Triple staining of γ-tubulin, microtubules, and nuclei reveal that three types of MTOCs initiate spindles in bryophytes. Polar organizers in liverworts and plastid MTOCs in hornworts are unique and nuclear envelope MTOCs in mosses appear like those in seed plants.
Abstract
Background and aims
As remnants of the earliest land plants, the bryophytes (liverworts, mosses and hornworts) are important in understanding microtubule organization in plant cells. Land plants have an anastral mitotic spindle that forms in the absence of centrosomes, and a cytokinetic apparatus comprised of a predictive preprophase band (PPB) before mitosis and a phragmoplast after mitosis. These microtubule arrays have no counterpart in animal cells and the nature of the plant microtubule organizing centre (MTOC) remained an enigma for many years until antibodies to γ-tubulin, an essential component of the MTOC in all eukaryotes, became available for tracing the origin of microtubule arrays.
Methodology
We used immunofluorescence techniques to colocalize γ-tubulin, microtubules and chromosomes in mitotic cells of a representative liverwort, moss and hornwort to study the organization of microtubules during mitotic cell division.
Principal results
The future division site is marked by a PPB in all taxa but the MTOCs initially generating the half spindles differ: polar organizers in the liverwort, plastid MTOCs in the hornwort, and nuclear envelope-associated MTOCs in the moss. By mid-prophase, the forming spindles become more similar as γ-tubulin begins to spread around the polar regions of the nuclear envelope.
Conclusions
Regardless of origin, mature metaphase spindles are identical and indistinguishable from the typical anastral spindle of higher plants with broad polar regions consisting of numerous subsets of converging microtubules. A curious phenomenon of plant spindles, true of bryophytes as well as higher plants, is the movement of γ-tubulin into the metaphase spindle itself. The bipolar arrays of phragmoplast microtubules are organized by diffuse γ-tubulin located at proximal surfaces of reforming nuclear envelopes. Phragmoplast development appears similar in the three taxa and to vascular plants as well.
Introduction
Plant microtubules underlie all phases of plant development, such as determination of the division plane, cell shaping and wall deposition, in addition to mitosis/meiosis and cytokinesis. Unlike animal cells, where microtubules are nucleated at discrete centriole-containing centrosomes, plant cells produce a bewildering assortment of microtubule arrays in the absence of centrosomes. Land plants have evolved dispersed (Wasteneys 2002) or pleiomorphic (Brown and Lemmon 2007) microtubule organizing centres (MTOCs) responsible for nucleating the diverse microtubule arrays associated with cell division and differentiation. An essential component of both centrosomes and the diverse MTOCs of plants is γ-tubulin, a protein universally associated with microtubule nucleation in eukaryotes (Schmit 2002; Binarová et al. 2006; Wiese and Zheng 2006). γ-Tubulin can now be followed by indirect immunofluorescence throughout the cell cycle as different microtubule arrays are organized (Brown and Lemmon 2007).
The origin, development and function of the diffuse anastral spindle of land plants are fundamental questions in evolutionary and developmental biology. In plants, somatic mitosis is concentrated in meristems or growing points and new cells are inserted into the framework of existing cells. As a consequence, a distinguishing feature of plant mitosis is the coordination of division plane and spindle assembly (Brown and Lemmon 2001, 2007; Wasteneys 2002). In vegetative cells of higher plants, the wall-related hooplike cortical microtubules of interphase are replaced by the preprophase band (PPB), which predicts the future plane of cytokinesis and interacts with microtubules of the forming prophase spindle. The PPB coordinates the spindle axis with the division plane and somehow prepares the division site (Gunning 1982; Mineyuki 1999, 2007). Spindle microtubules emanate from nuclear envelope-associated γ-tubulin that becomes concentrated at polar regions. The broad poles of the spindle consist of numerous subsets of converging microtubules called minipoles by Schnepf (1984). γ-Tubulin moves into the metaphase spindle and is most concentrated at spindle poles in anaphase. It then moves from poles (distal surfaces of telophase nuclei) to proximal surfaces of nuclei where it is involved in the nucleation of the bipolar array of phragmoplast microtubules that direct cell plate deposition during the completion of cytokinesis (Brown et al. 2004; Brown and Lemmon 2007).
Unlike the uniformity seen in ontogeny of mitotic spindles in seed plants, dramatic differences have been reported in bryophytes. Mitotic spindles of liverworts are initiated at polar organizers (POs) (reviewed by Brown et al. 2004). Spindle origin in the hornworts Phaeoceros laevis and Notothylas breutelii has been shown to be associated with a unique axial microtubule system (AMS) that forms in association with division of the single plastid (Brown and Lemmon 1988). Mitotic spindles in mosses were known to be anastral (Schnepf 1984; Doonan et al. 1987) but the pattern of spindle initiation was not known prior to this study.
Additionally, more information is needed to understand the differences and similarities between mitotic and meiotic spindle origin in the major groups of bryophytes. Whereas mitotic and meiotic spindle origin are similar in hornworts (Brown and Lemmon 1993) and in some liverworts (those with POs in meiosis, Brown and Lemmon 2006), this is not the case in mosses. In mosses, the vegetative tissues are polyplastidic, but sporocytes become monoplastidic and undergo monoplastidic meiosis with plastid MTOCs organizing the spindles of both first and second meiosis (Brown and Lemmon 1997). We undertook this study with the goal of providing concise data for direct comparison of spindle initiation and development in an example of each of the major taxa of bryophytes (liverworts, mosses and hornworts).
Materials and methods
The liverwort Reboulia hemisphaerica (L.) Raddi, the moss Physcomitrium pyriforme (Hedw.) Hampe and the hornwort Phaeoceros laevis (L.) Prosk. were collected on the campus of the University of Louisiana at Lafayette in February 2010 and processed immediately according to methods published previously (Brown and Lemmon 1995) as modified for liverworts (Brown and Lemmon 2006). Vegetative tissue from developing sporophytes was sliced directly into 4 % formaldehyde freshly prepared from paraformaldehyde in microtubule buffer (Brown and Lemmon 1995) and fixed overnight at 4 °C. Tissue was washed in buffer and treated in wall-digesting enzymes: 1 % cellulase and 0.5 % pectinase in dH2O for 30 min at room temperature. Tissue was squashed between two coverslips and both coverslips were covered with a thin agarose–gelatin film. The coverslips were subsequently rinsed in buffer and covered with 1 % Triton X-100 to permeabilize membranes. Following a thorough buffer wash, cells were incubated with a 1:160 000 dilution of mouse monoclonal antibody (G9) followed by a 1:100 dilution of rhodamine red anti-mouse IgG to label γ-tubulin, and a 1:100 dilution of rat monoclonal anti-α-tubulin followed by a 1:100 dilution of fluorescein anti-rat IgG to label microtubules. The G9 antibody was raised in mouse against bacterially expressed Schizosaccharomyces pombe γ-tubulin; its specificity in bryophytes has been characterized (Shimamura et al. 2004). Following several washes in water, nucleic acids were stained with 1 µM To-Pro-3 iodide (Molecular Probes, Eugene, OR, USA) or 4′,6-diamidino-2-phenylindole in dH2O, before mounting in Prolong antifade reagent (Molecular Probes). Nuclei/chromosomes of hornworts stained poorly with To-Pro-3 iodide. Fluorescence was examined with a Leica SR5 confocal laser scanning microscope. Series of images were sequentially collected in the Z-axis, and ImageJ software (http://rsbweb.nih.gov/ij/) was used to generate projections for illustrations. Adobe Photoshop (Adobe System Inc., San Jose, CA, USA) was used to process overall size, contrast, brightness and colour of the images. This was most important to allow visualization of the three signals in the overlaid composite images.
Results
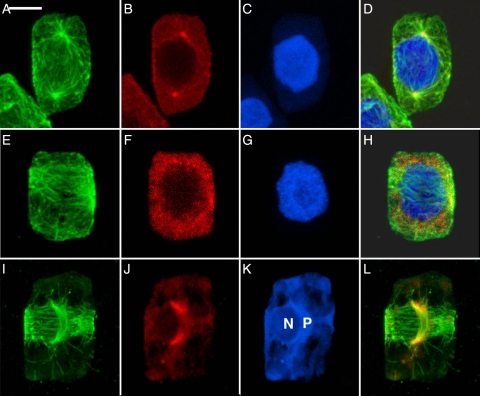
Spindles are initiated at different types of MTOCs in the three taxa. In the liverwort (Fig. 1A–D), astral microtubules emanate from distinct concentrations of γ-tubulin known as POs. The appearance of the POs at opposite poles precedes the organization of the PPB. In the moss (Fig. 1E–H), the PPB is well organized and the γ-tubulin is just beginning to concentrate in clumps around the nuclear envelope but spindle initiation is not yet apparent. In the hornwort (Fig. 1I–L), the unusual configuration of two perpendicular microtubule systems heralds initiation of the mitotic spindle (Fig. 1I). The PPB encircles the cell in the future plane of division and the AMS lies parallel to the isthmus of the dividing plastid and curves around one side of the adjacent nucleus. γ-Tubulin (Fig. 1J) is associated distinctly with the poles of the AMS.
Fig. 1.
Mitotic prophase in major taxa of bryophytes, each with a different pattern of spindle initiation. (A–D) The liverwort R. hemisphaerica. Scale bar = 5 µm. (E–H) The moss P. pyriforme. Scale bar = 4 µm. (I–L) The hornwort P. laevis. Scale bar = 5 µm. (A, E, I) Microtubules; (B, F, J) γ-tubulin; (C, G, K) nuclei; (D, H, L) composite. In the liverwort, POs nucleate astral microtubules at opposite poles before appearance of a PPB. In the moss, a PPB is formed and γ-tubulin begins to concentrate at the nuclear envelope. In the hornwort, the AMS forms in association with the isthmus of the dividing plastid (P) alongside the poorly stained nucleus (N) at right angles to the PPB.
Although PPBs mark the future division plane before mitosis in all bryophytes, the extent to which the division polarity is established and marked differs in the three groups. In the liverwort, the interaction of microtubules of the opposing half spindles emanating from the POs defines division polarity. The PPB is best seen just as the POs first begin to spread at polar regions (Fig. 2A), a stage occurring between that shown in Figs 1B and 3B. The PPB forms earlier in both the moss (Fig. 1E–H) and hornwort (Figs 1I–L and 2B). In hornworts, spindle polarity is clearly marked by the AMS, which reflects the position of the plastid, its long axis always at right angles to the division plane as marked by the girdling PPB. Microtubules of the PPB and forming spindle interact in the liverwort (Fig. 2A) and hornwort (Fig. 2B), but less so in the moss where the γ-tubulin is just beginning to concentrate at the nuclear envelope (Fig. 1F) at the time that the PPB (Fig. 1E) is broad but well organized.
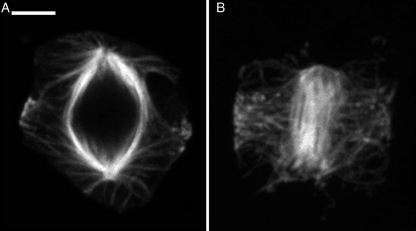
Fig. 2.
Interaction of microtubules of forming spindles with PPBs. Scale bar = 3.5 µm. (A) In the liverwort, astral microtubules emanating from spreading POs interact with the late forming PPB. Compare to early prophase in Fig. 1A and a later stage in Fig. 3A. (B) In the hornwort, microtubules emanating from tips of the elongated AMS (seen here in face view, as opposed to the side view seen in Fig. 1I) interact with the PPB.
Fig. 3.
Mid-late prophase in three major taxa of bryophytes showing similarity of the forming spindles. (A–D) The liverwort R. hemisphaerica. Scale bar = 5 µm. (E–H) The moss P. pyriforme. Scale bar = 4 µm. (I–L) The hornwort P. laevis. Scale bar = 5 µm. (A, E, I) Microtubules; (B, F, J) γ-tubulin; (C, G, K) nuclei; (D, H, L) composite. In the liverwort, POs lose their identity as γ-tubulin spreads around the nuclear envelope. In the moss, γ-tubulin is concentrated at the nuclear envelope, especially at the polar regions. In the hornwort, the AMS has nearly lost its identity as spindle microtubules are generated at its tips.
As chromosomes condense, the developing prophase spindles in all three taxa begin to show similarity (Fig. 3). In the liverwort, the γ-tubulin spreads from discrete POs (seen in Fig. 1B) to enclose rounded polar regions of the nucleus (Fig. 3B). In the moss, γ-tubulin appears more condensed around the nuclear envelope (Fig. 3F). In the hornwort, the spindle has become more symmetrical, although it still shows more concentration of γ-tubulin in the site of the AMS (Fig. 3J).
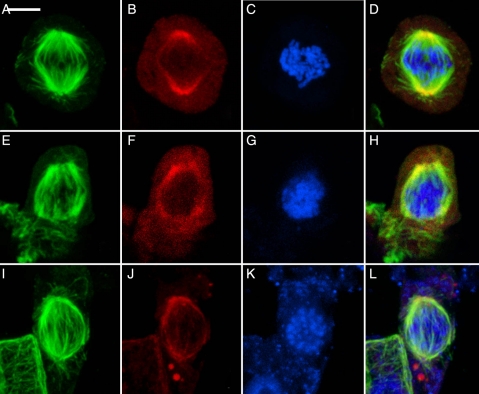
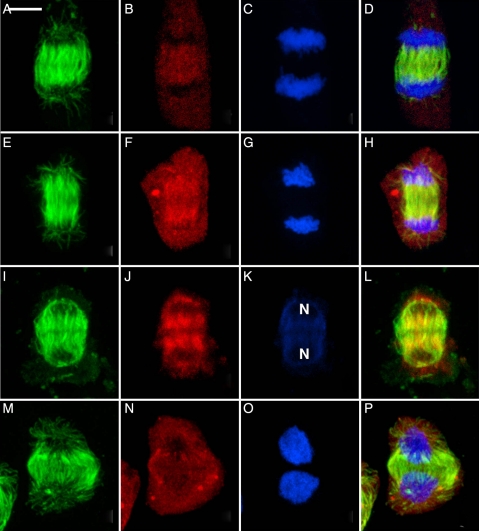
Despite their diverse origin, the mature metaphase spindles are remarkably alike in all three taxa (Fig. 4). The metaphase spindles exhibit minipoles that terminate in broad polar regions (Fig. 4A, E and I). Those of the hornwort (Fig. 4I–L) remain the most focused, perhaps because both poles remain associated with the plastid. By metaphase, the γ-tubulin has moved from previous polar distribution into the spindles (Fig. 4B, F and J). The size and shape of metaphase spindles appear to be related to the number of chromosomes (Fig. 4C, G and K). γ-Tubulin becomes more concentrated as kinetochore bundles shorten in anaphase, and it is typical for the spindles to become more focused. This results in very bright coincident occurrence of microtubules and γ-tubulin at the poles (not illustrated, but see Brown and Lemmon 2007 for examples).
Fig. 4.
Metaphase in three major taxa of bryophytes showing similarity of spindles. Metaphase spindle consists of minipoles terminating in broad polar regions, as is typical of land plants. In all, γ-tubulin has moved into the spindle itself and all traces of special structures associated with spindle initiation have disappeared. (A–D) The liverwort R. hemisphaerica. Scale bar = 5 µm. (E–H) The moss P. pyriforme. Scale bar = 4 µm. (I–L) The hornwort P. laevis. Scale bar = 5 µm. (A, E, I) Microtubules; (B, F, J) γ-tubulin; (C, G, K) nuclei; (D, H, L) composite.
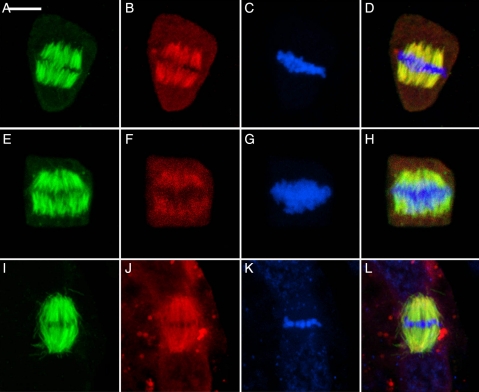
Following the arrival of sister groups of chromosomes at the poles (Fig. 5), γ-tubulin migrates to the proximal surfaces of the reforming nuclei where it is now involved in the generation of bipolar phragmoplast arrays. Again there is a tendency for γ-tubulin to extend along the microtubules. The interzonal phragmoplasts of the three bryophytes are virtually identical (Fig. 5A–L). The phragmoplasts expand centrifugally (Fig. 5M–P) to guide deposition of the cell plate to confluence with the division site previously established by the PPB.
Fig. 5.
Telophase showing the localization of γ-tubulin at the proximal faces of the reforming nuclei where it is associated with development of the bipolar phragmoplast arrays. Scale bar = 5 µm. (A, E, I, M) Microtubules; (B, F, J, N) γ-tubulin; (C, G, K, O) nuclei; (D, H, L, P) composite. (A–D) The liverwort R. hemisphaerica. (E–H) The moss P. pyriforme. (I–L) The hornwort P. laevis. The daughter nuclei, weakly stained by To-Pro-3, are labelled (N). (M–P) The expanded phragmoplast in R. hemisphaerica.
Discussion
The developmental process of mitotic cell division typical of all land plants is firmly established in the bryophytes. Preprophase bands of microtubules mark the future division plane before mitosis and the metaphase spindle is anastral consisting of minipoles terminating in broad polar regions. Most importantly, γ-tubulin consistently moves into the metaphase spindle even in the liverworts where it was initially concentrated into discrete POs. This phenomenon does not occur in animal and algal cells with centriolar centrosomes, but is typical of anastral spindles of land plants (Schmit 2002; Brown et al. 2004; Brown and Lemmon 2007). Although the functional significance is unknown, the phenomenon itself appears to be tied to the emancipation of γ-tubulin from tight association with centrosomes and the advent of the motile pleiomorphic plant MTOC.
Using the distribution of γ-tubulin as a marker, it appears that the three major evolutionary lines of bryophytes (liverworts, mosses and hornworts) employ different MTOCs in initiation of the mitotic spindle and those of the liverworts and hornworts are unique. Polar organizers are known to occur only in liverworts. The plastid-associated AMS is known only in hornworts. No such cytoskeletal structure has been seen in monoplastidic mitosis of lycopsids studied (Brown and Lemmon 1984); however, similar constructions are seen in monoplastidic meiosis (Brown and Lemmon 1993, 2001). The nuclear envelope-associated MTOC responsible for spindle initiation in mosses is most like the dispersed nuclear envelope-associated MTOC of higher plants. While it is tempting to conclude that each bryophyte group is characterized by a particular type of spindle organization, it must be realized that mitoses in certain early divergent groups such as Takakiopsida in the mosses and Haplomitriopsida in the liverworts have yet to be studied. Interestingly, monoplastidic meiosis has been reported in each (Renzaglia et al. 1994, 2007) and Takakia is most unusual in having monoplastidic mitosis in vegetative growth (Crandall-Stotler and Bozzola 1988).
Preprophase bands of microtubules occur in all three taxa but the time of appearance relative to the appearance of MTOCs associated with spindle initiation is different: after de novo synthesis of POs in the liverwort, before the nuclear envelope-associated MTOC is organized in the moss and concurrent with appearance of the AMS in the hornwort. Regardless of the type of spindle initiation, the metaphase spindles of all become anastral and consist of minipoles terminating in broad polar areas, as is typical of higher plants. Finally, distribution of γ-tubulin along the length of the spindle microtubules at metaphase is a phenomenon peculiar to the anastral spindles of land plants. The function of this phenomenon is unknown. The γ-tubulin, not anchored in centrosomes, may play a role in microtubule dynamics (Schmit 2002) or may simply be swept into the spindle. At telophase, however, the γ-tubulin does move in a directed manner to opposite faces of the reorganizing nuclei where it participates in nucleation of opposing sets of phragmoplast microtubules (Brown and Lemmon 2007). The cytokinetic apparatus tends to be generally similar to that described from a wide variety of euphyllophytes. Phragmoplasts are similar in structure/function; they always consist of bipolar arrays formed by interaction of opposing microtubules at the boundaries of cytoplasmic domains (Brown and Lemmon 2001, 2007).
Conclusions and forward look
The organization of mitotic spindles by three different types of MTOCs in bryophytes has broad evolutionary implications. It is indicative of the explosive radiation experienced by organisms that first made the transition to terrestrial life. The early land plants, responsible for initial greening of the Earth and making the land habitable for life as we know it, arose from charophycean algal ancestors that presumably had centrioles. Recent multigene phylogeny, as well as morphological considerations, supports the Coleochaetales as the closest living relatives of land plants (Finet et al. 2010). The Coleochaetales are themselves a diverse assemblage of thalloid and filamentous forms.
Modern-day Coleochaete has monoplastidic cells yet uses centrioles in mitosis (Marchant and Pickett-Heaps 1973; Brown et al. 1994). Although centrioles are produced de novo during spermatogenesis in plants with motile sperm, none use centrioles in either meiosis or vegetative mitosis. The extant bryophytes are the land plants most closely related to the early pioneers and appear to retain cytological features that provide clues to early evolution away from centriolar organization of spindles. The evolutionary advantage is difficult to discern but appears to be tied to the commitment to life on land, i.e. the production of airborne heavy-walled spores instead of motile meiospores and a vegetative body consisting of walled cells.
Based on extensive analyses of meiosis and spore wall development in bryophytes, we (Brown and Lemmon 2011) have hypothesized that the process of sporogenesis marked the transition from algae to plants and made possible the successful colonization of the land by extremely simple and small organisms. Meiosis appears to be a more conservative process than mitosis with all groups of bryophytes having members with monoplastidic meiosis. In monoplastidic meiosis, plastid MTOCs generate microtubules and organize spindles for both divisions of meiosis (Brown and Lemmon 1990, 1997, 2007, 2011). In mosses, cells undergoing vegetative mitosis are polyplastidic and spindles are organized by NE-MTOCs. In preparation for meiosis, the archesporial cells become monoplastidic and the MTOCs are plastid associated by the last proliferative division (Busby and Gunning 1988; Gambardella and Alfano 1990). We can now speculate on the evolution of microtubule systems in vegetative mitosis.
The spindles of plant cells are generally thought to be anastral and differ in this regard from those of all other eukaryotes (e.g. Liu et al. 2011). That the three groups of bryophytes differ in mode of spindle organization in itself is novel synthetic information with important evolutionary implications in understanding the cellular mechanisms underpinning evolution of form in early land plants. Mitotic plant cells are distinguished by cycling arrays of hooplike cortical microtubules in interphase, the predictive preprophase band (PPB), the spindle and the phragmoplast. This microtubule cycle is highly conserved and the component systems are typical of multicellular plant tissues of land plants from bryophytes to angiosperms (Brown and Lemmon 1993; Mineyuki 1999; Wasteneys 2002). It is clear that the variation in spindle organization in the bryophytes arose after divergence from the common ancestor.
As shown in this paper, the time of appearance of the PPB may vary slightly but the PPB consistently girdles the cell in the plane of future cytokinesis before the nucleus divides. It is present in all divisions where cells are intercalated into a pre-existing framework of cells and absent in reproductive cells (Brown and Lemmon 2001). It seems likely that this PPB mechanism evolved in an early common ancestor of land plants and was important in the elaboration of complex vegetative tissues.
Rigorous application of molecular systematics has done much to clarify relationships of the bryophytes (Renzaglia et al. 2007; Goffinet and Shaw 2008). An important implication from recent morphological and molecular studies is recognition of certain relictual groups such as the Haplomitriopsida in the liverworts and Takakiopsida in the mosses. Detailed studies of cell division of both mitosis and meiosis in these plants, as well as others such as Sphagnopsida, can be expected to yield valuable information not only in deciphering relationships, but also in understanding fundamental developmental processes in land plants.
Contributions by the authors
Both authors contributed to a similar extent overall.
Conflicts of interest statement
None declared.
Acknowledgements
We thank Dr T. Horio of the University of Kansas for the gift of the G9 antibody, without which this study could not have been conducted.
References
- Binarová P, Cenklová V, Procházková J, Doskocilová A, Volc J, Vrlik M, Bögre L. γ-Tubulin is essential for acentrosomal microtubule nucleation and coordination of late mitotic events in Arabidopsis. The Plant Cell. 2006;18:1199–1212. doi: 10.1105/tpc.105.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Plastid apportionment and preprophasic microtubule bands in monoplastidic root meristem cells of Isoetes and Selaginella. Protoplasma. 1984;123:95–103. [Google Scholar]
- Brown RC, Lemmon BE. Preprophasic microtubule systems and development of the mitotic spindle in hornworts (Bryophyta) Protoplasma. 1988;143:11–21. [Google Scholar]
- Brown RC, Lemmon BE. Monoplastidic cell division in lower land plants. American Journal of Botany. 1990;77:559–571. doi: 10.1002/j.1537-2197.1990.tb13588.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Diversity of cell division in simple land plants holds clues to the evolution of the mitotic and cytokinetic apparatus in higher plants. Memoirs of the Torrey Botanical Club. 1993;25:45–62. [Google Scholar]
- Brown RC, Lemmon BE. Methods in plant immunolight microscopy. Methods in Cell Biology. 1995;49:85–107. doi: 10.1016/s0091-679x(08)61448-x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. The quadripolar microtubule system in lower land plants. Journal of Plant Research. 1997;110:93–106. doi: 10.1007/BF02506848. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. The cytoskeleton and the spatial control of cytokinesis in the plant life cycle. Protoplasma. 2001;215:35–49. doi: 10.1007/BF01280302. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Polar organizers and girdling bands of microtubules are associated with γ-tubulin and act in establishment of meiotic quadripolarity in the hepatic Aneura pinguis (Bryophyta) Protoplasma. 2006;227:77–85. doi: 10.1007/s00709-006-0148-4. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. The pleiomorphic plant MTOC: an evolutionary perspective. Journal of Integrative Plant Biology. 2007;49:1142–1153. [Google Scholar]
- Brown RC, Lemmon BE. Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal–plant transition. New Phytologist. 2011;190:875–881. doi: 10.1111/j.1469-8137.2011.03709.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Graham LE. Morphogenetic plastid migration and microtubule arrays in mitosis and cytokinesis in the green alga Coleochaete orbicularis. American Journal of Botany. 1994;81:127–133. [Google Scholar]
- Brown RC, Lemmon BE, Horio T. γ-Tubulin localization changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma. 2004;224:187–193. doi: 10.1007/s00709-004-0061-7. [DOI] [PubMed] [Google Scholar]
- Busby CH, Gunning BES. Establishment of plastid-based quadripolarity in spore mother cells of the moss Funaria hygrometrica. Journal of Cell Science. 1988;91:117–126. [Google Scholar]
- Crandall-Stotler B, Bozzola JJ. Fine structure of the meristematic cells of Takakia lepidozioides Hattori et Inoue (Takakiophyta) Journal of the Hattori Botanical Laboratory. 1988;64:197–218. [Google Scholar]
- Doonan JH, Cove DJ, Cork FMK, Lloyd CW. Pre-prophase band of microtubules absent from tip-growing moss filaments, arises in leafy shoots during transition to intercalary growth. Cell Motility and the Cytoskeleton. 1987;7:138–153. [Google Scholar]
- Finet C, Timme RE, Delwiche CF, Marlétaz F. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Current Biology. 2010;20:2217–2222. doi: 10.1016/j.cub.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Gambardella R, Alfano F. Monoplastidic mitosis in the moss Timmiella barbuloides (Bryophyta) Protoplasma. 1990;156:29–38. [Google Scholar]
- Goffinet B, Shaw AJ. Bryophyte biology. 2nd edn. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Gunning BES. The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW, editor. The cytoskeleton in plant growth and development. London: Academic Press; 1982. pp. 229–292. [Google Scholar]
- Liu B, Chin-Min KH, Yuh-Ru JL. Microtubule reorganization during mitosis and cytokinesis: lessons learned from developing microgametophytes in Arabidopsis thaliana. Frontiers in Plant Science. 2011 doi: 10.3389/fpls.2011.00027. doi:10.3389/fpls.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant HJ, Pickett-Heaps JD. Mitosis and cytokinesis in Coleochaete scutata. Journal of Phycology. 1973;9:461–471. [Google Scholar]
- Mineyuki Y. The preprophase band of microtubules: its function as a cytokinetic apparatus in higher plants. International Review of Cytology. 1999;187:1–49. [Google Scholar]
- Mineyuki Y. Plant microtubule studies: past and present. Journal of Plant Research. 2007;120:45–51. doi: 10.1007/s10265-006-0063-y. [DOI] [PubMed] [Google Scholar]
- Renzaglia KS, Brown RC, Lemmon BE, Duckett JG, Ligrone R. Occurrence and phylogenetic significance of monoplastidic meiosis in liverworts. Canadian Journal of Botany. 1994;72:65–72. [Google Scholar]
- Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG. Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist. 2007;110:179–213. [Google Scholar]
- Schnepf E. Pre- and postmitotic reorientation of microtubule arrays in young Sphagnum leaflets: transitional stages and initiation sites. Protoplasma. 1984;120:100–112. [Google Scholar]
- Schmit AC. Acentrosomal microtubule nucleation in higher plants. International Review of Cytology. 2002;220:257–289. doi: 10.1016/s0074-7696(02)20008-x. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, Tomizawa K-I, Yoshimoto K, Deguchi H, Hosoya H, Horio T, Mineyuki Y. γ-Tubulin in basal land plants: characterization, localization and implication in the evolution of acentriolar microtubule organizing centers. The Plant Cell. 2004;16:45–59. doi: 10.1105/tpc.016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteneys GO. Microtubule organization in the green kingdom: chaos or self-order? Journal of Cell Science. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. Microtubule nucleation: γ-tubulin and beyond. Journal of Cell Science. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]