Abstract
Context
Patients with spinal cord injury (SCI) have many factors that are associated with pressure ulcer formation, including paralysis, loss of sensation, poor nutrition, anemia, and skin maceration related to incontinence. Treatment of these ulcers involves relieving pressure, improving nutrition and skin hygiene, treating infections, removing necrotic tissues, and applying the appropriate dressings. However, some cases are not responsive to the above treatment. Electrical stimulation (ES) is thought to enhance soft tissue healing through promotion of protein synthesis, inhibition of bacterial growth, facilitation of epithelial tissue migration, improvement of blood flow, and tensile strength. This data is mainly based on evidence from animal studies and very few rigorously controlled studies conducted in humans.
Objective
To demonstrate the effectiveness of ES in the treatment of recalcitrant pressure ulcers.
Methods
Retrospective case series describing the care of adults with SCI and recalcitrant pressure ulcers. ES was applied directly into the wound bed: 60 minutes per session, 3–5 times per week; with an intensity of 100 milliamperes and a frequency of 100 pulses per second. Polarity was negative initially and was switched weekly. The amplitude and wave form were maintained throughout.
Results
The long-standing (11–14 months) pressure ulcers were completely healed after 7 to 22 weeks of treatment with high-voltage ES.
Conclusion/clinical relevance
This case series demonstrates the effectiveness of ES for enhanced healing of Stage III–IV ulcers otherwise unresponsive to standard wound care. Further study is needed to identify the most effective protocol for ES therapy in the treatment of recalcitrant pressure ulcers.
Keywords: Electrical stimulation, Spinal cord injuries, Pressure ulcer, Wound healing
Introduction
Pressure ulcers are a significant health care problem in all patient care settings. They are a lifelong, serious complication of spinal cord injury (SCI) that has the potential to interfere with physical, psychological and social well-being, and overall quality of life. According to the Institute of Medicine, SCI research should prioritize the elimination of SCI-related complications, such as pressure ulcers.1 Pressure ulcers are wounds caused by unrelieved pressure from forces perpendicular (compression) or tangential (shear) to the tissue surface.1 This constant force can interfere with the pressure in capillaries and therefore, affect the exchange and elimination of nutrients and metabolites. Prolonged circulatory interference ultimately leads to cell death and in severe cases individuals can develop septic shock and ultimately organ failure.1
Pressure ulcers rank as the second highest cause of re-hospitalization after traumatic SCI.2 It is estimated that approximately 25–40% of patients with SCI will develop a pressure ulcer in their lifetime and that at 2-year follow-up the prevalence of pressure ulcers is 8.9%.1,3
Patients with SCI may have many factors that are associated with pressure ulcer formation, including paralysis, loss of sensation to pain and pressure, poor nutrition, anemia, and skin maceration related to incontinence. Healing time for pressure ulcers can vary greatly.
As Gardner et al.4 point out, there are several moderating variables that impact healing, including tissue perfusion, bacterial burden, and nutritional status, but few studies have provided data regarding these variables. Poor nutritional status has been correlated with the healing of pressure ulcers.5,6 Biochemical parameters useful in assessing nutritional status include prealbumin, total protein, albumin, hemoglobin, and hematocrit. According to the Consortium for Spinal Medicine, specific nutritional factors associated with wound healing include micronutrients such as zinc, vitamin C, vitamin A, and vitamin E.7
Treatment of these ulcers involves relieving pressure, improving nutrition and skin hygiene, treating infections, removing necrotic tissues, and applying the appropriate dressings. However, some cases are not responsive to the above treatment.
Electrical stimulation (ES) is thought to enhance soft tissue healing, but this is based chiefly on evidence from animal studies and very few rigorously controlled studies conducted in humans.
While the mechanisms that explain how ES promotes wound healing are poorly understood, some believe that ES imitates the natural electrical current that occurs in skin when it is injured.4 It has been shown that ES induces cellular actions in almost every phase of the wound healing cascade, including the stimulation of several fibroblast activities, such as enhanced collagen and deoxyribonucleic acid synthesis, adenosine triphosphate production and calcium influx, and an increased number of growth factor receptor sites.8 In vitro studies on macrophages, epithelial cells, and fibroblasts have demonstrated that ES promotes the migration and activation of key cells within the wound site. Additionally, in vivo studies involving animal models have shown that ES results in more collagen deposition, enhanced angiogenesis, greater wound tensile strength, and a faster wound contraction rate.8 ES has also been shown to improve tissue perfusion and reduce edema formation, indirectly stimulating healing by improving oxygen delivery to the tissue.9
There have been relatively few controlled studies conducted regarding the use of ES specifically for the healing of recalcitrant pressure ulcers. In Regan et al.'s9 review of therapeutic interventions for pressure ulcers, the ES studies focus on comparing the rate of healing with ES to either sham/placebo groups or to standard wound care groups. There is no discussion on the use of ES for recalcitrant wounds that have failed to respond to standard wound care and have persisted for longer than 11 months. Houghton et al.8 provide a recent single-blind randomly assigned study that compares healing rates for standard wound care versus ES plus standard wound care for pressure ulcers, but their study includes Stage II and non-recalcitrant wounds. In addition, their standard wound care group had a disproportionate amount of Stage II versus Stage III and IV wounds when compared to the Standard Wound Care (SWC) + Electrical Stimulation Treatment (EST) group. When you consider that all Stage II wounds were fully healed within 3 months for both groups, the comparison between both groups becomes skewed.
Case series
We report a retrospective case series describing the care of three adults with SCI and recalcitrant pressure ulcers.
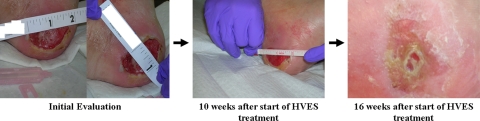
Patient A is a 49-year-old man with T4 AIS B paraplegia following a fall. He presented for outpatient physical therapy with a 14-month-old pressure ulcer that persisted despite conventional wound care management. He was noted to have a Stage IV pressure ulcer on his left heel measuring at 4.45 × 2.54 cm with moderate serosanguineous yellowish, non-foul smelling discharge, and a well-defined flat margin (Fig. 1). No tunneling was noted and the calculated wound surface area (WSA) was 11.30 cm2.
Figure 1.
Patient A's recalcitrant pressure ulcer at initial evaluation and the progression of wound healing after application of high voltage electrical stimulation (HVES). Source: Kennedy Krieger Institute.
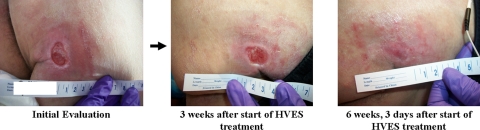
Case B is a 29-year-old man with T12 paraplegia AIS A due to a gun-shot wound. He presented with an 11-month-old Stage IV left ischial wound, despite conventional wound care, which measured 4 × 4 × 1 cm (16 cm2 WSA) (Fig. 2). The wound included no necrotic tissue, minimal non-foul smelling serosanguineous discharge, and no tunneling. The margin was flat and well defined with an area of maceration and scant granulation.
Figure 2.
Patient B's recalcitrant pressure ulcer at initial evaluation and the progression of wound healing after application of HVES. Source: Kennedy Krieger Institute.
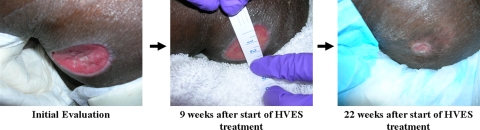
Patient C is a 51-year-old man with a C7 AIS A following a bicycle accident. He presented with an 11-month-old Stage III pressure ulcer over the right ischial tuberosity. Standard wound care management at a wound care clinic had yielded no significant improvement. The ulcer measured 3.6 × 2.0 cm (7.20 cm2 WSA), had minimal non-foul smelling serosanguineous drainage, no necrotic tissue, no induration, no tunneling, well-defined flat margins, and scant granulation.
All three patients were checked for specific biochemical parameters associated with the development and prolonged healing of pressure ulcers. Anemia, as assessed by hemoglobin levels below 12.0–14.0 g/dl, is associated with an increased incidence of pressure ulcers and serum total protein levels less than 6.4 g/dl have been associated with the development of pressure ulcers.10–12 Serum albumin levels lower than 3.5 g/dl have a higher incidence of pressure ulcers and low testosterone levels are associated with impaired healing of long-standing pressure ulcers.12–14
The patients' blood work tests included hemoglobin, total protein, albumin, zinc, total testosterone, and free testosterone. Their results were all within normal limits.
ES wound treatment protocol
The patients' wound care protocol included high-voltage electrical stimulation (HVES) treatments to the wound bed for 60-minute sessions 3–5 times per week. The following guidelines were used for initial wound healing: the wound cavity was filled with Hydrogel, a 2-inch round electrode was placed into the wound bed, and a dispersive electrode was placed over an area proximal to the wound (avoiding bony prominences). High voltage pre-programmed settings were used (twin peaked, monophasic, 10 microsecond pulse width) on the Empi 300 PV unit to administer the ES to the wound. These parameters were chosen based on information available in the users' manual for this device.15
Negative and positive polarities were alternated once weekly.8,16,17 As past literature has shown that different cell types are attracted to either a positive or negative polarity, alternating polarities produce improved results in both chemotaxis and wound closure times.17–29 Preset parameters included a 2 second ramp-up time, continuous on cycle; pulse rate of 100 Hz, and user chosen negative pre-programmed regimens (PPR 1) or positive (PPR 2) polarity. Following the ES treatment, the wound was dressed with a sterile Telfa pad and sterile gauze and was then secured in place with Hypafix tape. Treatment was administered 3–5 times per week and re-assessment was performed on a weekly basis by a physical therapist and physician.
Post-initiation
Periodically, each patient's wound was measured and WSA and percent decrease in wound surface area (% WSA↓) was calculated.
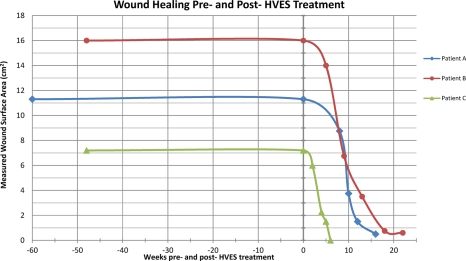
For Patient A, approximately 10 weeks after the initiation of HVES, there was significant reduction in drainage and WSA (62.82%) of the left heel decubitus ulcer (Graph 1, Table 1). There was granulation tissue within the wound bed and minimal drainage (Fig. 1). After 16 weeks, the WSA had decreased by 95.58% and ES to the wound bed was discontinued (Table 1). During this re-evaluation the wound demonstrated clean and dry edges, granulation, and epithelial tissues filled the wound bed without drainage. Wound care was continued and consisted of cleansing the wound with a saline solution and dressing it with a Telfa padding secured with Hypafix (Fig. 1). During a 12-month follow-up over the telephone, the patient reported that he no longer had a pressure ulcer.
Graph 1.
Patient A, B, and C's measured WSA pre- and post-application of HVES for recalcitrant pressure ulcers.
Table 1.
WSA and percent decrease in wound surface area (% WSA↓) for Patient A after application of HVES
| Duration of HVES treatment (weeks) | Wound surface area (cm2) | % WSA ↓ |
|---|---|---|
| 0 | 11.30 | |
| 8 | 8.75 | 22.59% |
| 10 | 3.75 | 66.82% |
| 12 | 1.50 | 86.73% |
| 16 | 0.50 | 95.58% |
For Patient B, approximately 9 weeks after initiation of HVES, there was significant reduction in wound size (57.81% WSA↓) and drainage of the wound (Graph 1). Upon discharge 22 weeks later, the wound was clean without macerated edges or undermining (Fig. 2, Graph 1). At that point, the WSA had decreased by 96.25% (Table 2) and ES was discontinued. Wound care consisted of cleansing the wound with a saline solution and dressing it with a Telfa padding secured with Hypafix (Fig. 2). During a 12-month follow-up, the patient reported that he no longer had a pressure ulcer.
Table 2.
WSA and % WSA↓ for Patient B after application of HVES
| Duration of HVES treatment (weeks) | WSA (cm2) | % WSA ↓ |
|---|---|---|
| 0 | 16.00 | |
| 5 | 14.00 | 12.50% |
| 9 | 6.75 | 57.81% |
| 13 | 3.50 | 78.13% |
| 18 | 0.75 | 95.31% |
| 22 | 0.60 | 96.25% |
For Patient C, after 6 and a half weeks of HVES treatment, the 11-month-old wound was completely healed (100% decrease in WSA – Table 3) and HVES treatment was discontinued (Graph 1, Fig. 3). A 6-month follow-up with the patient revealed no pressure ulcers.
Table 3.
WSA and % WSA↓ for Patient C after application of HVES
| Duration of HVES treatment (weeks) | WSA (cm2) | % WSA ↓ |
|---|---|---|
| 0 | 7.20 | |
| 2 | 6.00 | 16.67% |
| 4 | 2.25 | 68.75% |
| 5 | 1.50 | 79.17% |
| 6.5 | 0.00 | 100.00% |
Figure 3.
Patient C's recalcitrant pressure ulcer at initial evaluation and the progression of wound healing after application of HVES. Source: Kennedy Krieger Institute.
As Graph 1 demonstrates, despite conservative wound care management all three patients had wounds that had persisted for at least 11 months. For all three patients HVES initiation resulted in significant healing and a substantial decrease in wound surface (Graph 1).
Discussion
As a consequence of altered autonomic nervous system function, SCI patients whose neurological level is above T6 often lose the ability to perform reflexive sweating. For these patients, the functional properties of their skin are altered and they are unable to maintain a constant body temperature. Neurologically impaired skin undergoes a number of metabolic changes that usually do not stabilize for 3–5 years after the injury. Multiple reports describe a significant and rapid increase in the overall rate of collagen catabolism immediately after the trauma resulting in abnormal concentration of collagen metabolites.30,31 Rodriguez and Markowski32 report a decrease in the proportion of type I–type III collagen in the skin below the level of injury. The increased degradation of collagen affects the integrity of the skin.
The delivery of electrical current into refractory wounds for the purpose of enhancing tissue healing dates back to the seventeenth and twentieth centuries, when several reports described the use of electrostatically charged gold leaves in the treatment of skin lesions associated with small pox and wounds of various etiologies, including ischemic and venous insufficiency ulcers of the lower extremity.17 Since the mid-1960s, more research has been aimed at evaluating the effects of ES on healing of chronic wounds.17,33 ES as a wound healing modality is sometimes used to help heal Stage II and III ulcers in cases where surgery or other treatment methods are not indicated. For Stage IV ulcers, surgery is often the best method for covering chronically exposed bone with excellent results. In 2002, ES was approved for payment by the Centers for Medicare and Medicaid Services for the treatment of pressure ulcers and wounds that have not responded to standard wound treatment.17,34
Laboratory and clinical studies have shown evidence that ES promotes chemotaxis, helps reduce edema, inhibits bacterial growth, promotes protein and DNA synthesis in human fibroblasts, facilitates epithelial tissue migration, increases the migration of neutrophils and macrophages, and improves blood flow and tensile strength.4,17,35
Conclusion
This case series demonstrates the efficacy of HVES for the enhancement of Stage III–IV wound healing that has otherwise not responded to standard wound care. Recalcitrant pressure ulcers, for which the duration ranged from 8 to 14 months, were completely healed after 7–22 weeks of three times a week treatment with HVES. Further study is needed to identify the most effective protocol for HVES therapy in the treatment of long-standing pressure ulcers.
Acknowledgements
We acknowledge Cristina Sadowsky, MD and Varsha Gandhi, DPT for their invaluable support.
References
- 1.Liverman CT, Joy JE, Altevogt BM. (eds.). Spinal cord injury: progress, promise, and priorities. Washington, DC: National Academies Press; 2005. p. 13–106 [Google Scholar]
- 2.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004;85(11):1757–63 [DOI] [PubMed] [Google Scholar]
- 3.Kirshblum S, Gonzalez P, Cuccurullo S. Spinal cord injuries. In: Cuccurullo S. (ed.) Physical medicine and rehabilitation board review. New York: Demos Medical Publishing; 2004. p. 545–48 [Google Scholar]
- 4.Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen 1999;7(6):495–503 [DOI] [PubMed] [Google Scholar]
- 5.Ek AC, Unosson M, Larsson J, Von Schenck H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clin Nutr 1991;10(5):245–50 [DOI] [PubMed] [Google Scholar]
- 6.Strauss EA, Margolis DJ. Malnutrition with pressure ulcers: morbidity, mortality, and clinically practical assessments. Adv Wound Care 1996;9(5):37–40 [PubMed] [Google Scholar]
- 7.7 Consortium for Spinal Cord Medicine Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2001;24Suppl 1:S40–101 [DOI] [PubMed] [Google Scholar]
- 8.Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, et al. Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010;91(5):669–78 [DOI] [PubMed] [Google Scholar]
- 9.Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut JL. A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2009;90(2):213–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman CA. Risk factors for pressure ulcers in the spinal cord injured in the community. SCI Nurs 1995;12(4):110–4 [PubMed] [Google Scholar]
- 11.Salzberg CA, Byrne DW, Cayten CG, van Nuewerburgh P, Murphy JG, Viehbeck M. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil 1996;75(2):96–104 [DOI] [PubMed] [Google Scholar]
- 12.Tourtual DM, Riesenberg LA, Korutz CJ, Semo AH, Asef A, Talati K, et al. Predictors of hospital-acquired heel pressure ulcers. Ostomy Wound Manage 1997;43(9):24–40 [PubMed] [Google Scholar]
- 13.Bergstrom N, Braden B. A prospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc 1992;40(8):747–58 [DOI] [PubMed] [Google Scholar]
- 14.Spungeon A, Rasul M, Koehler K. Effect of anabolic steroid therapy on healing of long-standing pressure sores in patients with SCI. J Spinal Cord Med 1999;22(1):27 [Google Scholar]
- 15.300 PV Complete Electrotherapy System: Instruction Manual. St Paul, Minnesota: Empi; 2006.
- 16.Kloth LC, McCulloch JM. Promotion of wound healing with electrical stimulation. Adv Wound Care 1996;9(5):42–5 [PubMed] [Google Scholar]
- 17.Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005;4(1):23–44 [DOI] [PubMed] [Google Scholar]
- 18.Bourguignon G, Bourguignon L. Electric stimulation of protein and DNA synthesis inhuman fibroblasts. FASEB J 1987;1(5):398–402 [DOI] [PubMed] [Google Scholar]
- 19.Bourguignon G, Bergouignan M, Khorshed A. Effect of high voltage pulsed galvanic stimulation on human fibroblasts in cell culture. J Cell Biol 1986;103:344a [Google Scholar]
- 20.Bourguignon G, Wenche J, Bourguignon L. Electric stimulation of human fibroblasts causes an increase in Ca2+ influx and the exposure of additional insulin receptors. J Cell Physiol 1989;140(2):397–85 [DOI] [PubMed] [Google Scholar]
- 21.Orida N, Feldman J. Directional protrusive pseudopodial activity and motility in macrophages induced by extra-cellular electric fields. Cell Motil 1982;2(3):243–55 [DOI] [PubMed] [Google Scholar]
- 22.Canaday D, Lee R. Scientific basis for clinical application of electric fields in soft tissue repair. In: Brighton C, Pollack S. (eds.) Electromagnetics in biology and medicine. San Francisco, CA: San Francisco Press; 1991. p. 275–91 [Google Scholar]
- 23.Erickson C, Nuccitelli R. Embryonic fibroblastmotility and orientation can be influenced by physiological electric fields. J Cell Biol 1984;98(1):296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Onuma E, Hui S. Response of C3H/10T1/2 fibroblasts to an external steady electric field stimulation. Exp Cell Res 1984;155(1):92–104 [DOI] [PubMed] [Google Scholar]
- 25.Nishimura K, Isseroff R, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci 1996;109(Pt 1):199–207 [DOI] [PubMed] [Google Scholar]
- 26.Stromberg B. Effects of electrical currents on wound contraction. Ann Plast Surg 1988;21(2):121–3 [DOI] [PubMed] [Google Scholar]
- 27.Cooper M, Schliwa M. Electrical and ionic controls of tissue cell locomotion in DC electrical fields. J Neurosci Res 1985;13(1–2):223–44 [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt A, Szczypiorski P, Korytowski G. Effect of transcutaneous electrostimulation on the cell composition of skin exudate. ACTA Physiol Pol 1986;37(1):41–6 [PubMed] [Google Scholar]
- 29.Mertz P, Davis S, Cazzaniga A, Cheng K, Reich JD, Eaglstein VM. Electrical stimulation: acceleration of soft tissue repair by varying the polarity. Wounds 1993;5(3):153–9 [Google Scholar]
- 30.Claus-Walker J, Singh J, Leach CS, Hatton DV, Hubert CW, Di Ferrante N. The urinary excretion of collagen degradation products by quadriplegic patients and during weightlessness. J Bone Joint Surg 1977;59(2):209–12 [PubMed] [Google Scholar]
- 31.Rodriguez GP, Claus-Walker J. Biochemical changes in skin composition in spinal cord injury: a possible contribution to decubitus ulcers. Paraplegia 1988;26(5):302–9 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez GP, Markowski J. Changes in skin morphology and its relationship to pressure ulcer incidence in spinal cord injury. [ACRM abstract]. Arch Phys Med Rehabil 1995;76(6):593 [Google Scholar]
- 33.Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther 1988;68(4):503–8 [DOI] [PubMed] [Google Scholar]
- 34.Decision Memo for Electrostimulation for Wounds (CAG-00068N) Centers for Medicare and Medicaid Services Website. [document on the Internet]. 2002 [updated 2002; cited 2011 Apr 17]. Available from: http://www.cmms.hhs.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=27&ver=11&NcaName=Electrostimulation+for+Wounds&NCDId=190&ncdver=2&IsPopup=y&bc=AAAAAAAAEAAA& .
- 35.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11Suppl 1:S1–28 [DOI] [PubMed] [Google Scholar]