WJ, Lee DH, Jung YJ, Jeong JB, Kim JW, Kim BG, Lee KL, Lee KH, Park YS, Hwang JH, et al. Histologic characteristics of gastric polyps in Korea: emphasis on discrepancy between endoscopic forceps biopsy and endoscopic mucosal resection specimen. World J Gastroenterol. 2006;12:4029–4032. doi: 10.3748/wjg.v12.i25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CK, Chung IK, Lee SH, Kim SP, Lee SH, Lee TH, Kim HS, Park SH, Kim SJ, Lee JH, et al. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol. 2010;25:1507–1513. doi: 10.1111/j.1440-1746.2010.006367.x. [DOI] [PubMed] [Google Scholar]
- 49.Saraga EP, Gardiol D, Costa J. Gastric dysplasia. A histological follow-up study. Am J Surg Pathol. 1987;11:788–796. [PubMed] [Google Scholar]
- 50.Rugge M, Farinati F, Di Mario F, Baffa R, Valiante F, Cardin F. Gastric epithelial dysplasia: a prospective multicenter follow-up study from the Interdisciplinary Group on Gastric Epithelial Dysplasia. Hum Pathol. 1991;22:1002–1008. doi: 10.1016/0046-8177(91)90008-d. [DOI] [PubMed] [Google Scholar]
- 51.Fertitta AM, Comin U, Terruzzi V, Minoli G, Zambelli A, Cannatelli G, Bodini P, Bertoli G, Negri R, Brunati S. Clinical significance of gastric dysplasia: a multicenter follow-up study. Gastrointestinal Endoscopic Pathology Study Group. Endoscopy. 1993;25:265–268. doi: 10.1055/s-2007-1010311. [DOI] [PubMed] [Google Scholar]
- 52.Farinati F, Rugge M, Di Mario F, Valiante F, Baffa R. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients. A prospective study. I.G.G.E.D.--Interdisciplinary Group on Gastric Epithelial Dysplasia. Endoscopy. 1993;25:261–264. doi: 10.1055/s-2007-1010310. [DOI] [PubMed] [Google Scholar]
- 53.Di Gregorio C, Morandi P, Fante R, De Gaetani C. Gastric dysplasia. A follow-up study. Am J Gastroenterol. 1993;88:1714–1719. [PubMed] [Google Scholar]
- 54.Bearzi I, Brancorsini D, Santinelli A, Rezai B, Mannello B, Ranaldi R. Gastric dysplasia: a ten-year follow-up study. Pathol Res Pract. 1994;190:61–68. doi: 10.1016/s0344-0338(11)80497-8. [DOI] [PubMed] [Google Scholar]
- 55.Rugge M, Farinati F, Baffa R, Sonego F, Di Mario F, Leandro G, Valiante F. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107:1288–1296. doi: 10.1016/0016-5085(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 56.Kolodziejczyk P, Yao T, Oya M, Nakamura S, Utsunomiya T, Ishikawa T, Tsuneyoshi M. Long-term follow-up study of patients with gastric adenomas with malignant transformation. An immunohistochemical and histochemical analysis. Cancer. 1994;74:2896–2907. doi: 10.1002/1097-0142(19941201)74:11<2896::aid-cncr2820741103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Kokkola A, Haapiainen R, Laxén F, Puolakkainen P, Kivilaakso E, Virtamo J, Sipponen P. Risk of gastric carcinoma in patients with mucosal dysplasia associated with atrophic gastritis: a follow up study. J Clin Pathol. 1996;49:979–984. doi: 10.1136/jcp.49.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 59.Park SY, Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–970. doi: 10.1097/MEG.0b013e3283013d58. [DOI] [PubMed] [Google Scholar]
- 60.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]