Abstract
Objective
To compare different methods of assessing energy expenditure (EE) and physical activity (PA) in people with spinal cord injury (SCI) under community-dwelling conditions.
Methods
A reference standard encompassing the doubly labelled water (DLW) technique, heart rate monitoring (FLEX-HR), a multi-sensor armband (SenseWear Armband (SWA)), and two PA recall questionnaires were employed in 14 people with SCI to estimate EE and leisure-time PA.
Results
Mean total daily energy expenditure (TDEE) assessed by DLW, FLEX-HR, and SWA were 9817 ± 2491 kJ/day, 8498 ± 1516 kJ/day, and 11414 ± 3242 kJ/day, respectively. Physical activity energy expenditure (PAEE) quantified by DLW was 2841 ± 1626 kJ/day, 2935 ± 1732 kJ/day estimated from FLEX-HR, and 2773 ± 2966 kJ/day derived from SWA. After converting the PA recall questionnaire data to EE in kJ/day, PAEE for the Physical Activity Recall Assessment for People with Spinal Cord Injury (PARA-SCI) was 2339 ± 1171 kJ/day and for Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) 749 ± 1026 kJ/day. DLW-quantified PAEE was moderately associated with PARA-SCI (R2 = 0.62, P < 0.05), but not with the other estimates of PAEE (R2 ranged between 0.13 and 0.30, P > 0.05).
Conclusion
Our findings revealed that the PARA-SCI recall questionnaire was the best estimate of PAEE compared to the reference standard DLW approach. Although the between-method variability for SWA, FLEX-HR, and PASIPD-derived PAEE was small, there was a weak association between these methods and the criterion DLW technique. The best estimate of DLW-quantified TDEE was by FLEX-HR. SWA significantly overestimated TDEE in this population.
Keywords: Spinal cord injuries, Energy expenditure, Physical activity, Obesity, Basal metabolic rate, Doubly labelled water, Calorimetry, Sarcopenia, Disability, Wheelchair, Manual, Paraplegia, Tetraplegia
Introduction
Total daily energy expenditure (TDEE) is the energy used by an individual during a 24-hour period. TDEE can be partitioned into three main components: basal metabolic rate (BMR); the thermic effect of food (TEF); and physical activity (PA)-associated energy expenditure (PAEE). BMR is the largest fraction of TDEE comprising up to 75%, and is defined as the minimal energy cost during absolute rest.1 Although PAEE represents only 15–30% of TDEE, it is considered to be the most important component due to its high variability, which arises from lifestyle factors such as differences in body weight and the type or amount of PA undertaken.1
In people with spinal cord injury (SCI), BMR has been reported to be 14–27% lower than for able-bodied individuals.2 This lower energy expenditure (EE) at rest is likely due to a reduced fat-free mass (FFM) following obligatory sarcopenia after SCI.3 In addition, people with SCI engage in reduced levels of PA – up to 18% lower than for able-bodied individuals.4 Both lower BMR and reduced PAEE are consequences of chronic SCI, and these may be sustained across the lifespan, which predisposes the wheelchair-bound individual to adipose tissue accumulation (and overweight/obesity).5 In addition, numerous adverse metabolic sequelae such as hyperinsulinemia, insulin resistance, type 2 diabetes, dyslipidemia and cardiovascular disease are associated with obesity, and have been reported in this population.6
Participation in regular PA has the potential to elevate TDEE through increased PAEE, and to improve exercise capacity and physical fitness. These changes can lead to health benefits such as reduced risk factors for cardiovascular disease.7 Yet at present, there are no clear PA guidelines for individuals with SCI,8 partly through lack of consensus about activity assessment and EE measurement in this wheelchair-dependent population.2 A range of techniques have been previously employed under both community-dwelling and laboratory conditions, including indirect calorimetry,9 heart rate (HR) monitoring4,10,11 and PA recall questionnaires such as The Physical Activity Scale for Individuals with Physical Disabilities (PASIPD)12 or the Physical Activity Recall Assessment for People with Spinal Cord Injury (PARA-SCI).8 However, due to limitations with some of the techniques for this population, and the lack of comparative studies between the various techniques, there is insufficient evidence to determine which method most accurately estimates TDEE or PAEE in wheelchair users with SCI. For example, despite some initial research to assess EE after SCI, including HR monitoring4,11 and PA recall questionnaires,8,12 these methods have not been validated against a reference standard such as the doubly labelled water (DLW) technique. Additionally, the SenseWear Armband (SWA), which has been used to estimate EE in overweight children,13 in patients with cancer,14 and healthy children,15 has not been validated in SCI population.
Therefore, considering the importance of PA for general health and well-being, this study sought to compare predictive methods to assess PA and EE with the reference standard DLW technique.
Methods
Participants
Approval for this study was granted by the University of Sydney Human Research Ethics Committee. Fourteen people with SCI were recruited for this study. Inclusion criteria were: (i) aged 18–65 years; (ii) at least 1-year post-injury; and (iii) use of a manual wheelchair as their sole form of locomotion. Written informed consent was gained from all participants. Exclusion criteria were: non-treated pressure sores, any cardiovascular conditions, chronic neuropathic pain, and smoking.
Protocol
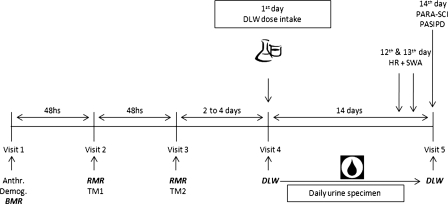
Participants visited the Clinical Exercise & Rehabilitation Unit on five occasions (Fig. 1). Anthropometric and demographic assessments and a BMR measurement were conducted during the first visit. At least 48 hours later, participants presented on two non-consecutive days for the treadmill tests, as part of the FLEX-HR EE-estimation protocol.16
Figure 1.
Protocol over the 5-day visit. Anthro. = anthropometric measurements; Demog. = demographic measurements; BMR = basal metabolic rate; RMR = resting metabolic rate; TM1 = first treadmill test; TM2 = second treadmill test; DLW = doubly labelled water; HR = heart rate monitoring; SWA = SenseWear Armband; PARA-SCI = Physical Activity Recall Assessment for People with Spinal Cord Injury; PASIPD = Physical Activity Scale for Individuals with Physical Disabilities.
On the fourth laboratory visit, a double-isotope labelled water dose was administered. Fourteen days later, participants attended for their final visit to complete two PA recall questionnaires (PASIPD and PARA-SCI) and to submit their daily urine samples. For two consecutive days during the 14-day period (between visits 4 and 5), participants wore a HR monitor and a multi-sensor armband (SWA) during their waking hours, for at least 12 hours duration. Our protocol, as characterized in Fig. 1, was based on previous studies for which similar methods had been used.17,18
Anthropometric and demographic characteristics (visit 1)
Body mass was taken to the nearest 0.1 kg using a digital scale (K Tron, K Tron International Inc, Scottsdale, AZ, USA), with participants wearing light clothing sitting in their wheelchairs. The mass of the wheelchair was later subtracted from the total mass of the participant plus the wheelchair. Stature was measured in a supine position using a metal measuring tape (Gulick Anthropometric Tape, G&S – Fibreflex, Inc, Danville, IL, USA). Body mass index (kg m2) was calculated from mass and stature. Body composition was measured using stable-isotope dilution to measure total body water19 and subsequently calculated fat mass and FFM using a two-compartment model. The following demographic information was collected: age, gender, neurological lesion level, severity of lesion, and time since injury.
Basal metabolic rate (visit 1)
BMR was measured following a 12-hour fast. Participants attended the laboratory between 7:00 and 8:00 a.m. and were instructed to lie supine and remain awake for 30-minute of quiet rest. Subsequently, BMR was measured over the next 30 minutes and then used for the TDEE sub-fractions of the FLEX-HR method.
To assess BMR, an open-circuit spirometry metabolic measurement system (Medical Graphics Corporation, St Paul, MN, USA) was used to perform breath-by-breath gas analysis. The Weir equation was used to calculate BMR (20.5 kJ/l O2).20 Simultaneously, HR was measured continuously via three-lead ECG (CR55 Portascope, Cardiac Recorders Ltd, City of Westminster, London, UK) and interfaced with data from the metabolic measurement cart.
EE reference standard
DLW technique (visits 4 and 5)
DLW is a reference standard technique to quantify EE (kJ) during free-living conditions of 7–14 days duration.21 Briefly, in the DLW technique, the rate of carbon dioxide production is estimated, by labelling water molecules (2H2O and H218O). The labelled water will distribute throughout the water pool and later, be eliminated from the body, in the urine. Knowing the rate of tracers ingested and eliminated, the total metabolic rate may be estimated from simplifying equations.19
Participants presented after a fast of at least 6 hours to maximize absorption of the tracer elements. A baseline urine specimen was collected and the DLW (10% enriched 18O and 99% D2O) was administered orally, with the dose based on body mass (1.35 g of DLW × body mass in kg).19 A second urine sample was collected 6 hours after the priming dose. Then, one specimen of urine was collected daily, over the next 2 weeks at approximately the same time of day as the first urine specimen. Participants collected the samples and stored them refrigerated at home in sterile plastic containers delivered by the researchers on the first day. All urine samples for the 14-day period were collected on the final day of each phase. Samples were analysed for 2H2O and H218O by isotope ratio by mass spectrometry as described in detail elsewhere.19 Total EE (kJ) over the two-week period was divided by 14 to estimate mean TDEE.
Calculation of sub-fraction EEs
The DLW technique quantifies only TDEE; therefore, to estimate PAEE we used the following approach: TDEE is the summation of BMR, TEF, and PAEE.22 TEF can be objectively measured via indirect calorimetry,23 or it can be considered as a constant 10% of TDEE.24 Therefore, PAEE was calculated using the formula:
where 0.9 is referred to as the TEF constant fraction of 10% of TDEE. TDEE was quantified by DLW and BMR by indirect calorimetry described earlier.
EE predictive methods
HR monitoring (FLEX-HR method) (visits 4 and 5)
The FLEX-HR method assesses EE from HR using individual calibration from linear regression of the HR and oxygen uptake (VO2).11 To calculate the FLEX-HR and to check the linearity of these two variables for each individual, HR and VO2 were measured in participants under the following conditions over two laboratory visits (Fig. 1):
(i) while lying down during a 30-min period of quiet rest;
(ii) while seated in their wheelchairs;
(iii) during an incremental speed and constant grade sub-maximal treadmill test (described below);
(iv) during a constant speed and incremental grade sub-maximal treadmill test (described below).
Incremental speed and constant grade sub-maximal treadmill test (visit 2)
This test involved the participant pushing their own wheelchair on a treadmill at six different velocities. Each stage lasted 5 minutes, at a constant grade (0.5%) and at increments of velocity by 1 km/h. The initial velocity was 1 km/h, and this progressed to 3.5 km/h in the final stage. A 3-minute recovery period was permitted between each stage. Expired respiratory gases and ventilation were collected and analysed using breath-by-breath indirect calorimetry.
Constant speed and incremental grade sub-maximal treadmill test (visit 3)
This test was of a similar format to that described above, but was at a constant speed of 2 km/h for all stages, starting at 0.5% gradient and increasing by 0.5% slope during each subsequent stage to final 3.0% grade.
After performing all resting and treadmill exercise tests, EE was predicted from HR by
Individual HR and VO2 linearity: The multiple resting and exercise bouts were used to verify the linearity of HR and VO2 for each participant. Linearity was calculated by deriving the calibration curves from linear regression of the lying, sitting, and 12 treadmill wheelchair exercise stages (i.e. 6 stages from the velocity-variable treadmill test and 6 stages from the gradient-variable treadmill test). The average of the last 3 minutes of steady-state rest and exercise stages was used to establish the linear regression between HR and VO2.
Calculating FLEX-HR: The multiple rest and exercise levels were used to calculate the FLEX-HR, which is determined as the mean of the highest HR at rest and the lowest during exercise plus 10 b/minute.16 In the present study, to calculate the individual FLEX-HR, we used the highest HR during lying and sitting, and the lowest HR from all treadmill stages and during steady-state exercise.
- Estimation of EE from FLEX-HR: Finally, in order to calculate EE, HR was recorded on a beat-by-beat basis during the waking hours at least 12-hour duration. Participants were instructed to use the HR monitor (Polar RS800CX, Polar Electro, Finland) over 2 days (12th and 13th days of the DLW period, Fig. 1), including a ‘more physically active day’ and a ‘less active day’, in order to assess the differences in PA patterns. During this observation period, participants also recorded their sleeping time. Minute-by-minute HR data were downloaded to a computer, and individual HR data points below and above FLEX-HR were identified, distinguishing the time spent in ‘sedentary’ versus ‘physically active’ phases. Participants were oriented to report their daily activities while wearing the SWA and HR monitor, and based on their report, the day was classified as more or less active. We subtracted the reported sleeping time and the time spent while the HR was above the FLEX-HR from 1440 minutes (24 hours) and assumed this difference as the HR below the FLEX-HR (sedentary phase). To estimate EE from HR, each individual's HR/VO2 slope and intercept regression equation was determined and thus, for every measured HR, there was a correspondent estimated VO2 value. Subsequently, EE from VO2 was derived based on the formulae by Weir (20.5 kJ/l O2).20 Finally, TDEE (kJ) for each day was individually calculated from HR as
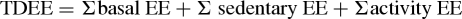
To calculate ‘basal EE’, individual reported sleeping time in minutes was converted to EE measured during BMR (visit 1). ‘Sedentary EE’ was the time spent when HR <FLEX-HR, and ‘activity EE’ was the time spent while HR was ≥FLEX-HR. Calculation for ‘activity EE’ was performed separately for each minute during the time that the participant wore the HR monitor.16
Multi-sensor armband (SWA method) (visits 4 and 5)
The SWA (SenseWear Software v7.0 BodyMedia®, Pittsburgh, PA, USA) incorporates a variety of bio-sensors (bi-axial accelerometer, galvanic skin resistance, heat flux, skin, and near-body temperature) and demographic characteristics (gender, age, height, weight) into proprietary algorithms to estimate EE.25 Participants were instructed to wear the SWA over 2 days (Fig. 1), including a ‘more physically active day’ and a ‘less active day’, in order to assess the differences in PA patterns. SWA was worn on the right arm over the triceps muscle during the waking hours of at least 12-hour duration. The SWA assessed EE in kcal and this was converted into SI units (kJ).
Estimation of EE from SWA
The SWA-estimated TDEE was input into a computerized software algorithm to express the PAEE using the METs system. The software was set up, by the authors, to identify the time spent in any physical energy cost at ≥3 METs.
Recall questionnaires method (visit 5)
Physical Activity Recall Assessment for People with Spinal Cord Injury
On the final visit, the PARA-SCI was administered to record lifestyle and leisure-time PA over the previous 3 days. The PARA-SCI is an interviewer-administered recall questionnaire tailored exclusively to people with SCI who use a wheelchair as their primary mode of locomotion.8 The PARA-SCI quantifies the duration of physical activities at three different intensities (mild, moderate, and heavy) and in two different categories: (i) leisure-time PA (i.e. activities done during free time), and (ii) lifestyle activities (i.e. activities done as part of daily routine such as household chores and work-related activities). The data are reported as minutes of total accumulated PA (min/day).8,26
Estimation of EE from PARA-SCI
To estimate PAEE, we summed mild, moderate, and vigorous of daily activities, assuming mild activity as 1.5 METs, moderate as 4.0 METs, and vigorous activities as 6.0 METs.17 Finally, we calculated the TDEE as:
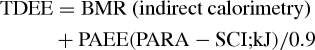 |
The Physical Activity Scale for Individuals with Physical Disabilities
The PASIPD is a 13-question instrument which requests information about the following 3 subscales: (i) leisure, (ii) household, and (iii) occupational PA over the preceding 7 days. Each scale item assesses the number of days and average hours per day of PA participation at different intensities. The scoring of the scale reflects a composite PASIPD score computed by multiplying the average hours per day by a MET value. The MET value are based on activity intensity, and eventually expressed the PA patterns as MET-h/day.12 This questionnaire was administered by an interviewer on the final visit.
Estimation of EE from PASIPD
To convert MET-h/day to PAEE we estimated the metabolic equivalent per hour for each person, on a daily basis, utilising an adapted method according to Besson et al.27 TDEE was calculated as:
Statistical analysis
Data are presented as mean ± SD. All analyses were performed using SPSS 19.0 and statistical differences were reported as exact probabilities. One-way ANOVA followed by post hoc Tukey tests were performed to determine whether there were differences in TDEE and PAEE between different methods to estimate EE. Coefficients of determination (R2) were used to determine the degree of association between DLW, FLEX-HR, SWA, PARA-SCI, and PASIPD estimates of EE. Finally, agreement between each method and DLW was assessed using the Bland–Altman analyses.
Results
Participant characteristics
The demographic and anthropometric characteristics of participants are presented in Table 1. Nine participants had complete spinal cord lesions and five possessed incomplete lesions. Level of lesion was distributed between paraplegia (71%) and tetraplegia (29%). The average age of participants was 40 ± 13 years, and they had a SCI for a mean of 10 ± 8 years. Average BMI was 25 ± 3 kg m2 suggesting that the participants were slightly overweight, with respect to able-bodied norms, and the mean fat mass estimated from stable-isotope dilution was 33 ± 9%, indicating obesity.
Table 1.
Demographic and anthropometric of individuals
| Subject | Age (years) | Gender | Stature (m) | Body mass (kg) | Injury level (ASIA scale) | TSI (years) | BMI (mass/stature2) | Body fat (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 43 | M | 1.66 | 52 | T5-A | 16 | 19 | 19 |
| 2 | 56 | M | 1.76 | 69 | T8-A | 16 | 22 | 32 |
| 3 | 40 | M | 1.82 | 96 | C7-A | 12 | 29 | 39 |
| 4 | 47 | M | 1.85 | 87 | T12-A | 5 | 25 | 39 |
| 5 | 39 | M | 1.88 | 97 | T12-A | 35 | 27 | 41 |
| 6 | 34 | F | 1.50 | 60 | T5-A | 5 | 27 | 45 |
| 7 | 65 | M | 1.71 | 82 | T11-A | 7 | 28 | 39 |
| 8 | 40 | M | 1.68 | 66 | C6-A | 11 | 23 | 43 |
| 9 | 30 | M | 1.80 | 72 | C4-C | 9 | 22 | 23 |
| 10 | 24 | M | 1.50 | 67 | T4-B | 4 | 30 | 25 |
| 11 | 60 | M | 1.83 | 83 | T10-C | 11 | 25 | 31 |
| 12 | 27 | M | 1.86 | 96 | T6-A | 4 | 28 | 33 |
| 13 | 30 | M | 1.95 | 100 | C4-C | 4 | 26 | 30 |
| 14 | 23 | M | 1.88 | 77 | T8-C | 5 | 22 | 18 |
| Mean ± SD | 40 ± 13 | 1.76 ± 0.14 | 79 ± 15 | 10 ± 8 | 25 ± 3 | 33 ± 9 |
TSI, time since injury; BMI, body mass index.
Averages are presented as mean ± standard deviation (Mean ± SD).
EEs estimated from reference and predictive methods
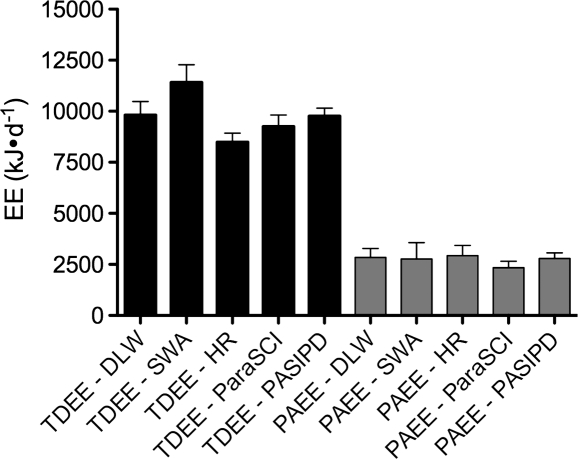
The mean TDEE measured by DLW, FLEX-HR, and SWA was 9817 ± 2491 kJ/day, 8498 ± 1516 kJ/day, and 11414 ± 3242 kJ/day, respectively. After being converted to kJ, the mean TDEE for PARA-SCI was 9259 ± 2094 kJ/day, and was 9766 ± 1462 kJ/day for PASIPD (Fig. 2). BMR measured by indirect calorimetry was 5995 ± 955 kJ/day. Although DLW-derived TDEE was overestimated by all predictive methods (except for FLEX-HR), only SWA demonstrated significant difference compared to DLW (about 16%, P = 0.02). For technical reasons, two individuals did not complete the FLEX-HR analysis.
Figure 2.
TDEE and PAEE (kJ/day ± SD) of reference-standard method and predictive techniques. TDEE = total daily energy expenditure; PAEE = physical Activity-associated energy expenditure; DLW = doubly labelled water; SWA = SenseWear Armband; FLEX-HR = Flex heart rate monitoring; PARA-SCI = Physical Activity Recall Assessment for People with Spinal Cord Injury; PASIPD = Physical Activity Scale for Individuals with Physical Disabilities.
The mean reference method-derived PAEE was 2841 ± 1626 kJ/day, but estimated to be 2935 ± 1732 kJ/day by FLEX-HR, and 2773 ± 2966 kJ/day by SWA (Fig. 2). PA scores estimated by PARA-SCI and PASIPD were 176 ± 58 min/day and 28 ± 10 MET-h/day, respectively. After these were converted to PAEE, the mean value for PARA-SCI was 2339 ± 1171 kJ/day, and PASIPD was 2749 ± 1026 kJ/day (Fig. 2). There were no significant differences in estimated PAEE between the reference standard and any of four predictive methodologies. (Note: 4.18 kJ = 1 kcal.)
Comparisons to reference standard method (DLW)
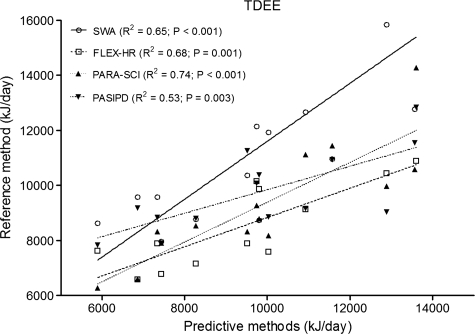
Fig. 3 portrays comparisons of TDEE and all other predictive methods. TDEE, quantified by DLW, was associated with FLEX-HR (R2 = 0.68, P = 0.001), SWA (R2 = 0.65, P < 0.001), PARA-SCI (R2 = 0.74, P < 0.001) and PASIPD (R2 = 0.73, P = 0.003) (Fig. 3).
Figure 3.
Comparison of TDEE between DLW (reference-standard method) and SWA, FLEX-HR, PARA-SCI, and PASIPD (predictive methods). TDEE = total daily energy expenditure; DLW = doubly labelled water; SWA = SenseWear Armband; FLEX-HR = Flex heart rate monitoring; PARA-SCI = Physical Activity Recall Assessment for People with Spinal Cord Injury; PASIPD = Physical Activity Scale for Individuals with Physical Disabilities. Shown in the legend are the coefficients of determination (R2) between DLW-derived TDEE and predicted TDEE.
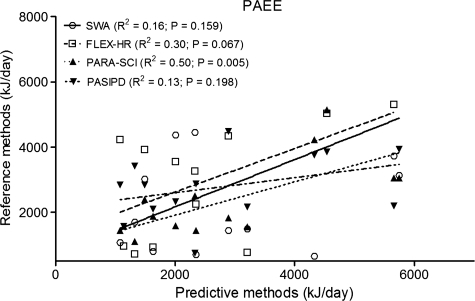
DLW PAEE (in kJ) was significantly associated only with PARA-SCI (R2 = 0.50, P = 0.005), but not with the other predictive methods (R2 ranged between 0.13 to 0.30, P > 0.05; Fig. 4). In addition, when the coefficient of determination was calculated between the DLW reference method and PARA-SCI PA index in min/day (R2 = 0.62, P = 0.001) or PASIPD PA index in MET-h/day (R2 = 0.13, P = 0.062), results were similar to PAEE in kJ.
Figure 4.
Comparison of PAEE between DLW (reference-standard method) and SWA, FLEX-HR, PARA-SCI, and PASIPD (predictive methods). PAEE = physical activity-associated energy expenditure; DLW = doubly labelled water; SWA = SenseWear Armband; FLEX-HR = Flex heart rate monitoring; PARA-SCI = Physical Activity Recall Assessment for People with Spinal Cord Injury; PASIPD = Physical Activity Scale for Individuals with Physical Disabilities. Shown in the legend are the coefficients of determination (R2) between DLW-derived PAEE and predicted PAEE.
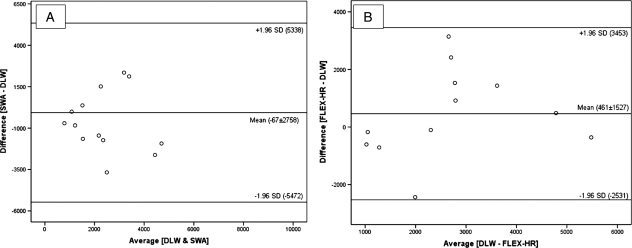
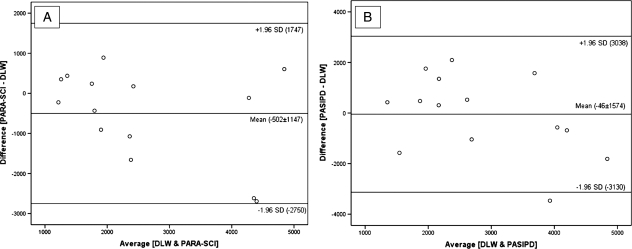
The degree of concordance between PAEE, derived from the difference between predictive methods (SWA, FLEX-HR, PARA-SCI, PASIPD) and the reference technique (DLW) has been graphically portrayed using Bland–Altman plots (Figs 5 and 6). For TDEE, the mean bias ± SD was 1597 ± 1915 kJ/day between DLW and SWA, −860 ± 1397 kJ/day between DLW and FLEX-HR, −557 ± 1275 kJ/day between DLW and PARA-SCI and −51 ± 1748 kJ/day for DLW and PASIPD. All data points (i.e. 100%) fell within the ±1.96SD boundaries for two of the predictive methods (FLEX-HR and PARA-SCI) and 93% of the data points fell with the ±1.96SD boundaries for SWA and PASIPD. For PAEE, the mean bias ± SD was −67 ± 2758 kJ/day between DLW and SWA, 461 ± 1527 kJ/day for DLW and FLEX-HR, −502 ± 1147 kJ/day between DLW and PARA-SCI and −46 ± 1574 kJ/day for DLW and PASIPD. All data points (100%) fell within the ±1.96SD boundaries for three of the predictive methods (FLEX-HR, SWA, and PARA-SCI) and 93% of the data points fell with the ±1.96SD boundaries for PASIPD.
Figure 5.
Bland and Altman plot of PAEE (kJ/day) using DLW and SWA (A) and of PAEE using DLW and FLEX-HR (B). PAEE = physical activity-associated energy expenditure; DLW = doubly labelled water; SWA = SenseWear Armband; FLEX-HR = Flex heart rate monitoring.
Figure 6.
Bland and Altman plot of PAEE (kJ/day) using DLW + indirect calorimetry and PARA-SCI (A) and of PAEE using DLW + indirect calorimetry and FLEX-HR (B). PAEE = Physical activity-associated energy expenditure; DLW = doubly labelled water; PARA-SCI = Physical Activity Recall Assessment for People with Spinal Cord Injury; PASIPD = Physical Activity Scale for Individuals with Physical Disabilities.
Discussion
DLW and indirect calorimetry
This study sought to contrast different indirect and predictive methodologies to estimate TDEE and PAEE against a commonly accepted ‘reference’ standard, the DLW technique, in a SCI population of wheelchair users. The DLW technique is both simple and non-invasive and has been validated in animal and human studies, providing accurate measurements of free-living EEs.19 However, the DLW technique has some limitations, including: (i) it is somewhat expensive and large sample studies might therefore be unfeasible and, (ii) only TDEE can be directly quantified, and therefore a combination with other techniques, such as indirect calorimetry, is necessary for the estimation of sub-fraction EE components.
Since this is the first report on DLW-quantified TDEE in SCI individuals, a direct comparison of our findings with those from previous reports in wheelchair-dependent populations is not possible. In able-bodied individuals though, Goran et al.28 reported a mean TDEE of 11908 ± 2165 kJ/day, approximately 24% higher than our data. Goldberg et al.29 investigated 10 healthy women and their results were 8% higher than the TDEE measured in individuals with SCI. Such differences might be expected, since the negative physiological sequelae after SCI and life style adaptations to a wheelchair, together promote reduced lean muscle tissue and lower levels of PA with concomitant effects upon TDEE.4,30 A more sedentary lifestyle can be an important factor for reduction in daily EEs, as was demonstrated in a study of DLW-quantified TDEE in adults with cerebral palsy.31 The mean TDEE for the entire group was 9346 kJ/day, yet when subdivided into ambulatory versus non-ambulatory individuals, the latter demonstrated a significantly lower TDEE (10307 ± 2107 kJ/day versus 8385 ± 2408 kJ/day for ambulatory and non-ambulatory, respectively). TDEE has been previously quantified in people with SCI using a respiratory chamber,23 which is considered another ‘reference standard’ technique. Those authors' findings for TDEE (7824 ± 305 kJ/day) were about 20% lower than observed in the current study. This disparity can be explained by methodological differences, since EEs assessed while confined in a respiratory chamber may underestimate 'free-living' EE by up to 47%.32
BMR measured by indirect calorimetry has been previously investigated in two SCI cohorts, and our findings closely replicate those prior investigations. For example, Buchholz et al.4 reported in 17 men with SCI, a BMR of 6499 kJ/day. BMR reported for 20 individuals with SCI by Yilmaz et al.33 was 6082 kJ/day. Both of these earlier reports are fairly close to the current study's findings of 5995 ± 955 kJ/day.
DLW and predictive methods of EE estimation
SWA method
The SWA overestimated TDEE by 16% compared to DLW-quantified TDEE (9817 ± 2491 and 11414 ± 3451 kJ/day, for DLW and SWA, respectively; P < 0.02). Conversely, SWA underestimated PAEE derived from the reference standard technique by 3%, although this difference was not statistically significant. Bland–Altman plots demonstrated that 93–100% of points for TDEE and PAEE lay between the ±1.96SD lines. Regression revealed a moderate association between SWA and DLW for TDEE (R2 = 0.65; P < 0.000), but a poor association between SWA and the reference method for PAEE (R2 = 0.16, P = 0.159) (Figs 3 and 4, respectively).
In this study, participants wore the SWA for at least 12 hours over 2 days; each with different characteristic self-reported PA routines. There was no difference between ‘less active’ and ‘more active’ days for TDEE (11328 ± 3145 versus 11499 ± 3449 kJ/day, respectively; P = 0.905) or PAEE (2716 ± 2941 versus 2838 ± 2995 kJ/day, respectively; P = 0.988). This suggested that TDEE and PAEE measurements for only 2 days within a 2-week survey period offered reasonably accurate information about the weekly EE of SCI individuals. A similar approach was used with DLW over 14 days and HR monitoring for 3 days in obese women with similar outcomes.17
To the authors' knowledge, the current study was the first to contrast SWA- and DLW-derived estimates of TDEE/PAEE in community-dwelling SCI. Although successfully deployed within other clinical populations, there is little previous information regarding the validity of SWA for EE assessment in wheelchair users. A conference report,34 wherein the authors compared SWA to indirect calorimetry in six people with SCI, described a good intraclass correlation between indirect calorimetry and SWA (0.79, P < 0.001). However, SWA overestimated EE at rest by 6%, during deskwork by 13%, during wheelchair propulsion at three different intensities by 88–138%, and during arm-cranking exercises in three different intensities by 27–55%. The preliminary findings of this conference abstract corroborate our data, revealing an overestimation of TDEE by SWA, but moderate to good measures of association between SWA and DLW.
In contrast, previous studies using SWA have demonstrated a good accuracy for assessing the EE of children during their typical activities of daily living,15 and a reasonably accurate estimate of EE in young people with cystic fibrosis and their healthy age-matched cohorts.25 Therefore, SWA would seem to be a promising approach for EE assessment after SCI in wheelchair users, as it is portable, comfortable, and metabolism can be easily assessed during daily activities under free-living environmental conditions. However, due to the significant overestimation of TDEE and poor association between DLW and SWA for PAEE, it is clear that specific algorithms for individuals who are wheelchair-ambulatory must be developed in order to improve the validity of using a SWA for PA promotion in this population.
FLEX-HR method
The FLEX-HR method uses the HR and VO2 linearity for a given individual to estimate their PAEE. Racette et al.17 compared the TDEE in obese women, quantified by DLW and estimated by HR and they observed only 1% difference between techniques. Livingstone et al.18 conducted a study in 14 community-dwelling adults, and the authors reported a negligible (less than 1%) difference between DLW-derived and HR-estimated TDEE. The current study employed a similar version of those protocols since they had demonstrated no significant differences between methods using DLW over 14 days and HR monitoring for only 3 days.
A strong HR-VO2 linearity has also been observed in wheelchair users with SCI.35,36 However, in a study conducted upon individuals with tetraplegia,37 the relationship between HR-VO2 was linear in only eight out of 18 SCI people. The non-linearity of HR-VO2 after tetraplegia is commonly attributed to their sympathetic dysfunction, resulting from spinal cord damage above the fourth thoracic neurological segment, evoking a deprivation of supraspinal sympathoadrenal control and a blunted HR response to cardioacceleration. Therefore, within the SCI population, two different patterns of HR response have been observed differing by injury level – a 'normal' HR response to increased exercise intensity in paraplegia and a blunted cardioacceleration in tetraplegia. Therefore, HR monitoring alone might either overestimate or underestimate the prediction of EE in sub-populations of SCI.
Evidence for this limitation to the use of HR monitoring has been provided by Mollinger et al.,38 who estimated TDEE in individuals with SCI, subdivided into four groups, according to their neurological injury level. The authors reported significant differences in TDEE estimated by the HR monitoring technique among groups. The higher lesion-SCI level group revealed the lowest values for TDEE (high tetraplegic group = 5567 kJ/day, low tetraplegic group = 8811 kJ/day, high paraplegia group = 10913 kJ/day, and low paraplegia group = 11 256 kJ/day).
Although these findings indicated an inverse relationship between neurological injury level and TDEE in persons with SCI, the HR monitoring technique was not compared to any reference standard, and therefore it was not possible to verify whether the FLEX-HR method over- or under-estimated TDEE between groups. So HR monitoring to predict EE may yield a certain inaccuracy if there is a blunted or an accelerated exercise-induced tachycardia that is not associated with EE variations. Alternatively, it might be reasonable to assume that higher lesion levels are associated with reduced PA due to decreased muscle mass under voluntary control.
Although not statistically significant, our findings revealed an overestimation by 13% of TDEE and by 3% of PAEE derived from FLEX-HR compared to DLW. The phenomenon observed in the previously cited research could explain our findings, as only four of 14 individuals in the current study met the criteria for blunted cardioacceleration to exercise. The association between DLW and FLEX-HR for TDEE (R2 = 0.68, P = 0.001) (Fig. 3) was better than that for PAEE (R2 = 0.30, P = 0.062) (Fig. 4), lending credence to the view that the FLEX-HR method of EE estimation during exercise or physical activities might be compromised by high-lesion neurological impairment after SCI.
Clearly, our data commend an individual analysis of the relationship between HR and VO2 when estimating EE during PA in wheelchair users, in part because of the atypical sympathetic response to exercise related to the injury level, in some people with upper motor neuron lesion SCI.
PA questionnaires method
Although preliminary evidence has validated the PARA-SCI as a measure of PA,8,26 this is the first study that directly compared the PARA-SCI to DLW estimates of PAEE. PARA-SCI revealed an acceptable association with the reference method for PAEE in SCI (R2 = 0.50, P = 0.005) (Fig. 4), and it was even somewhat better in its PA index score (R2 = 0.62, P = 0.001). Indeed, PAEE measured by PARA-SCI showed a better association with DLW than did FLEX-HR, SWA, or PASIPD. After the conversion of its PA activity index of min/day to kJ, statistical analysis revealed no significant differences between DLW and PARA-SCI, for TDEE (9817 ± 2491 kJ/day versus 9259 ± 2094 kJ/day for DLW and PARA-SCI, respectively) or for PAEE (2841 ± 1626 kJ/day and 2339 ± 1171 kJ/day for DLW and PARA-SCI). The Bland–Altman plots portrayed a good degree of concordance (‘goodness of concordance’ was defined according to the numbers of data points that lay between +1.96SD and −1.96SD of the mean difference between DLW and each predictive technique), and the least inter-individual variability in kJ of EE compared to all the other predictive methods (−558 ± 1275 kJ for TDEE and −501 ± 1147 kJ PAEE, respectively). Fig. 6 portrays the bias and standard deviations between DLW and PARA-SCI of PAEE. The findings of the current study clearly demonstrated that PARA-SCI was able to quantify the EE of daily PA patterns performed by wheelchair users when compared to 14-day DLW.
PASIPD non-significantly overestimated TDEE by 1% and underestimated PAEE by 3% in the current study. Despite its poor relationship with DLW for PAEE (R2 = 0.13, P = 0.062; Fig. 4), PASIPD revealed a barely acceptable association with DLW for TDEE (R2 = 0.53, P = 0.003; Fig. 3). However, a likely explanation for this finding was that in order to calculate TDEE from the original PASIPD PA index (in MET-h/day), measured BMR from indirect calorimetry was the other sub-fraction employed. Whereas BMR was the same sub-fraction used to calculate PAEE from DLW and, more importantly, BMR is quantitatively the ∼75% of TDEE, we therefore assume that TDEE from PASIPD is just a surrogate for BMR – limiting its utility for this population.
Conclusion
In this study, we compared EE determined by a combination of DLW and indirect calorimetry against some of other common predictive methods for PA and EE assessment. Our results revealed that SWA significantly overestimated TDEE by 16%, and the other techniques also overestimated TDEE by 13% (FLEX-HR), 6% (PARA-SCI), and 1% (PASIPD) when compared to DLW in our SCI subjects. FLEX-HR also overestimated PAEE by 3%, but this was not significantly different. Conversely, SWA, PARA-SCI, and PASIPD non-significantly underestimated PAEE (by 3, 18, and 3%, respectively) when compared to DLW-based estimates of daily PAEE. We conclude that, based on our results of this preliminary study, PARA-SCI was the best method for predicting PAEE, in wheelchair users of SCI aetiology.
Limitations of this study
The sample size of this study was limited, although with 14 SCI wheelchair users it represents one of the larger ones recently published in clinical populations. Recruitment of individuals with SCI is a well-known challenge for clinical researchers. Yet the results of this study are still highly relevant, since they lend credence to the PARA-SCI as a useful tool for PA and PAEE assessment, and they have revealed future directions for understanding TDEE and PAEE in the context of body adiposity accumulation for wheelchair users with SCI. A second limitation was to the experimental design. In the current study, for technical and practical reasons, we opted to use the SWA and FLEX-HR monitoring for 2 days comprising ‘more active’ and ‘less active’ self-reported sampling periods, in contrast to a longer period embracing the 14-day DLW assessment. However, in two other studies,17,18 a similar protocol was employed and those authors observed no differences between methods based on a 3-day periodic assessment of HR monitoring. Nevertheless, this study provided new information concerning the feasibility and validity of different methods to assess EE in individuals with SCI who are wheelchair-confined. This study can also be considered as laying an important foundation for understanding the links between PA, EE, and exercise prescription to reduce body adiposity in people with SCI.
Acknowledgements
The authors are grateful to Dr Patricia Ruell and Ms Connie Wishart for their technical support. Ricardo A. Tanhoffer is a doctoral candidate and has been awarded the ‘University of Sydney International Research Scholarship’ (USIRS).
References
- 1.Levine JA. Measurement of energy expenditure. Public Health Nutr 2005;8(7A):1123–32 [DOI] [PubMed] [Google Scholar]
- 2.Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 2004;7(6):635–9 [DOI] [PubMed] [Google Scholar]
- 3.Sedlock DA, Laventure SJ. Body-composition and resting energy-expenditure in long-term spinal-cord injury. Paraplegia 1990;28(7):448–54 [DOI] [PubMed] [Google Scholar]
- 4.Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res 2003;11(4):563–70 [DOI] [PubMed] [Google Scholar]
- 5.Bauman WA. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77 [DOI] [PubMed] [Google Scholar]
- 6.Nash MS, Mendez AJ. Nonfasting lipemia and inflammation as cardiovascular disease risks after SCI. Top Spinal Cord Inj Rehabil 2009;14(3):15–31 [Google Scholar]
- 7.Buchholz AC, Ginis KAM, Bray SR, Craven BC, Hicks AL, Hayes KC, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab – Physiologie Appliquee Nutrition Et Metabolisme 2009;34(4):640–7 [DOI] [PubMed] [Google Scholar]
- 8.Ginis KAM, Latimer AE, Hicks AL, Craven BC. Development and evaluation of an activity measure for people with spinal cord injury. Med Sci Sports Exerc 2005;37(7):1099–111 [DOI] [PubMed] [Google Scholar]
- 9.Jeon JY, Steadward RD, Wheeler GD, Bell G, McCargar L, Harber V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab 2003;88(1):402–7 [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki M, Irizawa M, Komura T, Kikuchi K, Sasaki H, Kai K, et al. Daily energy expenditure in active and inactive persons with spinal cord injury. J Hum Ergology 1992;21(2):125–33 [PubMed] [Google Scholar]
- 11.Hayes AM, Cover L, Myers J, Kiratli BJ. Predicting energy expenditure using Flex-HR in individuals with spinal cord injury. Med Sci Sports Exerc 2003;355 Suppl:S299. [Google Scholar]
- 12.Washburn RA, Zhu WM, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: Development and evaluation. Arch Phys Med Rehabil 2002;83(2):193–200 [DOI] [PubMed] [Google Scholar]
- 13.Backlund C, Sundelin G, Larsson C. Validity of armband measur- ing energy expenditure in overweight and obese children. Med Sci Sports Exerc 2010;42(6):1154–61 [DOI] [PubMed] [Google Scholar]
- 14.Cereda E, Turrini M, Ciapanna D, Marbello L, Pietrobelli A, Corradi E. Assessing energy expenditure in cancer patients: A pilot validation of a new wearable device. J Parenter Enteral Nutr 2007;31:502–7 [DOI] [PubMed] [Google Scholar]
- 15.Calabro MA, Welk GJ, Eisenmann JC. Validation of the SenseWear Pro Armband algorithms in children. Med Sci Sports Exerc 2009;41(9):1714–20 [DOI] [PubMed] [Google Scholar]
- 16.Ceesay SM, Prentice AM, Day KC, Murgatroyd PR, Goldberg GR, Scott W, et al. The use of heart-rate monitoring in the estimation of energy-expenditure – a validation-study using indirect whole-body calorimetry. Br J Nutr 1989;61(2):175–86 [DOI] [PubMed] [Google Scholar]
- 17.Racette SB, Schoeller DA, Kushner RF. Comparison of heart-rate and physical-activity recall with doubly labeled water in obese women. Med Sci Sports Exerc 1995;27(1):126–33 [PubMed] [Google Scholar]
- 18.Livingstone MBE, Prentice AM, Coward WA, Ceesay SM, Strain JJ, McKenna PG, et al. Simultaneous measurement of free-living energy-expenditure by the doubly labeled water and heart-rate monitoring. Am J Clin Nutr 1990;52(1):59–65 [DOI] [PubMed] [Google Scholar]
- 19.Bluck LD, Forsum E, Hills A, Kurpad A, Mokhtar N, Preston T, et al. (editor), Assessment of body composition and total energy expenditure in humans using stable isotope technique. Agency I-IAE Vienna: International Atomic Energy Agency; 2009 [Google Scholar]
- 20.Weir JBdV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoeller DA, van Santen E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol 1982;53(4):955–9 [DOI] [PubMed] [Google Scholar]
- 22.Tappy L, Binnert C, Schneiter P, Schneiter P. Energy expenditure, physical activity and body-weight control. Proc Nutr Soc 2003;62(3):663–6 [DOI] [PubMed] [Google Scholar]
- 23.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 1998;68(6):1223–7 [DOI] [PubMed] [Google Scholar]
- 24.Westerterp KR, Plasqui G. Physical activity and human energy expenditure. Curr Opin Clin Nutr Metab Care 2004;7(6):607–13 [DOI] [PubMed] [Google Scholar]
- 25.Dwyer TJ, Alison JA, McKeough ZJ, Elkins MR, Bye PTP. Evaluation of the SenseWear activity monitor during exercise in cystic fibrosis and in health. Respir Med 2009;103(10):1511–7 [DOI] [PubMed] [Google Scholar]
- 26.Latimer AE, Ginis KAM, Craven BC, Hicks AL. The physical activity recall assessment for people with spinal cord injury: Validity. Med Sci Sports Exerc 2006;38(2):208–16 [DOI] [PubMed] [Google Scholar]
- 27.Besson H, Brage S, Jakes RW, Ekelund U, Wareham NJ. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am J Clin Nutr 2010;91(1):106–14 [DOI] [PubMed] [Google Scholar]
- 28.Goran MI, Beer WH, Wolfe RR, Poehlman ET, Young VR. Variation in total energy-expenditure in young healthy free-living men. Metab Clin Exp 1993;42(4):487–96 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Sawyer MB, et al. Longitudinal assessment of the components of energy-balance in well-nourished lactating women. Am J Clin Nutr 1991;54(5):788–98 [DOI] [PubMed] [Google Scholar]
- 30.Bauman WA, Spungen AM, Wang J, Pierson RN. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev 2004;41(1):1–8 [DOI] [PubMed] [Google Scholar]
- 31.Johnson RK, Hildreth HG, Contompasis SH, Goran MI. Total energy expenditure in adults with cerebral palsy as assessed by doubly labeled water. J Am Dietetic Assoc 1997;97(9):966–70 [DOI] [PubMed] [Google Scholar]
- 32.Westerterp KR, Kester ADM. Physical activity in confined conditions as an indicator of free-living physical activity. Obes Res 2003;11(7):865–8 [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz B, Yasar E, Goktepe S, Alaca R, Yazicioglu K, Dal U, et al. Basal metabolic rate and autonomic nervous system dysfunction in men with spinal cord injury. Obesity 2007;15(11):2683–7 [DOI] [PubMed] [Google Scholar]
- 34.Hiremath SV, Ding D. Evaluation of activity monitors to estimate energy expenditure in manual wheelchair users. J Spinal Cord Med 2011;34(1):110–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hjeltnes N. Oxygen-uptake and cardiac-output in graded arm exercise in paraplegics with low-level spinal lesions. Scand J Rehabil Med 1977;9(3):107–13 [PubMed] [Google Scholar]
- 36.Hayes AM, Myers JN, Ho M, Lee MY, Perkash I, Kiratli BJ. Heart rate as a predictor of energy expenditure in people with spinal cord injury. J Rehabil Res Dev 2005;42(5):617–23 [DOI] [PubMed] [Google Scholar]
- 37.Valent LJM, Dallmeijer AJ, Houdijk H, Slootman J, Janssen TWJ, Hollander AP, et al. The individual relationship between heart rate and oxygen uptake in people with a tetraplegia during exercise. Spinal Cord 2007;45(1):104–11 [DOI] [PubMed] [Google Scholar]
- 38.Mollinger LA, Spurr GB, Elghatit AZ, Barboriak JJ, Rooney CB, Davidoff DD, et al. Daily energy-expenditure and basal metabolic rates of patients with spinal-cord injury. Arch Phys Med Rehabil 1985;66(7):420–6 [PubMed] [Google Scholar]