Abstract
Although the majority of smooth muscle neoplasms found in the uterus are benign, uterine leiomyosarcoma (LMS) is extremely malignant, with high rates of recurrence and metastasis. We earlier reported that mice with a homozygous deficiency for LMP2, an interferon (IFN)-γ-inducible factor, spontaneously develop uterine LMS. The IFN-γ pathway is important for control of tumor growth and invasion and has been implicated in several cancers. In this study, experiments with human and mouse uterine tissues revealed a defective LMP2 expression in human uterine LMS that was traced to the IFN-γ pathway and the specific effect of JAK-1 somatic mutations on the LMP2 transcriptional activation. Furthermore, analysis of a human uterine LMS cell line clarified the biological significance of LMP2 in malignant myometrium transformation and cell cycle, thus implicating LMP2 as an anti-tumorigenic candidate. This role of LMP2 as a tumor suppressor may lead to new therapeutic targets in human uterine LMS.
Smooth muscle tumors (SMTs) are commonly divided into benign leiomyoma (LMA) and malignant leiomyosarcoma (LMS) based on cytological atypia, mitotic activity, and other criteria. Uterine LMS is a very rare gynecologic malignancy in the female genital tract, having an estimated annual incidence of 0.64 per 100,000 women1. LMS accounts for approximately one-third of uterine sarcomas, of which only 53% are confined to the uterus2,3,4,5. Gynecological cancers, which include breast cancer and endometrial carcinomas, are strongly promoted by female hormones, but the rate of hormone receptor expression is reported to be significantly lower in human uterine LMS than in normal myometrium. These low receptor levels were found to correlate neither with the promotion of initial disease development, nor with the overall survival of patients with uterine LMS.
Although uterine LMS is sensitive to certain types of chemotherapy with gemcitabine or docetaxel, it is resistant to hormone therapy and radiotherapy, and thus surgical intervention is virtually the only means of treatment at this time6,7,8. It should be noted that when adjusting for stage and mitotic count, LMS has a significantly worse prognosis than carcinosarcoma11; the 5-year survival rate for patients with uterine LMS is 15%–25%. The development of efficient adjuvant treatments is expected to improve the outcome of this disease through the use of promising new molecular targeting therapies4,5,9,10. The determination of the malignant potential of smooth muscle neoplasms also represents a significant diagnostic conundrum with important therapeutic ramifications. However, the genetic changes underlying the neoplastic transformation of uterine smooth muscle cells have not been fully characterized. Moreover, diagnostic biomarkers that are able to distinguish between LMS and LMA have yet to be established.
The ubiquitin-proteasome degradation pathway is essential for many cellular processes, including cell cycle, regulation of gene expression, and response to oxidative stress. Therefore, individual expression of the low molecular weight protein (LMP)2, LMP7, and LMP10 (MECL-1) subunits are believed to contribute to the initiation and development of disorders. A recent study revealed a unique role for LMP7 in controlling pathogenic immune responses and provided a therapeutic rationale for targeting LMP7 in autoimmune disorders, especially rheumatoid arthritis12. It is also noteworthy that mice with a targeted disruption of LMP2, which is an interferon (IFN)-γ-inducible proteasome subunit, exhibited defects in tissue- and substrate-dependent proteasomal function, and that female LMP2-deficient mice spontaneously developed uterine LMS with a disease prevalence of 37% by 12 months of age13,14 (see Supplementary Fig. S1 online). Defective LMP2 expression is therefore likely to be one of the risk factors in the development of human uterine LMS as it is in LMP2-deficient mice14,15. The importance of the IFN-γ pathway in the transcriptional regulation of the LMP2 promoter has been established in another study, where defective LMP2 expression was attributable to a G871E mutation in the ATP-binding region of JAK1 in a SKN cell line established from a patient with uterine LMS15.
In the present study, we investigated whether LMP2 expression was markedly down-regulated in human uterine LMS tissues in comparison with both LMA and normal myometrium. Biological and histological findings showed that defective LMP2 expression contributed to abnormal cell proliferation, which directly correlated to tumor progression. Disruption of LMP2 expression stemmed from defects in the IFN-γ signaling pathway, specifically from somatic mutations in JAK1. Furthermore, LMP2 expression appeared to be responsible for the suppression of specific transformed phenotypes of human uterine LMS cells in an anti-oncogenic manner. Continued improvement of our knowledge of the molecular biology of uterine LMS may ultimately lead to novel diagnoses and therapies and improved outcome.
Results
Defective LMP2 expression in human uterine LMS
The effects of IFN-γ on LMP2 expression were examined using five cell lines15. LMP2 expression was not markedly induced by IFN-γ treatment in human uterine LMS cells, although cervical epithelial adenocarcinoma cell lines and normal human myometrium showed strong expression of LMP2 following IFN-γ treatment15. Furthermore, immunohistochemistry (IHC) revealed a pronounced loss in the ability to induce LMP2 expression in human uterine LMS tissue in comparison with normal myometrium located in the same section (Fig. 1a,c and see Supplementary Fig. S2 online). Of the 54 patients with uterine LMS that we examined, 46 were negative for LMP2 expression, 4 were focally positive, and 2 were partially positive (Fig. 1c). Two LMS patients were analyzed for LMP2 expression (Fig. 1c). LMP2 levels were also evaluated in skeletal muscle and rectal metastases from individual uterine LMS patients (see Supplementary Fig. S3 online), where surgical samples showed the presence of a mass measuring 3 cm in maximum diameter in the lumbar quadrate muscle without a fibrous capsule. All lymph nodes were negative for LMS metastases, and IHC analyses showed positivity for MIB1(Ki-67) and negativity for LMP2. The defective LMP2 expression detected in primary uterine LMS was also observed in the metastatic LMS of the skeletal muscle and rectum, indicating that the metastatic lesions conserved this biological characteristic of primary uterine LMS. (see Supplementary Fig. S3 online). In both Western blotting and RT-PCR experiments, LMP2 was expressed in normal myometrium but not in human uterine LMS (Fig. 1b), which was strongly supportive of the IHC findings. Although our research group has previously demonstrated that the abnormal expression of the ovarian steroid receptors TP53 and Ki-67 and mutations of TP53 were frequently associated with uterine LMS, defective LMP2 expression appeared to be more characteristic of uterine LMS (see Supplementary Table S1 online).
Figure 1. Defect in LMP2 expression in human uterine leiomyosarcoma (LMS) tissue.
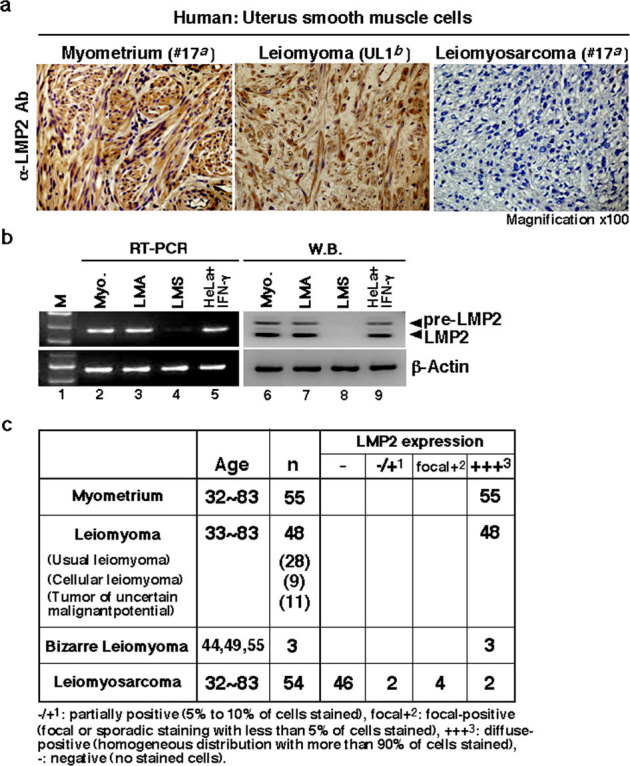
(a) Immunohistochemistry (IHC) of LMP2 in normal myometrium (patient #17a), uterine leiomyoma (LMA, patient UL1b) and uterine LMS (patient #17a) tissues located in the same tissue. For all samples, 5 μm sections of tissue were stained with anti-LMP2 antibody and revealed by peroxidase-conjugated anti-rabbit IgG antibody. (magnification x100) a,bDetails of patients with LMA or LMS are shown in Table. S1 and Fig. S6. (b) Cytosolic extracts were prepared from normal human myometrium (patient #17a), uterine leiomyoma (LMA, patient UL1b), and uterine LMS (patient #17a) tissues. Extracts of 50 μg were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The levels of LMP2 and β-actin were examined by immunoblot analysis with appropriate antibodies. Examinations of mRNA expression for LMP2, β2-m, and β-actin in normal myometrium (Myo.), uterine LMA, and uterine LMS were performed by reverse transcription-polymerase chain reaction (RT-PCR) with the appropriate primers indicated in the Materials and Methods section. The DNA products amplified by RT-PCR were loaded onto agarose gels. aDetails are shown in Table S1 and Table S2, bdetails are shown in Fig. S6. (c) IHC experiments individually performed at several medical facilities revealed a marked loss in the ability to induce LMP2 expression in human uterine LMS tissues compared to that in normal myometrium located in the same tissue section, as well as to that in LMA tissues. Normal total: 55 cases, LMA total: 48 cases, Bizarre Leiomyoma total: 3 cases, LMS total: 54 cases. The experiments were performed three times with similar results.
Key role of the IFN-γ-pathway for LMP2 expression in myometrium tissue
While it has been established that IFN-γ markedly enhances LMP2 production through JAK-STAT signaling, the NF-κB signaling pathway also reportedly induces LMP2 gene expression in an independent manner; inhibition of NF-κB signaling resulted in a decrease in LMP2 expression in human carcinoma cell lines and human lymphocytes16,17,18 (Fig. 2a and see Supplementary Fig. S4 online). However, the essential signaling pathway for LMP2 expression in myometrium is not yet clearly understood. We performed experiments with IFN-γ-deficient mice and TNF-α-deficient mice to elucidate the molecular mechanism of Lmp2 gene expression in myometrium. Although LMP2 expression was detected in several tissues (heart, ventriculus, esophagus, liver) obtained from IFN-γ- and TNF-α-deficient mice at a similar basal expression level as age-matched wild type mice (see Supplementary Fig. S5 online), the myometrium of IFN-γ-deficient mice had non-identical LMP2 expression in comparison with TNF-α-deficient mice and wild-type mice (Fig. 2b). IHC experiments revealed that IFN-γ was especially required for LMP2 expression in myometrium, and Western blotting and RT-PCR showed that IFN-γ-deficient mice had markedly decreased levels of LMP2 (Fig. 2c). Examination of mice lacking RelA or NF-κBp65 could not be performed due to embryonic lethality19.
Figure 2. Key role of the IFN-γ-pathway in LMP2 expression in normal myometrium.
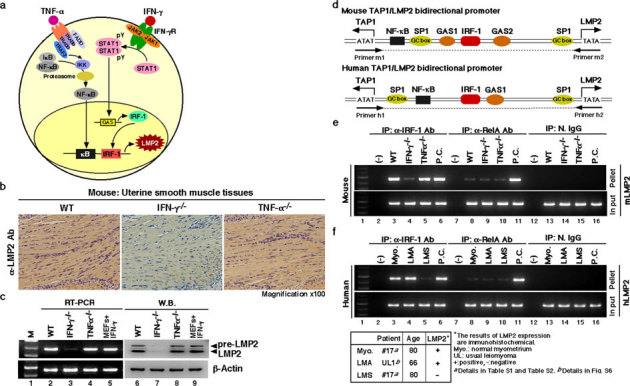
(a) Key role of the signaling pathway on LMP2 expression. (b) Immunohistochemical experiments with myometrium tissue sections derived from wild-type, IFN-γ-deficient, and TNF-α-deficient mice (2 months old) were carried out. (magnification x100) The results revealed that the IFN-γ signaling cascade was required for basal LMP2 expression. (c) Western blotting and RT-PCR experiments with myometrium tissue sections derived from wild-type and IFN-γ- and TNF-α-deficient mice (2 months old) were also performed. The results showed that IFN-γ-deficient mice had markedly reduced LMP2 levels in myometrium tissues. These findings support the notion that the IFN-γ pathway plays a key role in basal LMP2 expression. (d) Schematic representation of the LMP2/TAP1 bidirectional promoter, including NF-κB and IRF-1 binding sites. Chromatin immunoprecipitation (ChIP) analysis with antibodies against RelA (NF-κBp65) and IRF-1 was carried out. (e) ChIP assays showing that although mouse genomic DNA of the Lmp2 enhancer/promoter region was markedly amplified using immunoprecipitated TNF-α-deficient myometrium tissue with anti-IRF-1 antibody, amplified products were not detected using immunoprecipitated IFN-γ-deficient myometrium tissue with anti-IRF-1 antibody. The mouse genomic DNA of the LMP2 enhancer/promoter region was unclearly amplified using the immunoprecipitated materials with anti-RelA antibody. Positive control (P.C.): IFN-γ-treated mouse embryonic fibroblasts (lane 6), TNF-α-treated mouse splenocytes (lane 11). (f) ChIP assays showing that although human genomic DNA of the LMP2 enhancer/promoter region was markedly amplified using immunoprecipitated LMA tissue as well as normal myometrium tissue with anti-IRF-1 antibody, no-DNA amplification was detected in the immunoprecipitated LMS sample with anti-IRF-1 antibody. The DNA of the LMP2 enhancer/promoter region was not clearly amplified using any immunoprecipitated myometrium tissue materials with anti-RelA antibody. Positive control (P.C.): IFN-γ-treated mouse embryonic fibroblasts (lane 6), TNF-α-treated mouse splenocytes (lane 11). N.IgG, normal rabbit anti-serum was used as a negative control antibody. The experiments were performed four times with similar results.
To demonstrate whether LMP2 expression was positively regulated by the IFN-γ pathway in human and mouse myometria, chromatin immunoprecipitation (ChIP) was carried out on uterine organs obtained from patients and mice. IRF-1, an IFN-γ-inducible factor, induced LMP2 gene expression as part of an initiation complex with the LMP2 regulatory region of the genome (Fig. 2d). We next examined whether IRF-1 was necessary for LMP2 gene expression in human and mouse myometria by ChIP assays on uterine organs from patients and IFN-γ- and TNF-α-deficient and age-matched wild type mice. RelA did not clearly bind to the Lmp2 regulatory region of the genome in any mouse group (Fig. 2e). Conversely, IRF-1 bound to the Lmp2 regulatory region in TNF-α-deficient mice and wild-type mice, but a deficiency in IFN-γ resulted in undetectable IRF-1 occupancy in the Lmp2 regulatory region (Fig. 2e). RelA was not detected in the initiation complex with the LMP2 regulatory region in tumor tissue sections, LMA, LMS, or normal myometrium tissue sections derived from patient uterine organs (Fig. 2f). Although IRF-1 bound to the LMP2 regulatory region in normal myometrium and LMA patient tissue sections, LMS tissue sections demonstrated weak IRF-1 occupancy of the LMP2 regulatory region (Fig. 2f and see Supplementary Fig. S6 online). The ubiquitous nuclear factor SP1 directly binds to a GC box and positively regulates basal transcription of LMP2, which is TATA-less gene. SP1 was detected in the initiation complex of the regulatory region of the LMP2 gene in all tested tumor tissue sections (see Supplementary Fig. S6 online). Therefore, IFN-γ signaling was required to allow IRF-1 binding to the LMP2 regulatory region of the genome in human uterine organs. Taken together, these findings demonstrated that the IFN-γ signaling pathway likely played a key role in LMP2 expression in myometrium.
Mutations in IFN-γ signaling molecules in human LMS
Since the IFN-γ pathway was revealed to play a key role in basal LMP2 expression in normal human myometrium (Fig. 2), we next focused on whether the defect in LMP2 expression in uterine LMS was attributable to mutations or deletions in IFN-γ signaling factors. Following IFN-γ binding to the type II IFN receptor, Janus-activated kinase (JAK) 1 and JAK2 are activated and phosphorylate signal transducer and activator of transcription (STAT) 1 on the tyrosine residue at position 701 (Tyr701) and the serine residue at position 727 (Ser727)20,21 (see Supplementary Fig. S4 online). Tyrosine-phosphorylated STAT1 forms homodimers that translocate to the nucleus and bind to IFN-γ-activated site (GAS) elements in the promoters of IFN-γ-regulated genes20,21 (see Supplementary Fig. S4 online). The phosphorylation of Ser727 is required for full transcriptional activation of LMP220,21.
Sequence analysis demonstrated that the loss of IFN-γ responsiveness in a human uterine LMS cell lines was attributable to inadequate JAK1 kinase activity due to a G781E mutation in the ATP-binding region15. Genetic alterations in tyrosine kinases have previously been firmly implicated in tumorigenesis, but only a few serine/threonine kinases are known to be mutated in human cancers21,22,23,24,25,26,27. The examination of 19 LMS tissue sections and patient-matched normal tissue controls was performed to identify somatic (tumor-specific) mutations in the catalytic domains of the IFN-γ-signaling factors JAK1, JAK2, STAT1, and LMP2 enhancer/promoter region. As the catalytic domains of these genes were the most likely to harbor mutations that activate the gene product, we focused on exon stretches containing kinase domains, transcriptional activation domains, and the enhancer/promoter region for the LMP2 gene. The cDNA fragments of these molecules were amplified and directly sequenced. Comparison of cDNA sequences obtained from LMS tissue sections, normal tissue sections, the catalytic domains of JAK1 (JAK1 Accession, M64174.1; JAK2 Accession, AF005216.1; STAT1 Accession, NM_007315), and the LMP2 enhancer/promoter region (NCBI Reference, homo sapiens chromosome 6 NT_007592.15) demonstrated the presence of a total of eight point mutations that were confirmed by re-sequencing in both the sense and antisense directions. Overall, nearly 36.8% (7/19) of uterine LMS tissues had somatic mutations in the ATP-binding region or kinase active site of JAK1 (see Supplementary Fig. S7 online). Furthermore, 31.6% (6/19) of uterine LMS tissues had somatic mutations in essential sites of the LMP2 enhancer/promoter region required for LMP2 transcriptional activation (see Supplementary Table S2–S3 online). No somatic mutations at Tyr701 or Ser727, which are needed for STAT1 transcriptional activation, were found in uterine LMS. Over a quarter (5/19) of uterine LMS tissues unexpectedly had mutations in the STAT1 intermolecular region, an area not yet reported to be of importance for transcriptional activation or other biological functions (see Supplementary Table S2–S3 online). No somatic mutations in the catalytic domains of JAK2 were detected in uterine LMS (see Supplementary Table S2–S3 online). There were 7 of 19 tumors without detectable mutations in the JAK1, JAK2 or LMP2 promoter region in our tested samples, which implied that further experiments might uncover additional somatic mutations in the catalytic subunit (p110) of PI3K or PKC-δ. MOTIF Search profiling28 and NCBI's Conserved Domain Database and Search Service, v2.17 analysis29, revealed that the somatic mutations identified in the catalytic domains of these genes resulted in impaired activation of tyrosine kinases or transcriptional factors.
In a recent report, a comparative genomic hybridization (CGH)-based analysis of LMS patients using a high resolution genome-wide array gave gene-level information about the amplified and deleted regions that may play a role in the development and progression of human uterine LMS. Other reports showed that among the most intriguing changes in genes were losses of JAK1 (1p31-p32) and LMP2 (6p21.3)32,33,34. It has also been demonstrated that a correlation exists between the development of malignant tumors and ethnic background, so we conducted CGH experiments with tissue samples obtained from Japanese patients in order to obtain gene-level information. Our results showed that LMS having a clear functional loss at JAK1 (1p31-p32) and LMP2 (6p21.3) also harbored one nonsense mutation and one deletion, suggesting a possible homozygous loss of function (see Supplementary Fig. S8 online). The discovery of these mutational defects in a key cell-signaling pathway may be important in understanding the pathogenesis of human uterine LMS.
Defective LMP2 expression due to somatic mutations in catalytic domains of JAK1
It appeared likely that IFN-γ-inducible LMP2 expression was impaired due to somatic mutations in the catalytic domains, ATP-binding region, or kinase active site of JAK1 of the IFN-γ-pathway in uterine LMS. To evaluate whether a mutation resulted in the loss of a transcriptional response to IFN-γ, wild-type JAK1 (JAK1wt) or JAK1 mutant expression vectors were transiently introduced into JAK1-null cells. Western blotting and RT-PCR analyses showed that although exogenous JAK1wt restored the IFN-γ inducibility of LMP2 expression, phospho-JAK1(p-JAK1) and phospho-STAT1(p-STAT1) could not be induced by any exogenous JAK1 mutant except JAK1-G986P (Fig. 3). Similarly, electrophoresis mobility shift assays (EMSA) demonstrated that the DNA-binding activity of STAT1 and IRF-1 to specific regions was absent in the presence of any exogenous JAK1 mutant except JAK1-G986P but was rescued by inclusion of JAK1wt (Fig. 3).
Figure 3. Somatic JAK1 mutations prevent LMP2 expression.
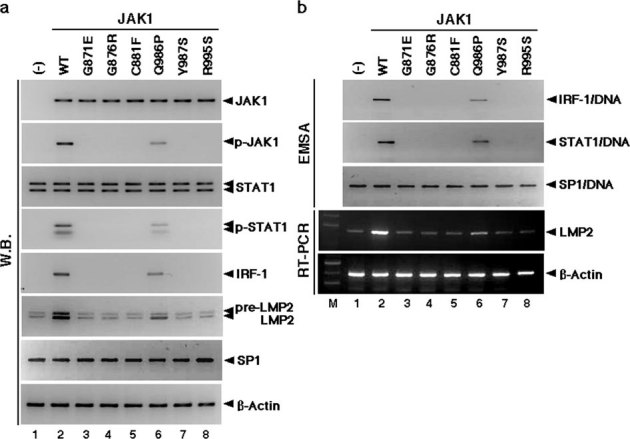
(a) JAK1 mutants resulted in defective activation of downstream IFN-γ pathways, as described in Table S3. Activation of JAK1, STAT1, and IRF-1 for LMP2 expression by IFN-γ in cells expressing JAK1WT and JAK1 mutants. Protein lysates of JAK1-deficient cells expressing wild-type and JAK1 mutants were analyzed by Western blotting (W.B.) using appropriate antibodies. JAK1 and STAT1 phosphorylation after 15 minutes of stimulation was consistently increased in cells expressing JAK1WT and JAK1G986P, but phosphorylated proteins were not detected in cells expressing other JAK1 mutants. IRF-1 and LMP2 expression was markedly activated in cells expressing JAK1WT and JAK1G986P. There was no difference in the expression of JAK1, STAT1, SP1, or β-Actin among cells expressing wild-type and JAK1 mutants. (b) Defective DNA-binding activities of IRF-1 and STAT1 suggest that LMP2 expression is attributable to mutations in the catalytic domains of JAK1. DNA-binding activities of SP1 were detected in all tested samples. RT-PCR supported W.B. results. The experiments were performed three times with similar results. Direct sequencing demonstrated that the mutations detected in the catalytic sites were indeed somatic mutations (details in Supplementary Fig. S7 online).
Immunoblotting revealed that IFN-γR1, a component of the IFN-γ receptor, was expressed in LMS tissue sections at levels similar to those in normal tissue sections from patients (see Supplementary Fig. S9 online). Since the uterine LMS tissue sections had levels of surface IFN-γR1 chains comparable to those in normal tissue sections derived from patient uterine organs, the loss of IFN-γ responsiveness could not be attributable to inadequate surface expression of this component. IHC experiments with antibodies against JAK1 and STAT1 showed strongly positive cells in uterine LMS tissue sections and myometrium (see Supplementary Fig. S10 online). In contrast, although p-STAT1 was weakly positive in normal myometrium tissue sections, it was essentially negative in uterine LMS tissue sections from the same patients (see Supplementary Fig. S10 online). Therefore, the loss of IFN-γ responsiveness in uterine LMS may have been due to the inadequate kinase activity of JAK1 stemming from mutations in its catalytic domains.
Biological significance of hLMP2 in uterine leiomyosarcoma tumorigenesis
Although cell proliferation has been demonstrated to be strongly inhibited by IFN-γ-induced JAK1 kinase activation35, it is difficult to demonstrate tumorigenicity in JAK1-deficient mice because they die perinatally36. Therefore, the differential responsiveness to genetically modified stable LMP2 expression of the SKN human uterine LMS cell line was investigated to determine whether reintroducing LMP2 to a LMS cell line would affect its tumorigenic properties for development of uterine LMS and if the observed effect was due to the immunoproteosomal function of the protein. SKN cells (approximately 5×l05 cells) were transfected with 5 μg of control vector DNA (pCEM9), pCEM9-LMP2wt, or pCEMp-LMP2K33A, which has no effect on immunoproteasome function due to non-incorporation into the 20 S proteasome (see Supplementary Fig. S11 online), and selected in medium containing 1 mg/ml of G418. The efficiencies of neo-marker transfer with these three plasmids were comparable (see Supplementary Table S4 online). However, in the case of pCEM9-LMP2wt or pCEM-LMP2K33A, approximately 78% (LMP2wt) or 76% (LMP2K33A) of the total G418-resistant colonies were relatively compact and appeared dark when observed under a phase-contrast microscope after 6–7 days of selection. These partially flat (P/F type) colonies consisted of cells with increased attachment to the substrate, while the majority of other transformed (T type) colonies looked similar in cell morphology to the cells observed in the control dishes, albeit slightly smaller in size (Fig. 4a and see Supplementary Fig. S12 online). After 2 to 3 weeks when most of the colonies outgrew and detached from the substrate, some colonies consisting of flatrevertant (F type) cells were found (Fig. 4a and see Supplementary Fig. S12–S13 online) at frequencies of approximately 29.5% (pCEM9-LMP2wt) or 24.8% (pCEM-LMP2K33A) of the total number of G418-resistant colonies initially observed (see Supplementary Table S4 online). No colonies of this flatrevertant morphology were observed in the cultures transfected with control DNA (Table 1 and see Supplementary Table S4 online).
Figure 4. Biological activity of hLMP2 in uterine leiomyosarcoma (LMS).
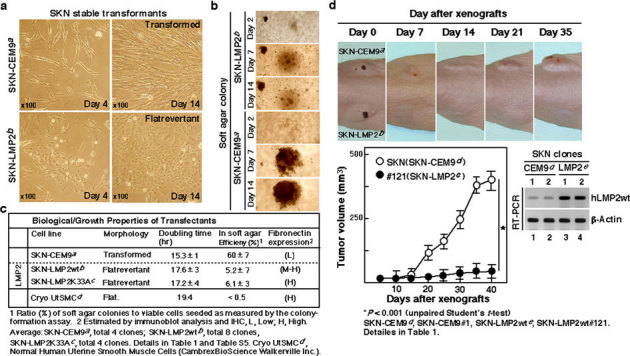
(a) Phase-contrast micrographs of the parental transformed SKN-CEM9 (T type) clone and flat revertants of the SKN-LMP2 (F type) clone. (magnification x100) (b) Phase-contrast micrographs of the colony formations of the SKN-CEM9 (T type) clone and SKN-LMP2 (F type) clone in soft agar. (magnification x40) (c) The table indicates the biological properties of the transfectants, whose details are described in Table 1 and Supplementary Table 5. (d) Changes in the human uterine LMS cell line, SKN transfectants, the SKN-CEM9 (T type) clone, and the SKN-LMP2 (F type) clone xenograft volumes in mice (n = 8). Representative photographs of xenografts in mice (Upper panel). Tumor growth of SKN-LMP2 was markedly reduced in comparison with that of the control transfectant SKN-CEM9 (T type) clone. Tumor growth kinetics after subcutaneous injection of the SKN-CEM9 (T type) clone and SKN-LMP2 (F type) clone (Lower panel). RT-PCR experiments reveal hLMP2 mRNA expression in tumors (Lower right panel). The experiments were performed four times with similar results.
Table 1. Biological Properties of the Transfectants.
| Cell line | Molphology | PDTa | SoftAgarColony Efficiency(%)bSizec | Tumori-genecityd | Protein Expressione | Inco LMP2f | iTNF-α Cell death | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMP2 | LMP7 | Calponin | Fibronectin | ||||||||
| SKN | hTransformed | 15.0 | 53 | 11–29 | +++ | W | L | - | L | - | Sen. |
| SKN-CEM9#1 | Transformed | 15.2 | 67 | 10–26 | +++ | W | L | - | L | - | Sen. |
| SKN-CEM9#2 | Transformed | 15.2 | 63 | 11–28 | ++ | W | L | - | L | - | Sen. |
| LMP2wt#230 | P.Flat. | 15.4 | 6.2 | 5–9 | + | M | L | L | M | + | Resi. |
| LMP2wt#231 | P.Flat. | 16.8 | 6.4 | 6–10 | + | M | L | L | M | + | Resi. |
| LMP2wt#196 | Flatrevertant | 17.3 | 5.4 | 4–9 | + | H | H | M | H | + | Resi. |
| LMP2wt#198 | Flatrevertant | 17.4 | 5.6 | 5–10 | + | H | H | H | H | + | Resi. |
| LMP2wt#120 | Flatrevertant | 17.5 | 4.9 | 4–11 | +/- | H | H | M | H | + | Resi. |
| LMP2wt#121 | Flatrevertant | 17.3 | 4.7 | 5–9 | +/- | H | M | M | H | + | Resi. |
| LMP2wt#122 | Flatrevertant | 17.6 | 5.4 | 4–12 | + | H | M | M | M | + | Resi. |
| LMP2wt#124 | Flatrevertant | 17.5 | 5.9 | 6–10 | + | H | M | M | H | + | Resi. |
| LMP2wt#125 | Flatrevertant | 17.8 | 5.8 | 5–12 | +/- | H | M | M | H | + | Resi. |
| LMP2wt#127 | Flatrevertant | 17.3 | 4.5 | 5–10 | + | H | M | M | H | + | Resi. |
| LMP2K33A#1 | Flatrevertant | 17.5 | 6.2 | 5–13 | + | H | M | M | H | - | Sen. |
| LMP2K33A#2 | Flatrevertant | 16.8 | 6.1 | 6–11 | +/- | H | M | M | H | - | Sen. |
| LMP2K33A#3 | Flatrevertant | 17.3 | 5.9 | 5–10 | +/- | H | M | M | H | - | Sen. |
| LMP2K33A#4 | Flatrevertant | 17.2 | 6.4 | 6–8 | + | H | M | M | H | - | Sen. |
| CryoUTSMCg | Flatrevertant | 19.4 | <0.5 | - | - | M | M | H | H | - | Resi. |
aPDT: Population Doubling Time.
bRatio(%) of soft agar colonies/number of cells plated that have an ability to form colonies on plastic substrate.
cDiameter of colonies as expressed by the number of cells lined up across the colonies; determined on day 21. The experiments were repeated three times with similar results.
dCells (1x107) were inoculated subcutaneously into 7–8-weeks old nude mice, and the mice were periodically examined for evidence of tumors, -, no evidence of tumors; +/-, tumors of <0.5 cm diameter; +, tumors of 0.5–1.0 cm diameter; ++, tumors of 1.0–1.5 cm diameter, +++, tumors of 1.5–2.0 cm diameter, in two inoculated mice. Experiments were terminated at 5 weeks after inoculation.
eEstimated by immunoblot analysis. W, weak expression; L, low expression; M, medium expression; H, high expression; -, no evidence of expression.
fIncorporation of LMP2 into proteasome complexes. Proteasome was isolated by GST-UbL beads in accordance with manufacturer's recomendation (Merck Ltd. Darmstadt, Germany). Immunoblot analysis was performed. -, no evidence of expression; +, Detectable expression of LMP2 by immunoblot analysis.
gCryo UtSMC: normal human uterine liomyosarcoma cell line (Cambrex BioScience Walkersville, Inc. MD, USA).
hMorphology. After 2 to 3 weeks, when most of the colonies outgrew and detached from the substrate, some colonies consisting of very flat cells were found at frequencies of around a few percent of the total number of G418-resistant colonies initially observed in Table S4. Microscopic characteristics such as flat cell morphology and transformed cell morphology were analyzed. The number of flat cells or transformed cells in 10 consecutive high-power-fields was counted. Transformed, no evidence about appearance of flat cells; Flatrevertant, transformed cell number is less than 10% of total cell number; P.Flat., Partially Flatrevertant, transformed cell number is less than 30% of total cell number; P.Tras., Partially Transformed, transformed cell number is higher than 30% of total cell number.
iTNF-α induced cell death. Sen., Sensitive to TNF-α-induced cell death; Resi., Resistant to TNF-α-induced cell death.
We next isolated and expanded representative colonies of each type and analyzed their growth properties and the occurrence and expression of LMP2. The growth rate, expressed as doubling time, of typical SKN-LMP2wt (F type) colonies (8 clones) or SKN-LMP2K33A (F type) colonies (4 clones) was generally lower than that of SKN-LMP2wt (P/F type) colonies (2 clones) and control SKN-CEM9 (T type) colonies (4 clones) (Fig. 4c, Table 1 and see Supplementary Table S5 online). FACS analysis demonstrated that LMP2 expression might have induced G1 arrest in the cell cycle of SKN-LMP2 (F type) colonies (see Supplementary Fig. S14 online).
In trials evaluating the anti-tumorigenic properties of LMP2wt or LMP2K33A, the efficiency of colony formation and size of the colonies in soft agar were greatly reduced in the F type clones and P/F type clones and were significantly reduced in the morphologically similar T type SKN-LMP2wt or SKN-LMP2K33A transfectant clones compared with those in the SKN-CEM9 (T type) clone (Fig. 4b,c, Table 1 and see Supplementary Table S5 online). Tumor growth was clearly observed in control mice inoculated with the SKN-CEM9 (T type) clones; however, a reduction in tumor growth was observed in mice inoculated with the SKN-LMP2wt (F type) clone or SKN-LMP2K33A (F type) clone (Fig. 4d, Table 1 and see Supplementary Fig. S15 online). Since both wild type and mutant LMP2 blocked tumorigenesis, it became necessary to rule out a toxic effect of LMP2 overexpression in a control cancer cell line. Additional experiments demonstrated no toxic effect of either wild type LMP2 or mutant LMP2K33A overexpression in a HeLa control cancer cell line (see Supplementary Fig. S16 and Supplementary Table S6–S7 online). It is noteworthy that although calponin h1 may function as a tumor suppressor in uterine LMS, calponin h1-deficient mice do not exhibit uterine LMS37,38. To examine the connection between LMP2 and calponin h1 in tumorigenesis, we analyzed the expression pattern of calponin h1 in SKN-CEM9 (T type) clones and SKN-LMP2wt (F type) clones. Our results revealed that calponin h1 expression was dependent on the presence of LMP2, suggesting that the anti-oncogenic function of calponin h1 may be involved with that of LMP2 in human uterine LMS (Table 1 and see Supplementary Table S5 online).
The dysregulation of apoptotic mechanisms has also been associated with many human malignancies. Whereas the mechanical link of the NF-κB family with LMP2 for anti-apoptotic functions has been reported, other lines of evidence suggest that NF-κB activity is modulated by tumor suppressors, including TP53 and ARF, whereby NF-κB subunits repress, rather than activate, the expression of tumor-promoting genes39,40,41,42,43,44. The nuclear NF-κB activation in LMS tissue samples was reduced as in SKN-CEM9 (T-type) clones in comparison with myometrium and LMA tissue samples, and thus they were susceptible to TNF-α-induced apoptosis due to defective NF-κB expression. Tumors grew more slowly in mice inoculated with the SKN-p50p65 (T type) clones, which had exogeous NF-κB function, compared with SKN-CEM9 (T type) clones (see Supplementary Table S5 online). These findings suggested that NF-κB activation was not involved in the tumorigenesis of human uterine LMS, and strengthened the overall notion that the suppression of cell proliferation, tumorigenesis, and morphological change of uterine LMS cells observed under stable LMP2 expression was attributable to the biological function of single-molecule LMP2 only, without involvement of its immunoproteasome function.
Discussion
Recent molecular targeting therapies against tumors have achieved remarkable results. To improve the prognosis of human uterine LMS, studies are being performed to identify the roles of key pro- and anti-oncogenic factors that have important functions in tumor pathogenesis and may serve as molecular targets for treatment. For this purpose, several research groups have shown that cell cycle regulatory factors and known pro-oncogenic factors, such as the brain-specific polypeptide PEP-19 and c-kit, may be associated with the pathogenesis of human uterine LMS45,46,47,48.
LMP2-deficient mice are reportedly prone to spontaneous development of uterine LMS14. The percentage of mice with overt tumors increased with age after six months, with a cumulative disease prevalence of 40% in female mice by 14 months of age and no apparent plateau at this late observation time. In the present study, histopathological experiments demonstrated a high correlation between a loss of LMP2 and malignancy of uterine tumors developing in myometrium. Thus, LMP2 may constitute a diagnostic biomarker that is able to distinguish between LMS and LMA. Recent reports have also shown a loss in the IFN-γ-inducible expression of LMP2 in a LMS culture cell line13. It is intriguing that only LMP2-deficient mice spontaneously develop uterine LMS, and the individual expression of LMP2, LMP7, and LMP10/MECL-1 subunits have been reported to contribute to the initiation and development of disorders12,14,15. Our histopathological examinations with IFN-γ-deficient and TNF-α-deficient mice revealed that the IFN-γ pathway is especially required for LMP2 expression in normal myometrium. Therefore, organ-specific LMP2 functions might be one of the factors influencing disease development in mice and humans.
The functionally inactivated K33A mutant of LMP2 has the same morphology in vitro as the wt transfectant, suggesting that the action of LMP2 is not only through its role in immunoproteasomes, but also as a single subunit. The relative amounts of LMP2 and LMP7 messaging vary significantly among tissues of mice and humans, as well as among different cell lines49. Given the fact that LMP2 levels in proteasomes showed greater variation among tissues than did LMP7 levels, it may be possible that the constitutive levels of both proteins can also differ in the proteasomes of mouse and human tissues49. The proteasome subunits that are not incorporated into complexes are believed to individually mediate gene transcriptional activation together with other co-factors50; for instance, LMP2 is also reportedly required for estrogen receptor-mediated gene transcription as well as for estrogen-stimulated cell cycle progression51. Other reports have demonstrated the nuclear localization of single-unit LMP2 in various mammalian tissues and cell types49,52, but the biological functions of LMP2 in the nucleus are not clearly understood. Our additional results have revealed an interaction between LMP2wt or LMP2K33A with RNA polymerase II holoenzymes that is capable of responding to a transcriptional activator or co-factor, suggesting a model in which activators function, in part, through direct interactions with the holoenzyme. Unfortunately, no evidence of LMP7 association with holoenzyme components was observed. Further experiments may provide a more complete description of the tumorigenic phenotype of SKN cells by nonproteosomal LMP2.
Genetic alterations in tyrosine kinases have been firmly implicated in tumorigenesis, but only a few serine/threonine kinases are known to be mutated in human cancers53,54,55. For example, mice carrying a homozygous deletion of Pten alleles developed widespread smooth muscle cell hyperplasias and abdominal sarcomas, and JUN oncogene amplification and overexpression blocked adipocytic differentiation in highly aggressive sarcomas56,57,58,59. The molecular basis for genetic alterations remains unclear, and therefore we performed genetic studies to explore such a genotype-tumorigenesis correlation. Our findings demonstrated that the IFN-γ signaling pathway played a key role in LMP2 expression in myometrium. Intriguingly, we observed a significantly higher representation of somatic mutations in the catalytic domain of JAK1 in uterine LMS compared with patient-matched myometrium. Although heterozygosity for somatic mutations was demonstrated to be significantly more frequent in uterine LMS, the evaluation of a genotype-tumorigenesis correlation might not be statistically significant between heterozygotes and homozygotes due to our small sample size. In addition, genetic alteration in other malignant tumors reportedly occur in genes of the MAPK signaling, p53 signaling, Wnt signaling, cell cycle, and mTOR pathways53,54,55,56,57,58,59. Thus, genetic analyses of other signaling pathways are also needed to explore the genotype-tumorigenesis correlation for this disease. From our data, we can infer a heterogeneous genetic background of JAK somatic mutations, but we cannot definitively conclude tumorigenesis by genotype.
Proteasome subunits are assembled in the cytoplasm, and most subunits, including LMP2, do not contain a nuclear localization signal. It is thus unclear how proteasomes enter the nucleus57,58. LMP2 molecules reportedly associate with cellular factor(s) and then regulate cellular processes, including cell cycle and the regulation of gene expression. Recent studies indicate that a tuned balance of proteolysis by LMP2-incorporating proteasomes and cellular function(s) by LMP2-associated complexes with cellular factor(s) is instrumental for normal cellular processes and, when deregulated, leads to the development of disease. The downregulation of MHC-related molecules, including LMP2, is one of the biological mechanisms used by tumor cells to evade host immunosurveillance60. Furthermore, LMP2 expression appears to suppress cell transformation, proliferation, and tumorigenesis in uterine LMS cells, which suggests that LMP2 plays a key role as a tumor suppressor in uterine LMS.
Defective LMP2 expression is likely to be one of the risk factors in the development of human uterine LMS as it is in the LMP2-deficient mouse. Overall, nearly 96% (52/54) of our uterine LMS patients had alterations in LMP2 expression, and thus this protein represents a strong candidate target for therapeutic intervention. As there remains no effective therapy for unresectable uterine LMS, our results may lead to the development of diagnostic approaches and specific molecular therapies to treat this disease.
Methods
Immunohistochemistry (IHC)
IHC staining for LMP2, ER, PR, p53, and Ki-67 was performed on serial human uterine LMS sections. Antibodies for ER (ER1D5), PR (PR10A), p53 (DO-1), and Ki-67(MIB-1) were purchased from Immunotech (Marseille, France). The antibodies for Stat1, p-Stat1, and NF-κBp50 were purchased from Santa Cruz Biotechnology Inc. (CA, USA). The antibody for Jak1 was purchased from Chemicon International (CA, USA). The antibody for NF-κBp65(RelA) was purchased from Applied Biological Materials, Inc. (BC, Canada). The antibody for fibronectin was purchased from Rockland Inc. (PA, USA). The antibody for α-Smooth muscle actin (SMA) was purchased from Covance Research Products, Inc. (Princeton, New Jersey). The LMP2 antibody was produced by SIGMA-Aldrich Israel Ltd. (Rehovot, Israel) (see Supplementary Fig. 2 online). IHC was performed using the avidin-biotin complex method as described previously15,41. Briefly, one representative 5-μm tissue section was cut from a paraffin-embedded sample of a radical hysterectomy specimen from each patient with uterine LMS. Uterine tissue sections from adult C57BL/6J, IFN-γ−/−, and TNF-α−/− mice on C57BL/6J genetic backgrounds (10 weeks old), which were donated by Dr. Y. Tagawa (Tokyo Inst. of Tech., Yokohama, Japan, details in Supplemental Material), were fixed with 1% buffered formalin, and paraffin sections of mouse uterine tissues were analyzed using IHC staining with and without either antibody to LMP2 or normal rabbit IgG as a negative control. Sections obtained from patients or gene-deficient mice were deparaffinized and rehydrated in graded concentrations of alcohol, incubated with normal mouse serum for 20 min, and then incubated at room temperature for 1 h with primary antibody. Afterwards, sections were incubated with a biotinylated secondary antibody (Dako, CA, USA) and then exposed to a streptavidin complex (Dako). The completed reaction was revealed by 3, 3′-diaminobenzidine, and the slide was counterstained with hematoxylin. Normal myometrium portions in the specimens were used as positive controls. Negative controls consisted of tissue sections incubated with normal rabbit IgG instead of the primary antibody. These experiments were registered at Shinshu University in accordance with local guidelines (approval no. M192).
Reverse Transcription-polymerase Chain Reaction Analysis (RT-PCR)
The expression of LMP2 and β-actin transcripts was examined using RT-PCR. Total RNA was prepared from human uterine LMS tissues and normal myometrium tissues using TRIzol reagent according to the manufacturer's protocol (Invitrogen Co., CA, USA). The RNA was reverse-transcribed with the Superscript II enzyme (Invitrogen Co.), and single stranded cDNA was used for amplification. LMP2 and β-actin transcripts were subjected to PCR with the appropriate primer sets following a program of 35 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1.5 min with an additional 5 min for the extension of transcripts15. The primers were used as follows: LMP2 (F 5′-gggatagaactggagg aacc-3′, R 5′-agatgacacccccgcttgag-3′), LMP7 (F 5′-gaacacttatgcctacggggtc-3′, R 5′-tttctactttcac ccaaccatc-3′), Calponin h1 (F 5′-ggaaggcagctgaggttgtg-3′, R 5′-cccaagct cagggctctggcctggc-3′), and β-Actin (F 5′-tccggagacggggtca-3′, R 5′-cctgcttgctgatcca-3′). These experiments were conducted at Shinshu University in accordance with local guidelines (approval no. 4737, no. 150, and no. M192).
Chromatin Immunoprecipitation (ChIP) Analysis
Cells prepared from tissues were washed in PBS with protease inhibitors and centrifuged. The cells were resuspended in 1% SDS, 10 mM EDTA, and 50 mM Tris-HCl, pH 8.0, with protease inhibitors and sonicated. Samples were diluted (1/10 in 0.01% SDS, 1.1% Triton-X100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 8.0, and protease inhibitor), precleared with protein G beads and salmon sperm DNA, and centrifuged. The supernatants were incubated with primary antibody against the protein of interest between 4 h and overnight at 4°C. Antisera were anti-RelA (Upstate, MA), anti-IRF-1 (Santa Cruz, CA, USA), anti-SP1 (Santa Cruz) or normal rabbit IgG (Santa Cruz). After multiple washes, samples were eluted and cross-links reversed with 5 μl of 5 M NaCl. Samples were purified before PCR amplification. Primer sequences were as follows: human LMP2 (F 5′-cgagaagctcagccatttaggggaaag cga-3′, R 5′-cgcccgcagcatccctgcaaggcaccgctc-3′) and mouse Lmp2 (F 5′-tgcgcagccatcgagcgtga gctgtccaga-3′, R 5′-gcccgcagcatctca gcagagcggggctcg-3′).
Author Contributions
TH and AH performed most of the experiments and contributed to data analysis, discussion, and writing of the paper; KS, NH, MK, TI, TS, OI, RN, NY and YK performed all pathological analyses; HA performed all gene-profiling analyses; YT contributed new reagents/analytic tools; TH, TS and IK designed and supervised the study, secured funding, and analyzed the data. TH and AH contributed equally to this work.
Supplementary Material
Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy
Acknowledgments
We sincerely appreciate the donation of LMP2-deficient breeding mice and scientific cooperation of Prof. Susumu Tonegawa (Picower Institute of Learning and Memory, M.I.T). We thank Dr. Isamu Ishiwata (Ishiwata Clinic, Ibaraki, Japan) for generously providing the uterine LMS cell line and also thank Dr. Yoshi Adachi (Shinshu University, Nagano, Japan) for generously providing the uterine HeLa cell line. We also thank BEX Corporation (Tokyo, Japan) for assistance with constructing plasmid vectors. We also appreciate Mr. Trevor Ralph for critical reading the manuscript. This work was supported by grants from the Ministry of Education, Culture, Science and Technology, the Japan Science and Technology Agency, The Foundation of Takeda Medical Research, The Foundation for the Promotion of Cancer Research, Kanzawa Medical Research Foundation, and The Ichiro Kanehara Foundation.
References
- Zaloudek C. & Hendrickson M. R. Mesenchymal tumors of the uterus, in Kurman RJ. (ed): Blaustein's Pathology of the Female Genital Tract (ed 5). New York, Springer-Verlag 561–578 (2002). [Google Scholar]
- Gadducci A., et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol. Oncol. 62, 25–32 (1996). [DOI] [PubMed] [Google Scholar]
- Nordal R. & Thoresen S. Uterine sarcomas in Norway 1956–1992: incidence, survival and mortality. Eur. J. Cancer 33, 907–311 (1997). [DOI] [PubMed] [Google Scholar]
- Brooks S. E., Zhan M., Cote T. & Baquet C. R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 93, 204–208 (2004). [DOI] [PubMed] [Google Scholar]
- Dusenbery K. E., Potish R. A., Argenta P. A. & Judson P. L. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am. J. Clin. Oncol. 28, 295–300 (2005). [DOI] [PubMed] [Google Scholar]
- Wu T. I., et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol. Oncol. 100, 166–172 (2006). [DOI] [PubMed] [Google Scholar]
- Leitao M. M., et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer 101, 1455–1462 (2004). [DOI] [PubMed] [Google Scholar]
- Perez E. A., Pusztai L. & Van de Vijver M. Improving patient care through molecular diagnostics. Semin. Oncol. 31, 14–20 (2004). [DOI] [PubMed] [Google Scholar]
- Lin J. F. & Slomovitz B. M. Uterine sarcoma. Curr. Oncol. Rep. 10, 512–518 (2008). [DOI] [PubMed] [Google Scholar]
- Amant F., Coosemans A., Debiec-Rychter M., Timmerman D. & Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 10, 1188–1198 (2009). [DOI] [PubMed] [Google Scholar]
- Miettinen M. & Fetsch J. F. Evaluation of biological potential of smooth muscle tumours. Histopathology 48, 97–105 (2006). [DOI] [PubMed] [Google Scholar]
- Muchamuel T., et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature Med. 15, 781–788 (2009). [DOI] [PubMed] [Google Scholar]
- Van Kaer L. et al. Altered peptidase and viral-specifi c T cell response in LMP2 mutant mice. Immunity 1, 533–541 (1994). [DOI] [PubMed] [Google Scholar]
- Hayashi T. & Faustman D. L. Development of spontaneous uterine tumors in low molecular mass polypeptide-2 knockout mice. Cancer Res. 62, 24–27 (2002). [PubMed] [Google Scholar]
- Hayashi T., Kobayashi Y., Kohsaka S. & Sano K. The mutation in the ATP-binding region of JAK1, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene 25, 4016–4026 (2006). [DOI] [PubMed] [Google Scholar]
- Marques J., Brucet M., Lloberas J. & Celada A. STAT1 regulate lipopolysaccharide- and TNF-α- dependent expression of transporter associated with antigen processing 1 and low molecular mass polypeptide 2 genes in macrophages by distinct mechanisms. J. Immunol. 173, 1103–1110 (2004). [DOI] [PubMed] [Google Scholar]
- Sizemore N., et al. Inhibitor of κB kinase is required to activate a subset of interferon-γ-stimulated genes. Proc. Natl. Acad. Sci. USA 101, 7994–7998 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas A., et al. CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-κB and of NF-κB-mediated de novo synthesis of IRF-1. Mol. Cell Biol. 28, 6208–6222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Sha W. C., Bronson R. T., Ghosh S. & Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376, 167–170 (1995). [DOI] [PubMed] [Google Scholar]
- Parmar S. & Platanias L. C. Interferons. Cancer Treat. Res. 126, 45–68 (2005). [DOI] [PubMed] [Google Scholar]
- Platanias L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature Rev. Immunol. 5, 375–386 (2005). [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P. & Hunter T. Oncogenic kinase signalling. Nature 411, 355–365 (2001). [DOI] [PubMed] [Google Scholar]
- Bardelli A., et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science 300, 949 (2003). [DOI] [PubMed] [Google Scholar]
- Futreal P. A., et al. A census of human cancer genes. Nature Rev. Cancer 4, 177–183 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P., et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 431, 525–526 (2004). [DOI] [PubMed] [Google Scholar]
- Wang Z., et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science 304, 1164–1166 (2004). [DOI] [PubMed] [Google Scholar]
- Parsons D. W., et al. Colorectal cancer: Mutations in a signalling pathway. Nature 436, 792 (2005). [DOI] [PubMed] [Google Scholar]
- Hernando E., et al. The AKT-mTOR. pathway plays a critical role in the development of leiomyosarcomas. Nature Med. 13, 748–753 (2007). [DOI] [PubMed] [Google Scholar]
- Mariani O., et al. JUN. Oncogene Amplification and Overexpression Block Adipocytic Differentiation in Highly Aggressive Sarcomas. Cancer Cell 11, 361–374 (2007). [DOI] [PubMed] [Google Scholar]
- MOTIF Search profiling. http://motif.genome.jp
- NCBI's Conserved Domain Database and Search Service, v2.17 analysis. http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml
- Larramendy M. L., Kaur S., Svarvar C., Böhling T. & Knuutila S. Gene copy number profiling of soft-tissue leiomyo- sarcomas by array comparative genome hybridization. Cancer Genet. Cytogen 169, 94–101 (2006). [DOI] [PubMed] [Google Scholar]
- Svarvar C., et al. Do DNA copy number changes differentiate uterine from non-uterine leiomyosarcomas and predict metastasis? Modern Pathol. 19, 1068–1082 (2006). [DOI] [PubMed] [Google Scholar]
- Beck A. H., et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene 29, 845–854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexl V., et al. Jak1 deficiency leads to enhanced Abelson-induced B-cell tumor formation. Blood 101, 4937–4943 (2003). [DOI] [PubMed] [Google Scholar]
- Rodig S. J., et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93, 373–383 (1998). [DOI] [PubMed] [Google Scholar]
- Horiuchi A., et al. Reduced expression of calponin h1 in leiomyosarcoma of the uterus. Lab. Invest. 78, 839–846 (1998). [PubMed] [Google Scholar]
- Hriuchi A., Nikaido T., Taniguchi S. & Fujii S. Possible role of calponin h1 as a tumor suppressor in human uterine leiomyosarcoma. J. Natl. Cancer Inst. 91, 790–796 (1999). [DOI] [PubMed] [Google Scholar]
- Greten F. R. & Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 206, 193–199 (2004). [DOI] [PubMed] [Google Scholar]
- Pacifico F. & Leonardi A. NF-kappaB in solid tumors. Biochem. Pharmacol. 72, 1142–1152 (2006). [DOI] [PubMed] [Google Scholar]
- Perkins N. D. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 14, 64–69 (2004). [DOI] [PubMed] [Google Scholar]
- Chen F. & Castranova V. Nuclear factor-kappaB, an unappreciated tumor suppressor. Cancer Res. 67, 11093–11098 (2007). [DOI] [PubMed] [Google Scholar]
- Hayashi T. & Faustman D. Essential role of human leukocyte antigen-encoded proteasome subunits in NF-κB activation and prevention of tumor necrosis factor-alpha-induced apoptosis. J. Biol. Chem. 275, 5238–5247 (2000). [DOI] [PubMed] [Google Scholar]
- Wang H. X., et al. Proteasome subunit LMP2 is required for matrix metalloproteinase-2 and -9 expression and activities in human invasive extravillous trophoblast cell line. J. Cell Physiol. 206, 616–623 (2006). [DOI] [PubMed] [Google Scholar]
- Kanamori T., et al. PEP-19 overexpression in human uterine leiomyoma. Mol. Hum. Reprod. 9, 709–717 (2003). [DOI] [PubMed] [Google Scholar]
- Wang L., et al. The proto-oncogene c-kit is expressed in leiomyosarcomas of the uterus. Gynecol. Oncol. 90, 402–406 (2003). [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja S. K., et al. Analysis of fumarate hydratase mutations in a population- based series of early onset uterine leiomyosarcoma patients. Int. J. Cancer 119, 283–287 (2006). [DOI] [PubMed] [Google Scholar]
- Mittal K. R., et al. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod. Pathol. 10, 1303–1311 (2009). [DOI] [PubMed] [Google Scholar]
- Frentzel S., et al. The major histocompatibility complex encoded beta-type proteasome subunits LMP2 and LMP7. Evidence that LMP2 and LMP7 are synthesized as proproteins and that cellular levels of both mRNA and LMP-containing 20S proteasomes are differentially regulated. Eur. J. Biochem. 216, 119–26 (1993). [DOI] [PubMed] [Google Scholar]
- Choi H. S., Seol W. & Moore D. D. A component of the 26S proteasome binds on orphan member of the nuclear hormone receptor superfamily. J. Steroid Biochem. Biol. 56, 23–30 (1996). [DOI] [PubMed] [Google Scholar]
- Zhang H., et al. The catalytic subunit of the proteasome is engaged in the entire process of estrogen receptor-regulated transcription. EMBO J. 25, 4223–33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits E. A., Benham A. M., Plougastel B., Neefies J. & Trowsdale J. Dynamics of proteasome distribution in living cells. EMBO J. 16, 6087–94 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futreal P. A., et al. A census of human cancer genes. Nature Rev. Cancer 4, 177–183 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E., Sawada K. & Salgia R. Tyrosine kinase mutations in human cancer. Curr. Mol. Med. 7, 77–84 (2007). [DOI] [PubMed] [Google Scholar]
- Pajares M. J., et al. Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol. 8, 349–357 (2007). [DOI] [PubMed] [Google Scholar]
- Hernando E., et al. The AKT-mTOR. pathway plays a critical role in the development of leiomyosarcomas. Nature Med. 13, 748–753 (2007). [DOI] [PubMed] [Google Scholar]
- Mariani O., et al. JUN. Oncogene Amplifi cation and Overexpression Block Adipocytic Differentiation in Highly Aggressive Sarcomas. Cancer Cell 11, 361–374 (2007). [DOI] [PubMed] [Google Scholar]
- Barretina J., et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nature Genet. 8, 715–721 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibon F., et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nature Med. 16, 781–787 (2010). [DOI] [PubMed] [Google Scholar]
- Mikecz A. V. The nuclear ubiquitin-proteasome system. J. Cell Science 119, 1977–1984 (2006). [DOI] [PubMed] [Google Scholar]
- Swann J. B. & Smyth M. J. Immune surveillance of tumors. J. Clin. Inve. 117, 1137–1196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy


