Abstract
Insect metamorphosis is regulated by ecdysteroids, which induce molts, and juvenile hormone (JH), which inhibits metamorphic changes. The molecular action of ecdysteroids has been thoroughly studied, but that of JH is poorly understood, with data currently only being available for holometabolous species, like Drosophila melanogaster and Tribolium castaneum. We studied the function of Krüppel homolog 1 (Kr-h1) in Blattella germanica, a hemimetabolous model. Kr-h1 is a Zn finger transcription factor whose function as transductor of the antimetamorphic action of JH has recently been demonstrated in D. melanogaster and T. castaneum. The RNAi experiments reported herein indicated that Kr-h1 transduces the antimetamorphic action of JH also in B. germanica, thereby suggesting that this role is an ancestral condition that has been conserved in insect evolution from hemimetabolous to holometabolous species.
Insect metamorphosis has fascinated mankind since the time of Aristotle, some two thousand years ago. Much later, Renaissance entomologists established that post-embryonic changes are most spectacular in insects like beetles, moths and flies, which undergo a dramatic morphological transformation from larva to pupa and adult, a phenomenon nowadays known as holometaboly, which is typical in endopterygote species. Exopterygote insects, such as locusts and cockroaches, also transform from last nymphal instar to adult, although the transformation is not as radical as the nymphs are relatively similar to the adult stage. However, they undergo qualitative metamorphic changes, such as formation of mature wings and external genitalia, amongst others, in a type of metamorphosis known as hemimetaboly1,2,3. Understanding insect metamorphosis at molecular level is still a challenging mystery because we still only have a few pieces of the puzzle. From an endocrine point of view, metamorphosis is regulated by two kinds of hormones, namely molting hormone, which induces molts, and juvenile hormone (JH), which modulates the quality of the molt: to an immature stage when it is present, and to the adult when it is absent; JH therefore plays a crucial repressive role in insect metamorphosis1,4,5.
The effect of the commonest molting hormone, namely 20-hydroxyecdysone, is mediated by a cascade of transcription factors and starts upon its binding to the heterodimeric receptor composed by the ecdysone receptor and the ultraspiracle (or RXR), both of which belong to the nuclear receptor superfamily. This activates expression of a hierarchy of transcription factors, such as E75, E78, HR3, HR4 and FTZ-F1, which regulate the genes that underlie the cellular changes associated with molting and metamorphosis6,7. Most of the reported data on this cascade of transcription factors refer to Drosophila melanogaster, the fruit fly, a holometabolous insect that shows many highly derived characters, which has been the most thoroughly studied species from the point of view of molecular endocrinology8,9. From this point of view, the most widely studied hemimetabolous model is the German cockroach Blattella germanica, and results indicate that the transcription factors involved in 20-hydroxyecdysone signalling are generally conserved, although the functions of some of them and the precise epistatic relationships between them may differ with respect to D. melanogaster10,11,12,13,14.
Conversely, the molecular mechanisms underlying the action of JH are poorly understood, and our current understanding relies completely on holometabolous models4. An important player is Methoprene tolerant (Met), a transcriptional regulator of the basic helix-loop-helix (bHLH) -Per-Arnt-Sim (PAS) domain family that was discovered in D. melanogaster15 where it binds JH at physiological concentrations. This and other characteristics4,16 suggest that Met plays the role of JH receptor, or is a component of a heterodimeric JH receptor. Key functional evidence that Met is required for the repressor action of JH on metamorphosis was not obtained in D. melanogaster but in the beetle Tribolium castaneum, a holometabolous insect that shows fewer highly derived characters than D. melanogaster, where the RNAi of Met induced larvae to undergo precocious metamorphosis17,18.
The transcription factor Krüppel homolog 1 (Kr-h1), whose antimetamorphic action has recently been demonstrated in D. melanogaster19 and T. castaneum20, is another important transducer of the JH signal. In D. melanogaster, the adult epidermis of head and thorax derive from imaginal discs21, whereas that of the abdomen derives from larval histoblasts that start proliferating after puparium formation and give the pupal epidermal cells22,23. Ectopic application of JH prior to the prepupal stage prevents the normal differentiation of the abdominal epidermis, and the bristles that normally occur in the dorsal midline of the adult fly become shorter or simply do not form24,25. Moreover, ectopic expression of Kr-h1 in the abdominal epidermis during metamorphosis causes missing or short dorsal midline bristles, just as in the experiments with JH treatment, thereby suggesting that, in D. melanogaster, Kr-h1 mediates the antimetamorphic action of JH19. In T. castaneum, RNAi experiments have shown that Kr-h1 represses metamorphosis and that it works downstream of Met in the JH signalling pathway20. Kr-h1 therefore appears to be the more distal transcription factor in the JH signalling pathway whose role as mediator of the antimetamorphic action of JH has been conserved from beetles to flies, within the Endopterygota ( = Holometabola) insect subclass.
As all functional data on the signalling pathway of JH have been obtained in holometabolous models, research in hemimetabolous species is needed if we aim at elucidating the evolution of insect metamorphosis. In light of this, we studied the function of Kr-h1 in B. germanica, a polyneopteran exopterigote that shows a gradual morphological transformation along the life cycle, which is representative of hemimetabolous metamorphosis1,2. Methodologically, our strategy was to knockdown Kr-h1 by RNAi, a technique that has been shown to be highly effective for silencing gene expression in B. germanica26, and then to examine the phenotype obtained.
Results
The sequence of Krüppel-h1 is highly conserved in insects
Cloning of Kr-h1 cDNA in B. germanica was accomplished by a RT-PCR approach, combining the use of degenerate primers based on Kr-h1 conserved motifs to obtain a partial sequence, and 5’-RACE and 3’-RACE experiments to complete it. These amplifications rendered a cDNA of 2269 bp (GenBank accession number HE575250) where the putative start codon is preceded by in-frame stop codons, thus suggesting that a full-length open reading frame had been obtained. Database BLAST searches indicated that it encoded an ortholog of Kr-h1, which we called BgKr-h1. The conceptual translation rendered a 658 amino acid protein sequence containing the eight classical C2H2 zinc fingers towards the C-terminal region, and the “A” (LPLRKR) and “B” (RSRSVIHYA) motifs towards the 3’end in the N-terminal region, which are typical of Kr-h1 proteins27.
The alignment of the Kr-h1 protein sequences (Supplementary Fig. 1 online) indicates that the most conserved region is the Zn finger domain, where the most apparent feature is an insertion-deletion of 25–47 amino acids located between the first and second Zn finger, which distinguishes the dipterans (that show the insertion) from the other insect orders. The percentage of identity of the BgKr-h1 Zn finger domain with respect to non-dipteran species is very high, ranging from 90% (with Apis mellifera), to 82% (with Nasonia vitripennis), whereas it is lower when compared with dipteran sequences, ranging from 68% (with Aedes aegypti) to 63% (with D. melanogaster). The percentage of identity in the Zn finger domain is also high with the homolog that we found in the crustacean Daphnia pulex, whose sequence presents only seven Zn fingers but having between 60 and 70% identity with the equivalent region in insects.
Krüppel-h1 expression decays suddenly in the last instar nymph and is up-regulated by juvenile hormone
To gain a first insight into the possible involvement of BgKr-h1 in cockroach metamorphosis, we studied its expression pattern in the whole body of females during the three last nymphal instars. The results (Fig. 1a) show that BgKr-h1 mRNA levels generally oscillate between 10 and 20 copies per 1000 copies of BgActin-5c but suddenly vanish one day after the molt to the sixth (last) nymphal instar. This down-regulation of BgKr-h1 expression coincides with decreasing levels of circulating JH (Fig. 1a), according to analytical data previously obtained in our laboratory28. Moreover, expression is not confined to a single or few tissue types, but is ubiquitously distributed amongst practically all tissues, especially the muscle, epidermis (pronotum, mesonotum and metanotum samples) and ovaries (Fig. 1b). Pattern coincidence suggests that BgKr-h1 expression is induced by JH, therefore to test this hypothesis we treated freshly emerged sixth instar female nymphs with JH III, and measured BgKr-h1 transcript levels 6 h, 2 days, 4 days and 6 days later. The results obtained indicate that JH up-regulates BgKr-h1 expression and that the stimulatory effect lasts practically the entire sixth instar nymph, although with decreasing intensity (Fig. 1c). A number of JH-treated specimens were left alive until the next molt, and they molted into adultoids with nymphal features (Supplementary Fig. 2 online), as expected.
Figure 1. Expression of BgKr-h1 mRNA in Blattella germanica determined by qRT-PCR.
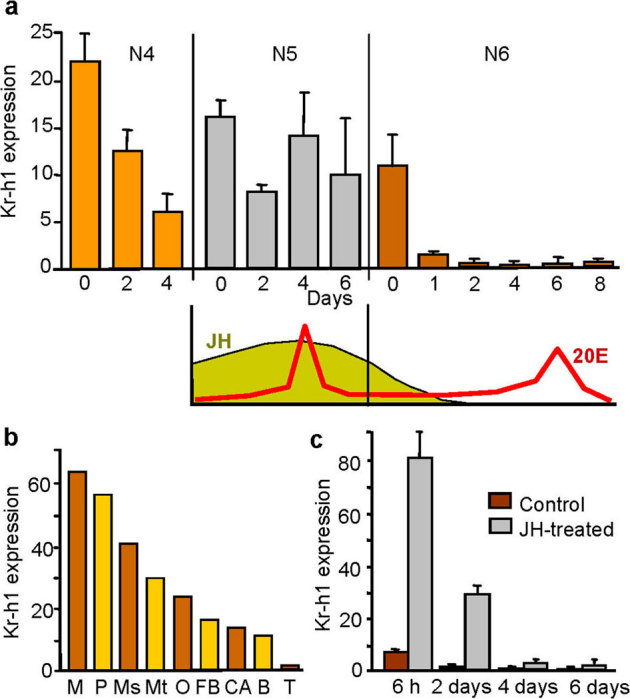
(a) Expression in female whole body in the three last nymphal instars: N4, N5 and N6. Relative titers of juvenile hormone III (JH) and 20-hydroxyecdysone (20E) in N5 and N6 are indicated below, according to Treiblmayr et al.28 and Romaña et al.39, respectively. (b) Expression in different tissues of females in day 0 of N6: muscle (M), pronotum (P), mesonotum (Ms), metanotum (Mt), ovaries (O), brain (B), fat body (FB), corpora allata (CA), and in testicles (T) from males of the same age. (c) Effect of the application of 20 µg of JH on freshly emerged N6 on BgKr-h1 mRNA levels. Data in (a) and (c) represent the mean ± SEM, and are indicated as copies of BgKr-h1 mRNA per 1000 copies of BgActin-5c; each point represents 4 biological replicates. Data in (b) represent a pool of 5 specimens. In (c), differences of JH-treated with respect to controls were statistically significant in all cases (p < 0.05), according to the REST software tool40.
RNAi of Krüppel-h1 in fifth instar female nymphs results in precocious metamorphosis after the next molt
We approached the study of BgKr-h1 function in B. germanica by RNAi. In a first set of experiments, we injected a single 3-µg dose of dsRNA targeting BgKr-h1 (dsKr-h1) into the abdomen of freshly emerged fifth (penultimate) instar female nymphs. Controls received the same dose of unspecific dsRNA (dsMock). Transcript monitoring at 48 h intervals indicated that BgKr-h1 levels were significantly lower (52%) in dsKr-h1-treated specimens than in controls 6 days after the treatment (Fig. 2a). dsMock-treated (control) specimens (n = 40) molted to normal sixth instar nymphs ca. 6 days after the treatment. Females treated with dsKr-h1 (n = 41) required, on average, two or three days more than controls to perform the next molt (Fig. 2b), and this molt rendered individuals with adult features. About 71% of the specimens (Fig. 2c) had a general morphology and coloration intermediate between a sixth (last) instar nymph and an adult; of note, the structure of the latero-basal expansions of the mesonotum and metanotum (which correspond to the mesonotal and metanotal wing pads) was flexible and membranous, as in mature wings and tegmina (Fig. 2d). Most of these intermediates (86%) died between 6 and 10 days after the molt. In contrast, some of them (14%) were able to molt again (around day 9 of this sixth instar), although they were unable to properly shed the exuvia, and this resulted in mechanically deformed adults, with the wings well patterned but not well extended (Supplementary Fig. 3 online). The remaining 29% of the treated specimens (Fig. 2c) had the typical morphology and coloration of an adult, although they were smaller (having the size of a normal sixth instar female nymph) and their wings were membranous and well patterned, although not well extended (Fig. 2d). These precocious adults lived much longer than the nymph-adult intermediates (between 2 and 3 months, as average) and did not molt again.
Figure 2. Effects of BgKr-h1 depletion in fifth nymphal instar (N5) of Blattella germanica females.
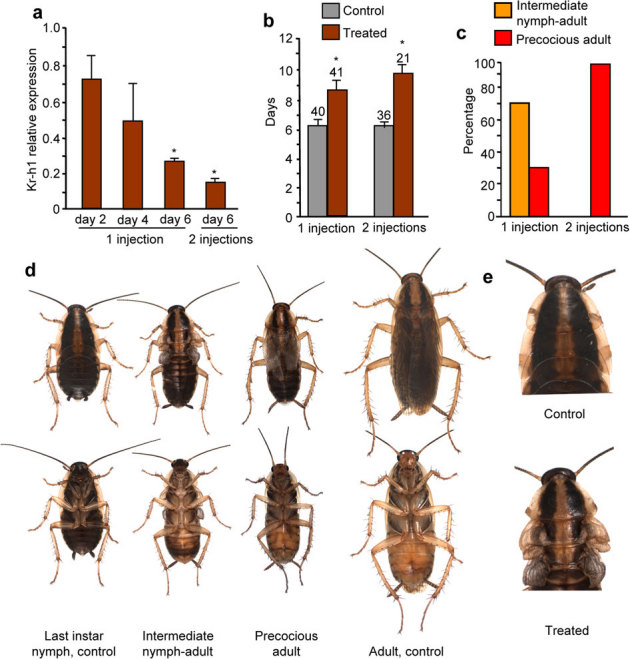
Females received 1 injection (3 µg-dose, on day 0 of N5), or 2 injections (3 µg each, on day 0 and day 3 of N5, respectively) of dsMock (control) or dsKr-h1 (treated). (a) Effects on BgKr-h1 mRNA levels measured by qRT-PCR on days 2, 4 and 6 of N5 in single-injection experiments, or on day 6 of N5 in two-injection experiments. (b) Length (days) of N5 in control and treated female specimens. (c) Percentage of specimens showing the intermediate nymph-adult phenotype or the precocious adult phenotype in the 1- or 2-injection experiments. (d) Dorsal and ventral view of phenotypes resulting from 1- or 2-injection experiments, compared with control females in last nymphal instar and with the adult stage. (e) Dorsal part of the thorax in the intermediate nymph-adult phenotype, showing the membranous structure of the wing pads. Data in (a) represent 4 biological replicates (mean ± SEM) and are normalized against the dsMock females (reference value = 1); the asterisk indicates statistically significant differences with respect to controls (p < 0.05), according to the REST software tool40. Replicates in (b) are indicated at the top of each bar; the asterisk indicates that differences with the respective controls are statistically significant (student’s t-test, P<0.001).
In a second set of experiments we injected two doses of 3 µg each of dsKr-h, one into freshly emerged fifth instar female nymphs, and the other one three days later. Controls were equivalently treated with dsMock. Six days after the treatment, BgKr-h1 mRNA levels were significantly lower (72%) in dsKr-h1-treated specimens than in controls (Fig. 2a). Females treated with dsKr-h1 (n = 21) took between three and four days more than controls (n = 36) to complete the fifth instar (Fig. 2b). In contrast with controls, which molted normally to the sixth nymphal instar, all dsKr-h1-treated specimens molted to precocious adults with imperfectly extended wings, in a similar manner to the 29% fraction of the single-injection experiments reported above (Fig. 2d). None of these precocious adults molted again.
RNAi in fourth instar female nymphs results in precocious metamorphosis after two molts
In order to test whether precocious metamorphosis could also be provoked in younger instars, we carried out experiments equivalent to those just described but using fourth instar female nymphs. Single-injection treatments were carried out on freshly emerged fourth (antepenultimate) instar female nymphs, and four days after the injection BgKr-h1 mRNA levels were already significantly lower (72%) in dsKr-h1-treated specimens than in controls (Fig. 3a). The length of the fourth instar was the same (ca. 5 days) in dsKr-h1-treated (n = 25) and in controls (n = 15), and all specimens molted normally to fifth instar. However, the length of the fifth instar in the dsKr-h1-treated specimens was almost twice that for the controls (Fig. 3b). After the next molt, control specimens became normal sixth instar nymphs, whereas six out of 25 specimens (24%) of the ds-Kr-h1-treated group (Fig. 3c) gave a phenotype intermediate between a sixth instar nymph and an adult, showing the typical membranous structure of the wing pads (Fig. 3d). These six intermediates died between 8 and 10 days after the molt. The remaining 19 dsKr-h1-treated specimens (76%) (Fig. 3c) molted into precocious adults with imperfectly extended wings (Fig. 3d), which did not molt again.
Figure 3. Effects of BgKr-h1 depletion in fourth nymphal instar (N4) of Blattella germanica females.
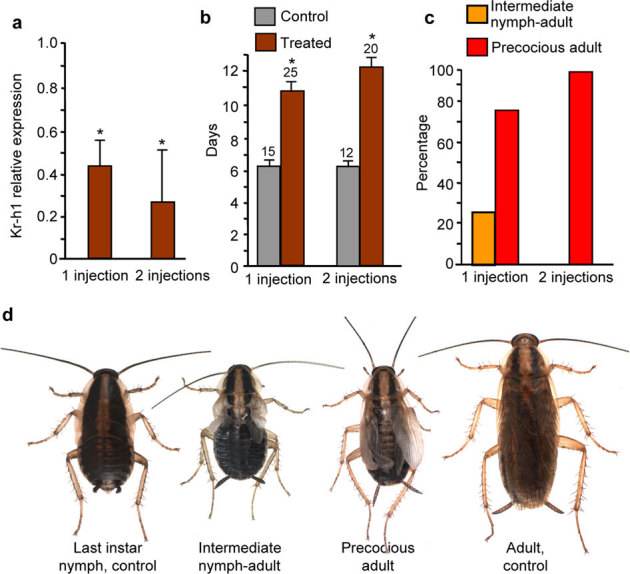
Females received 1 injection (3 µg-dose, on day 0 of N4), or 2 injections (3 µg each, on day 0 and day 3 of N4, respectively) of dsMock (control) or dsKr-h1 (treated). (a) Effects on BgKr-h1 mRNA levels measured by qRT-PCR on day 4 of N4 in 1- or 2-injection experiments. (b) Length (days) of N5 in control and treated specimens. (c) Percentage of specimens showing the intermediate nymph-adult phenotype or the precocious adult phenotype in the 1- or 2-injection experiments. (d) Dorsal view of phenotypes resulting from 1- or 2-injection experiments, compared with control females in last nymphal instar and with the adult stage Data in (a) represent 4 biological replicates (mean ± SEM) and are normalized against the dsMock females (reference value = 1); the asterisk indicates statistically significant differences with respect to controls (p < 0.05), according to the REST software tool40. Replicates in (b) are indicated at the top of each bar; the asterisk indicates that differences with the respective controls are statistically significant (student’s t-test, P<0.001).
Two-injection experiments also induced a significant decrease (82%) of BgKr-h1 mRNA levels four days after the first injection (Fig. 3a), and the timing of the fourth and fifth nymphal instars was similar to that found in the single-injection experiments: no differences in the length of the fourth instar between dsKr-h1-treated (n = 20) and controls (n = 12), and a significant increase in the length of the fifth instar in the dsKr-h1-treated specimens (Fig. 3b). After the next molt, all dsKr-h1-treated specimens became precocious adults with imperfectly extended wings (Fig. 3d), which did not molt again. Controls molted to normal sixth instar nymphs.
Males are more sensitive to the silencing effects of Krüppel-h1 RNAi
A few male nymphs, all of which yielded precocious adults after two molts, were inadvertently included in the experiments involving injecting a single dose of dsKr-h1 into fourth instar female nymphs. This prompted us to test whether males could be more sensitive than females to the silencing effects of RNAi on BgKr-h1. First, we obtained data regarding BgKr-h1 mRNA levels on selected days of the penultimate and last nymphal instars in males. Our determinations indicated that the expression pattern (Fig. 4a) is similar to that of females (Fig. 1a), with a sudden decrease after the first day of the last nymphal instar.
Figure 4. Expression of BgKr-h1 and effects of its depletion in Blattella germanica males.
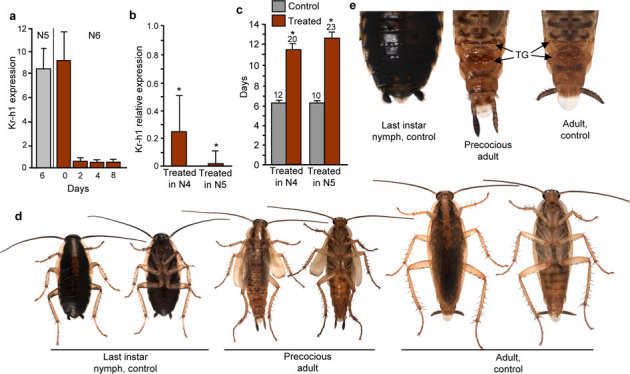
Males received a single dose of 3 µg on day 0 of fourth (N4) or fifth (N5) nymphal instar of dsMock (control) or dsKr-h1 (treated). (a) Expression of BgKr-h1 in male whole body in selected days of N5 and N6. (b) Effects of dsKr-h1 on BgKr-h1 mRNA levels measured by qRT-PCR in specimens treated on N4 (transcript measured on day 4) or on N5 (transcript measured on day 6). (c) Length (days) of N5 in male specimens treated in N4 or N5. (d) Phenotype (dorsal and ventral view) resulting from the experiments carried out on N4 after two molts or in N5 after one molt. (e) Tip of the abdomen showing the area occupied by the tergal glands (TG) in the seventh and eighth tergites. Data in (a) represent the mean ± SEM, and are indicated as copies of BgKr-h1 mRNA per 1000 copies of BgActin-5c; each point represents 4 biological replicates. Data in (b) represent 4 biological replicates (mean ± SEM) and are normalized against the dsMock females (reference value = 1); the asterisk indicates statistically significant differences with respect to controls (p < 0.05), according to the REST software tool40. Replicates in (c) are indicated at the top of each bar; the asterisk indicates that differences with the respective controls are statistically significant (student’s t-test, P<0.001).
We then used the design of single-injection RNAi experiments by administering a 3-µg dose of dsKr-h1 into freshly emerged fourth instar male nymphs (controls received the same dose of dsMock). This treatment induced a significant decrease (75%) of BgKr-h1 mRNA levels four days after the injection (Fig. 4b). Moreover, the fourth instar had the same length in dsKr-h1-treated and in control males, and all specimens molted normally to the fifth instar, whereas the length of the latter in dsKr-h1-treated males was practically twice that of the controls (Fig. 4c). All dsKr-h1-treated specimens (n = 20) then molted into precocious adults, with the same shape and coloration as a normal adult male, but with the size of a sixth nymphal instar, the membranous wings present but not well extended, and the tergal glands (which are absent in nymphs but present in adult males as paired pouches on the tergites 7 and 8) readily apparent (Fig. 4d). Of note, the posterior margin of tergite 7 was somewhat shorter and notched in these precocious adults, which allowed seeing directly the paired glands of the tergite 8, whereas they are partially hidden by the well developed tergite 7 in normal adults (Fig. 4e). Male precocious adults resulting from dsKr-h1 treatments did not molt again. Control specimens (n = 12) became normal sixth instar nymphs after the fifth molt.
Finally, we carried the same experiments but treating freshly emerged fifth instar male nymphs. In this case, the treatment induced a remarkable decrease (93%) of target transcript levels (Fig. 4b), much higher than that observed in females in equivalent experiments, and the length of the fifth instar was higher in treated than in controls (Fig. 4c), as in the former experiments. All dsKr-h1-treated specimens (n = 23) molted into precocious adults at the next molt, showing the same features as those described in the experiments carried out on males in the fourth nymphal instar (see Fig. 4d). Controls (n = 10) molted to normal sixth instar nymphs.
Discussion
The amino acid sequence of BgKr-h1, the Krüppel homolog 1 of the cockroach B. germanica (which undergoes a nymphal-adult transformation that is representative of hemimetabolous metamorphosis) is similar to those of ortholog sequences of other insects from holometabolous orders (i.e. those that undergo a full metamorphosis). Considering all sequences included in the alignment (Supplementary Fig. 1 online), the degree of conservation is high, even when comparing the sequence of B. germanica with that of the holometabolous insect D. melanogaster, which shows many highly derived characters. The similarity is particularly high in the Zn finger domain, where the most complex binding capacity of the molecule resides. Indeed, multiple-adjacent C2H2 zinc finger proteins bind 25–75% of the Zn fingers to DNA, whereas the remainder may bind to proteins and RNA, including dsRNA and DNA-RNA heterocomplexes29. The high degree of conservation found in the C2H2 zinc finger domain in all studied species suggests that the Kr-h1 function might have been generally conserved across the insect class. The discovery of a Kr-h1 homolog in the crustacean D. pulex opens the question of its function, which remains to be investigated.
Our mRNA determinations showed that BgKr-h1 is ubiquitously expressed in different tissues, whereas time-course studies indicated that expression vanishes after the first day of the last instar nymph. Similar decreases in Kr-h1 expression have been observed in the transition from the “propupa” to the “pupa” of thrips species (Frankliniella occidentalis and Haplothrips brevitubus)30, which show a variant of hemimetaboly called neometaboly1,31. Similar decreases of Kr-h1 expression occur between the prepupal and pupal stages in holometabolous models, such as the fly D. melanogaster19 and the beetle T. castaneum20. The coincidence of the patterns of BgKr-h1 and that of circulating JH (which decreases during the first days of the last nymphal instar28) in the cockroach B. germanica (Fig. 1a), suggested that BgKr-h1 expression is induced by JH. This hypothesis was assessed in experiments that showed that treatment of last instar nymphs with JH readily induced the re-expression of BgKr-h1. Early works on D. melanogaster reported that Kr-h1 is partially regulated by 20-hydroxyecdysone and, in turn, modulates ecdysteroid-dependent metamorphic processes32,33. However, more recent work revealed that Kr-h1 expression is induced by JH in the fly D. melanogaster19, the beetle T. castaneum20, and the thrips F. occidentalis and H. brevitubus30, as is also the case in B. germanica (present work).
The results of RNAi experiments indicate that knockdown of BgKr-h1 in B. germanica in juvenile stages induces a precocious metamorphosis after the penultimate (fifth) nymphal instar. Of note, when the RNAi was carried out in the fourth nymphal instar, two molts were needed before the occurrence of precocious metamorphosis. Pioneering experiments have shown that metamorphosis can be precociously induced by dissecting out the corpora allata, i.e., the JH-producing glands. However, when such allatectomy is performed in very young larvae, one or two additional molts are currently needed before precocious adult features appear34. More recent studies have shown that depletion of JH through overexpression of JH esterase fails to cause premature pupation in larvae of the lepidopteran Bombyx mori if they are younger than third instar35. Similarly, metamorphic changes after RNAi treatment targeting Met in young larvae of T. castaneum typically require two or three molts before giving precocious adult features17. These observations, and those described herein in B. germanica, suggest that the immature insect must achieve a critical weight (thus a minimum time of postembryonic growth) in order to be able to metamorphose when JH vanishes, either in hemimetabolous as well as in holometabolous species. Connected with this issue is the observation showing that the stage previous to precocious metamorphosis in dsKr-h1-treated specimens, i.e., the fifth (normally the penultimate) nymphal instar is prolonged by 40–100% before molting, compared with the same instar in controls. Again, this delay could be necessary to reach a minimal critical weight or (perhaps more plausibly, given that there are no delays in the fourth nymphal instar) to unfold the developmental transition between the nymph and the adult.
RNAi was found to be more efficient in males than in females of B. germanica. Thus, the penetrance of the precocious adult phenotype in males using a single injection was 100%, irrespective of the stage of treatment, whereas in females it was between 29%, when females were treated in the fifth nymphal instar, and 76% when treated in the fourth nymphal instar (Supplementary Table 1 online). The experiments with males made clearer that there is a rough correlation between the percentage of transcript decrease after dsKr-h1 treatment and that of precocious adults obtained in the experiment. Moreover, the fact that target transcript decrease was higher in males than in females, especially in fifth nymphal instar (compare figures 2a and 4b), suggests that RNAi machinery26 was more efficiently induced in males than in females after dsKr-h1 treatment, an aspect that may deserve further research.
According to the functional studies performed on D. melanogaster19 and T. castaneum20, Kr-h1 was considered the more distal transductor of the JH hierarchy whose antimetamorphic action had been conserved from beetles to flies, within the endopterygote suborder, whose species show holometabolous metamorphosis. Now, the results of the RNAi experiments reported herein indicate that BgKr-h1 plays the equivalent role in B. germanica, an exopterygote polyneopteran species showing an hemimetabolous mode of metamorphosis1. This suggests that the repressor role of Kr-h1 on metamorphosis is an ancestral condition that has been conserved from hemimetabolous to holometabolous species. Thus, the ancestral role of Kr-h1 can be a useful starting point to study the mechanisms underlying the evolutionary transition from hemimetaboly to holometaboly, which still remain a challenging enigma.
Methods
Insects
The specimens of B. germanica used in the experiments were obtained from a colony reared in the dark at 30 ±1°C and 60–70% RH. They were anaesthetized with carbon dioxide prior to injection treatments, dissections and tissue sampling.
Cloning of Kr-h1 cDNA
The B. germanica Kr-h1 homolog was obtained following a RT-PCR strategy using degenerate primers designed on the basis of conserved motifs from insect Kr-h1 sequences, and cDNA from one- to six-day-old fifth instar female nymphs of B. germanica as a template. The primers were: forward, 5'- GVCAYTACCGNACNCAYACBGGBGA -3'; reverse, 5'- TTBAGCACRTGRTTGTAGCCRAAG -3'. The sequence of the amplified fragment (419 bp) was highly similar to the equivalent region in known insect Kr-h1 sequences. Then, the sequence was completed by 5' and 3' RACE (5'- and 3'-RACE System Version 2.0; Invitrogen) using the same template. For 5'-RACE, reverse primer was 5'- CCTTGCCACAAATGACACAA -3' and the nested primer was 5'-AATGATTTGCTGCAATACTCGC -3. For 3'-RACE, forward primer was 5'-CTTGTCATACACATGCGCACTCATACAG-3' and the nested primer was 5'-GGAGAAACCCTATTCTTGTG -3'. All PCR products were subcloned into the pSTBlue-1 vector (Novagen) and sequenced.
RNA Extraction and retrotranscription to cDNA
All RNA extractions were performed using the miRNeasy Mini Kit (Qiagen). A 500-ng sample from each RNA extraction was treated with DNAse (Promega) and reverse transcribed with Superscript II reverse transcriptase (Invitrogen) and random hexamers (Promega). RNA quantity and quality was estimated by spectrophotometric absorption at 260 nm using a Nanodrop Spectrophotometer ND-1000® (NanoDrop Technologies).
Determination of mRNA levels by quantitative real-time PCR
Quantitative real time PCR (qRT-PCR) reactions were carried out in triplicate in an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories), using SYBR®Green (Power SYBR® Green PCR Master Mix; Applied Biosystems). A template-free control was included in all batches. The primers used to detect Kr-h1 mRNA were as follows: forward, 5’- GCGAGTATTGCAGCAAATCA -3’ and reverse, 5’- GGGACGTTCTTTCGTATGGA -3’. The efficiency of this primer set was first validated by constructing a standard curve through four serial dilutions. mRNA levels were calculated relative to BgActin-5c (Accession number AJ862721) expression, using the Bio-Rad iQ5 Standard Edition Optical System Software (version 2.0). Results are given as copies of mRNA per 1000 copies of BgActin-5c mRNA.
Treatments with juvenile hormone III in vivo
To study the effect of juvenile JH upon Kr-h1 expression, JH III (Sigma-Aldrich), which is the native JH of B. germanica36, was applied topically to freshly emerged last instar nymphs at a dose of 20 µg per specimen in 1 µL of acetone. The commercial JH III is a mixture of isomers containing ca. 50% of the biologically active (10R)-JH III, thus the active dose applied was around 10 µg per specimen. Controls received 1 µL of acetone.
RNA interference
The detailed procedures for RNAi experiments were as described previously37. A dsRNA encompassing a 320-bp fragment located between nucleotides 611 and 930 (dsKr-h1), in the Zn finger domain, was designed. The primers used to generate the templates to prepare the dsKr-h1 were as follows: forward, 5’- GAATCTCAGTGTGCATAGGCG -3’ and reverse, 5’- CCTTGCCACAAATGACACAA -3’. The fragments were amplified by PCR and cloned into the pSTBlueTM-1 vector. A 307-bp sequence from Autographa californica nucleopoyhedrovirus (Accession number K01149, from nucleotide 370 to 676) was used as control dsRNA (dsMock). The dsRNAs were prepared as reported previously37. A volume of 1 µL of dsRNA solution (3 µg/µL) was injected into the abdomen of specimens at chosen ages and stages. Control specimens were treated with the same dose and volume of dsMock.
Sequence comparisons
Insect sequences labelled as Krüppel homolog 1 were obtained from GenBank, and the list was enlarged by BLAST search using the B. germanica BgKr-h1 sequence as query. Finally, the protein sequences included in the analysis were the following (GenBank accession number and annotation details, if needed, in parenthesis). Acromyrmex echinatior (EGI66600.1), Acyrtosiphon pisum (XP_001946194, annotated as hypothetical protein), Aedes aegypti (EAT46451, annotated as Zinc finger protein), Anopheles gambiae (EAA13888.4, annotated as Zinc finger protein, incomplete at the 3’ end of the ORF), Apis mellifera (NP_001011566.1), Blattella germanica (HE575250), Bombyx mori (NP_001171332.1), Bombus terrestris (ACX50259.1), Camponotus floridanus (EFN62423.1), Culex quinquefasciatus (XP_001863529.1, annotated as Zinc finger protein), Drosophila melanogaster (CAA06543, Kr-h1 isoform B), Frankliniella occidentalis (BAJ41258.1, Kr-h1 isoform B), Nasonia vitripennis (XP_003425921.1), Solenopsis invicta (EFZ20948.1, annotated as Zn finger protein), Spodoptera littoralis (EZ981183.1, nucleotide sequence from transcriptome shotgun assembly), Striacosta albicosta (EZ585624.1, nucleotide sequence from transcriptome shotgun assembly), Tribolium castanenum (NP_001129235.1), Pediculus humanus (XP_002428656.1, annotated as Kr-18). In addition, we found a Kr-h1 orthologue of the Crustacean Daphnia pulex (EFX82007.1, annotated as Zn finger protein), which served as a reference for the sequences comparison. The sequences were aligned using the MAFFT program38, with default parameters, and visualized using Geneious Software.
Author Contributions
X.B. initially conceived and designed the research. J.L. carried out the experiments and the sequences comparison. X.B. wrote the manuscript. Both authors discussed the results, decided about subsequent experiments and commented on the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
We are grateful to Jose Castresana, Maria-Dolors Piulachs and Jia-Hsin Huang for helpful discussions and comments on the manuscript. Support for this research was provided by the Spanish MICINN (grant CGL2008-03517/BOS to X.B. and predoctoral fellowship to J.L.) and by the CSIC (grant 2010TW0019, from the Formosa program, to X.B.).
References
- Bellés X. in Encyclopedy of Life Sciences (ELS) (John Wiley and Sons, Ltd., 2011). [Google Scholar]
- Grimaldi D. & Engel M. S. Evolution of the insects. (Cambridge University Press, 2005). [Google Scholar]
- Heming B. S. Insect Development and Evolution. (Comstock Publishing Associates, 2003). [Google Scholar]
- Riddiford L. M. Juvenile hormone action: a 2007 perspective. J Insect Physiol 54, 895–901 (2008). [DOI] [PubMed] [Google Scholar]
- Truman J. W. & Riddiford L. M. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol 47, 467–500 (2002). [DOI] [PubMed] [Google Scholar]
- King-Jones K. & Thummel C. S. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet 6, 311–323 (2005). [DOI] [PubMed] [Google Scholar]
- Nakagawa Y. & Henrich V. C. Arthropod nuclear receptors and their role in molting. FEBS J 276, 6128–6157 (2009). [DOI] [PubMed] [Google Scholar]
- Thummel C. S. Ecdysone-regulated puff genes 2000. Insect Biochem Mol Biol 32, 113–120 (2002). [DOI] [PubMed] [Google Scholar]
- Yin V. P. & Thummel C. S. Mechanisms of steroid-triggered programmed cell death in Drosophila. Semin Cell Dev Biol 16, 237–243 (2005). [DOI] [PubMed] [Google Scholar]
- Mane-Padros D. et al.. The hormonal pathway controlling cell death during metamorphosis in a hemimetabolous insect. Dev Biol 346, 150–160 (2010). [DOI] [PubMed] [Google Scholar]
- Cruz J., Martin D. & Belles X. Redundant ecdysis regulatory functions of three nuclear receptor HR3 isoforms in the direct-developing insect Blattella germanica. Mech Dev 124, 180–189 (2007). [DOI] [PubMed] [Google Scholar]
- Cruz J., Nieva C., Mane-Padros D., Martin D. & Belles X. Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev Dyn 237, 3179–3191 (2008). [DOI] [PubMed] [Google Scholar]
- Mane-Padros D. et al.. The nuclear hormone receptor BgE75 links molting and developmental progression in the direct-developing insect Blattella germanica. Dev Biol 315, 147–160 (2008). [DOI] [PubMed] [Google Scholar]
- Martin D., Maestro O., Cruz J., Mane-Padros D. & Belles X. RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J Insect Physiol 52, 410–416 (2006). [DOI] [PubMed] [Google Scholar]
- Ashok M., Turner C. & Wilson T. G. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci U S A 95, 2761–2766 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A. A. & Wilson T. G. in Gene Duplication (ed Friedberg F.) Ch. 18, 333–352 (Intech, 2011). [Google Scholar]
- Konopova B. & Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A 104, 10488–10493 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R., Tan A. & Palli S. R. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech Dev 125, 601–616 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi C., Zhou X. & Riddiford L. M. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev 125, 91–105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi C., Namiki T. & Shinoda T. Kruppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol 325, 341–350 (2009). [DOI] [PubMed] [Google Scholar]
- Fristrom D. & Fristrom J. W. in The development of Drosophila melanogaster (eds M. Bate & A. Arias Martinez) 843–897 (Cold Spring Harbor Laboratory Press, 1993). [Google Scholar]
- Madhavan M. M. & Madhavan K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J Embryol Exp Morphol 60, 1–31 (1980). [PubMed] [Google Scholar]
- Ninov N., Chiarelli D. A. & Martin-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development 134, 367–379 (2007). [DOI] [PubMed] [Google Scholar]
- Ashburner M. Effects of juvenile hormone on adult differentiation of Drosophila melanogaster. Nature 227, 187–189 (1970). [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. & Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol 82, 172–183 (1991). [DOI] [PubMed] [Google Scholar]
- Belles X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 55, 111–128 (2010). [DOI] [PubMed] [Google Scholar]
- Shpigler H. et al.. The transcription factor Kruppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol Biol 10, 120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiblmayr K., Pascual N., Piulachs M. D., Keller T. & Belles X. Juvenile hormone titer versus juvenile hormone synthesis in female nymphs and adults of the German cockroach, Blattella germanica. J. Insect Sci 6, 47 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S. Three classes of C2H2 zinc finger proteins. Cell Mol Life Sci 58, 625–635 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi C., Tanaka M., Miura K. & Tanaka T. Developmental profile and hormonal regulation of the transcription factors broad and Kruppel homolog 1 in hemimetabolous thrips. Insect Biochem Mol Biol 41, 125–134 (2011). [DOI] [PubMed] [Google Scholar]
- Sehnal F., Svacha P. & J Z. in Metamorphosis (eds L. I. Gilbert, J. R. Tata, & B. G. Atkinson) 3–58 (Academic Press, 1996). [Google Scholar]
- Beckstead R. B., Lam G. & Thummel C. S. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol 6, R99 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecasse F., Beck Y., Ruiz C. & Richards G. Kruppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev Biol 221, 53–67 (2000). [DOI] [PubMed] [Google Scholar]
- Wigglesworth V. B. The physiology of insect metamorphosis. (The University Press, 1954). [Google Scholar]
- Tan A., Tanaka H., Tamura T. & Shiotsuki T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci U S A 102, 11751–11756 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps F., Casas J., Sánchez F. J. & Messeguer A. Identification of juvenile hormone III in the hemolymph of Blattella germanica adult females by gas chromatography–mass spectrometry. Arch. Insect Biochem. Physiol. 6, 181–189 (1987). [Google Scholar]
- Ciudad L., Piulachs M. D. & Belles X. Systemic RNAi of the cockroach vitellogenin receptor results in a phenotype similar to that of the Drosophila yolkless mutant. FEBS J 273, 325–335 (2006). [DOI] [PubMed] [Google Scholar]
- Katoh K., Asimenos G. & Toh H. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537, 39–64 (2009). [DOI] [PubMed] [Google Scholar]
- Romaña I., Pascual N. & Bellés X. The ovary is a source of circulating ecdysteroids in Blattella germanica (L.) (Dictyoptera, Blattellidae). Eur. J. Entomol. 92, 93–103 (1995). [Google Scholar]
- Pfaffl M. W., Horgan G. W. & Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30, e36 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


