Abstract
The generation of a robust population of memory T cells is critical for effective vaccine and cell-based therapies to prevent and treat infectious diseases and cancer. A series of recent papers have established a new, cell-intrinsic approach in which small molecules target key metabolic and developmental pathways to enhance the formation and maintenance of highly functional CD8+ memory T cells. These findings raise the exciting new possibility of using small molecules, many of which are already approved for human use, for the pharmacologic induction of immunologic memory.
INTRODUCTION
Immunologic memory is a hallmark of the adaptive immune system and provides for enhanced host protection by increasing the precursor frequency and functionality of antigen-specific B and T lymphocytes. CD8+ memory T cells, a subset of antigen-experienced T cells that recognizes intracellular-derived antigens, play a critical role in the immune response against cytosolic pathogens and cancers (1, 2). Previous strategies to enhance the generation of CD8+ T cell memory have focused on the modulation of the extent and quality of antigenic stimulation as well as the provision of co-stimulation, cytokines, and agonists of Toll-like receptors, a group of innate immune receptors that recognize structurally conserved microbial motifs (3). Each of these factors acts in a cell-extrinsic fashion to initiate receptor-mediated signaling cascades that ultimately result in increased memory formation and maintenance. Recently, we and others have reported in a series of papers a new approach to boost CD8+ T cell memory in a cell-intrinsic manner through the pharmacologic modulation of key metabolic and developmental pathways (4–6). In this Perspective, we highlight the intracellular pathways amenable to manipulation by small molecules that might enhance both the number and functionality of CD8+ memory T cells. We refer to this class of reagents as cell-intrinsic modulators of memory formation, or CIMMs, to distinguish them from classical adjuvants that function in a cell-extrinsic manner to enhance T cell function. To offer a framework for understanding how CIMMs might augment memory CD8+ T cell development, we begin by briefly discussing our current knowledge of the ontogeny of CD8+ T cell memory formation.
T CELL MEMORY FORMATION
During a primary immune response, naïve CD8+ T cells are recruited in specialized lymphoid organs and are specifically stimulated by pathogen- or tumor-associated antigens presented by professional antigen-presenting cells (APCs). Upon activation, specific T cells undergo a multi-log clonal expansion to generate large numbers of effector T cells capable of migrating to peripheral tissues in order to eliminate cells bearing the target antigen. Most of the responding population will undergo apoptosis, but a minority will persist as antigen-experienced memory T cells. CD8+ memory T cells are phenotypically and functionally diverse and have been broadly divided into two major subsets termed central memory T cells (TCMs) and effector memory T cells (TEMs), on the basis of their expression of the lymph node–homing molecules CD62L and CCR7 (7). TCMs express high surface levels of CD62L and CCR7 and are capable of secreting interleukin (IL)–2, a key cytokine that modulates the expansion and the effector functions of lymphocytes, whereas TEMs lack these lymph node–homing molecules, preferentially reside in the periphery, and are impaired in their ability to release IL-2. Although TEMs can provide a first line of defense in peripheral sites of pathogen entry (8), such as the lung, skin, and mucosal surfaces, TCMs might be more efficient than TEMs in clearing systemic infections and treating tumors, as a result of their enhanced proliferative capacity and greater polyfunctionality (9, 10). More recently, a population of memory CD8+ T cells has been described that displays robust self-renewal and the multipotent capacity to derive TCM, TEM, and effector T cells after serial transplantation, prompting the label of memory stem cells (TSCMs) (6, 11). Phenotypically, these cells exhibit a CD44low CD62Lhigh phenotype like naïve T cells but co-express stem cell antigen– 1 (SCA-1) as well as high levels of the antiapoptotic molecule B cell lymphoma 2 (Bcl-2) and the β chain of the IL-2 and IL-15 receptor CD122. The therapeutic potential of TSCMs has just begun to be explored, but new findings indicate that this T cell subset might be more effective than other T cell memory subsets (6). Thus far, TSCMs have been described in mice during allogeneic graft-versus-host responses or after activation of the Wnt signaling cascade (6, 11). Recently, Turtle and colleagues identified a human CD8+ memory T cell population that shares some phenotypic and functional characteristics with hematopoietic stem cells, such as the expression of the stem cell marker c-Kit and the ability to efflux cellular toxins through the ATP-binding cassette (ABC)–superfamily multidrug efflux protein ABCB1 (12). However, the prevalence of this population within the more differentiated TEM subset makes them unlikely to represent the human counterpart of the TSCMs described in mice, which phenotypically resemble naïve T cells.
The ontogeny of memory CD8+ T cells has been the subject of numerous investigations, but the lineage relationships between effector and memory subsets remain controversial (13). What is clear, however, is that CD8+ T cells at the peak of the primary response are heterogeneous with respect to their differentiation state and capacity to become memory T cells. Detailed analyses of the phenotype of responding CD8+ T cells have revealed that surface expression of the killer cell lectin-like receptor G1 (KLRG1) and the α chain of the receptor for IL-7 (IL-7Rα) can distinguish CD8+ T cells fated to die or survive as long-lived memory cells (14, 15). Specifically, IL-7RαhighKLRG1low CD8+ T cells have a greater potential to persist and enter into the memory T cell pool, whereas IL-7R αlowKLRG1high CD8+ T cells represent terminally differentiated, senescent effector T cells destined to undergo apoptotic cell death.
The distribution of cells expressing these patterns of surface receptors is an integrated function of antigenic stimuli and inflammatory cues present during T cell priming. Cells receiving high-intensity signaling can undergo terminal effector differentiation mediated by the expression of the transcription factors T-bet (14) and eomesodermin (16), the antagonist of E-protein inhibitor of DNA binding 2 (Id2) (17), and the transcriptional repressor B lymphocyte– induced maturation protein 1 (Blimp1) (18). High-intensity signaling concurrently triggers reductions in the expression of pro-memory transcription factors such as Bcl-6 (19), Bcl-6b (20), T cell factor–1 (6), and the polycomb group member B lymphoma Mo-MLV insertion region 1 homolog (Bmi-1) (21). Thus, strong T cell activation can promote terminal differentiation at the expense of memory formation.
SHIFTING THE BALANCE TOWARD MEMORY WITH CIMMS
Enhancing fatty acid metabolism
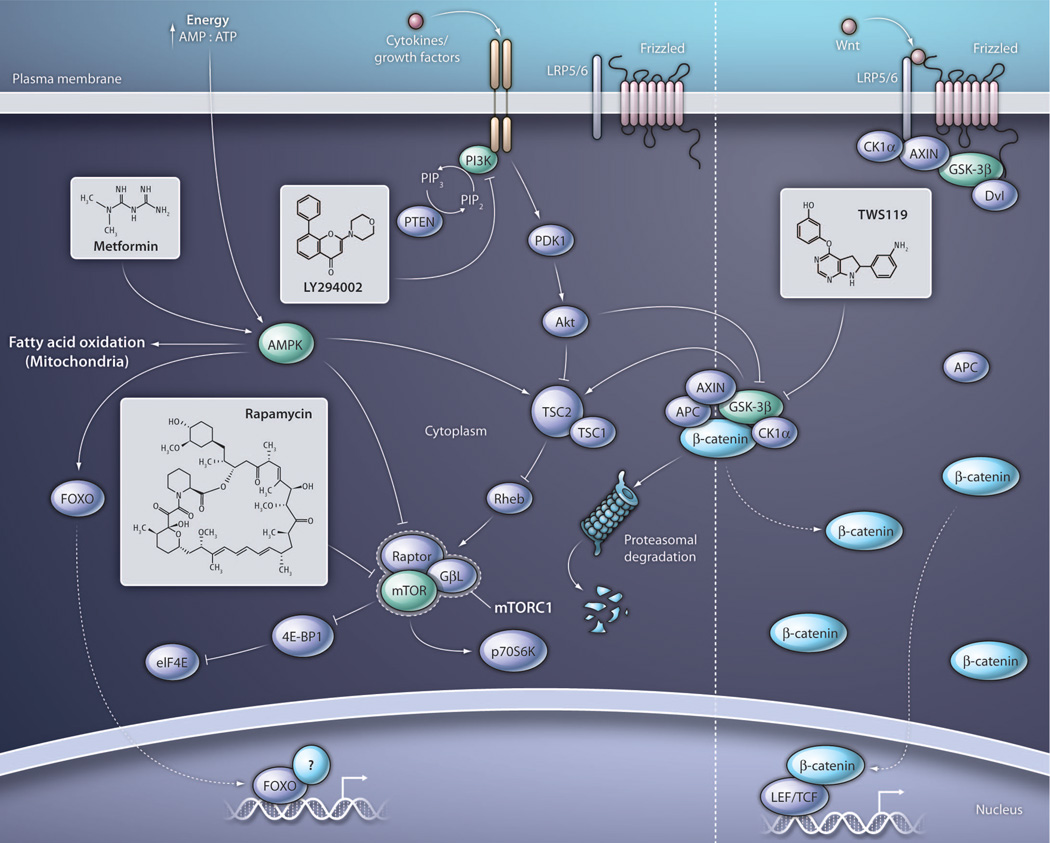
The balance of these pro-differentiation and pro-memory factors in vaccinology has historically been tilted by using different antigen schedules, doses, and adjuvants (3), but it might now be possible to modulate these signals in a cell-intrinsic manner by targeting key pathways with CIMMs. Recently, Pearce and colleagues found that the activity of metabolic pathways involved in fatty and aliphatic amino acid metabolism controls the generation of CD8+ T cell memory independently of the magnitude of the effector response (5). Fatty acid oxidation (FAO) occurs within mitochondria and is regulated in part by adenosine monophosphate kinase (AMPK), a key sensor of cellular energy stores whose activity is promoted physiologically by the adenosine monophosphate:adenosine triphosphate (AMP:ATP) ratio and pharmacologically by the biguanide metformin (Fig. 1) (22). During an immune response, naïve T cells switch to anabolic glycolytic metabolism to provide the energy necessary to sustain high rates of proliferation and the acquisition of effector functions (23). As T cells transition from an activated effector state to a quiescent memory state, catabolic FAO is preferentially used for energy production. To facilitate this conversion, metformin was provided during the contraction phase, resulting in increased secondary responses and tumor prevention (5). Similarly, enhanced recall responses were observed with rapamycin, an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1) historically used for its immunosuppressive properties to prevent the rejection of solid organ and allogeneic hematopoietic stem cell transplants (24).
Fig. 1. Pharmacologic targets for the enhancement of CD8+ T cell memory formation.
The activity of AMPK can be modulated by the AMP:ATP ratio or triggered by the biguanide metformin, resulting in inhibition of mTORC1 as well as augmentation of fatty acid oxidation and increased transcriptional activity of FOXO protein. FOXO protein acts in tandem with intranuclear binding partners to regulate gene transcription, but its binding partners involved in the modulation of pro-memory genes are incompletely elucidated. The PI3K-AKT pathway is activated by cytokine receptor engagement as well as T cell receptor and co-stimulatory receptor signaling (not shown) resulting in the downstream activation of mTORC1. PI3K-AKT activity can phosphorylate FOXO protein, promoting its cytosolic sequestration (not shown). PI3K can be inhibited by LY294002 or a number of small molecules which have recently entered clinical trials. In addition to indirect inhibition through modulation of upstream targets, mTORC1 may also be directly inhibited by rapamycin and its analogs. The activity of GSK-3β inhibits mTORC1 through activation of TSC2 as well as targets β-catenin for proteasomal degradation. Inhibition of GSK-3β using TWS119 mimics Wnt signaling by promoting β-catenin accumulation and translocation into the nucleus, where it binds to partners including LEF and TCF to promote gene transcription. PTEN, phosphatase and tensin homolog; PDK1, pyruvate dehydrogenase kinase 1; Rheb, Ras homolog enriched in brain; GβL, G protein beta subunit–like; 4E-BP1, phosphorylated heat- and acid-stable protein regulated by insulin 1; p70S6K, p70 ribosomal protein S6 kinase 1; APC, adenomatous polyposis coli; CK1α, casein kinase 1, alpha 1; Dvl, disheveled; LRP5/6, low-density lipoprotein receptor–related protein 5/6.
Restraining mTOR-mediated protein translation
mTORC1 is a central metabolic node positively regulated by the activity of phosphoinositol 3-kinase (PI3K) and inhibited by the AMPK and glycogen synthase kinase–3 (GSK-3) pathways. Activation of mTORC1 promotes the translation of some mRNAs, such as those containing long and structured 5′ untranslated region sequences via the p70 ribosomal protein S6 kinase 1 (p70S6K) and the eukaryotic translation initiation factor 4E (eIF4E) (Fig. 1) (24). These mRNAs oft en encode proteins involved in promoting cell growth and proliferation. Contemporaneously with Pearce, Araki and colleagues also found that rapamycin could enhance CD8+ T cell memory (4). In a detailed analysis, the authors found that the timing of drug administration was a critical determinant for the numbers, phenotype, and function of memory CD8+ T cells. Provision of rapamycin during the priming and expansion phase augmented the absolute numbers of memory T cells as a result of increased frequencies of IL-7RαhighKLRG1low CD8+ T cell memory precursors present at the peak of the responses. Conversely, administration during the contraction phase enhanced TCM formation and secondary responses. Systemic administration of rapamycin can modulate many host populations, including professional APCs such as dendritic cells and suppressive T cell populations such as CD4+ forkhead box P3+ (FOXP3) regulatory T cells, in addition to the responding CD8+ T cells (24). Using retroviral transduction of short-hairpin RNAs targeting key components of the mTOR pathway into antigen-specific CD8+ T cells, Araki et al. could recapitulate the effect of rapamycin, demonstrating that the drug operated in a cell-intrinsic manner. Whether the primary effect of rapamycin is to enhance FAO as suggested by Pearce, or to restrain protein translation as shown by Araki, remains to be fully elucidated.
Inhibiting PI3K activity
An alternative pharmacologic approach for down-modulating mTORC1 activity might be the inhibition of PI3K, a pathway that transduces multiple pro-differentiation signals from the engagement of cytokine, co-stimulatory, and T cell receptors (Fig. 1) (24). PI3K inhibitors have yet to be explored as CIMMs, but recent findings indicate that these small molecules can sustain the expression of the lymph node–homing markers CD62L and CCR7 that are associated with highly functional TCMs (25). The PI3K inhibitor LY294002 inhibits the mTOR-mediated down-regulation of Krüppel-like factor–2 (Klf-2), a key transcriptional modulator for the expression of lymph node–homing receptors (25). PI3K inhibitors might also enhance CD8+ memory T cell formation by preventing PI3KAKT–mediated cytosolic sequestration of forkhead box O1 (FOXO1), a transcription factor that controls the expression of Klf-2 and pro-memory factors such as BCL6 and IL-7Rα (26, 27). It should be noted that in addition to increasing FAO, metformin might also augment CD8+ T cell memory formation by increasing FOXO transcriptional activity through AMPK (27).
Activating the Wnt/β-catenin signaling pathway
Other than metabolic pathways, pharmacologic manipulation of key developmental pathways, such as Wnt, can potently enhance the formation of CD8+ T cell memory (6). Wnt signaling negatively regulates the activity of GSK-3β, resulting in the accumulation and translocation of β-catenin into the nucleus, where it binds and activates the transcription factors lymphoid enhancer factor (LEF) and T cell factor (TCF) (Fig. 1). Wnt-mediated blockade of GSK-3β can also result in the activation of mTORC1 through the inhibition of the tuberous sclerosis complex 2 (TSC2) (28) (Fig. 1). Depending on the context, Wnt proteins can promote stem self-renewal and maintenance of pluripotency through their effects on β-catenin (29, 30), or they can induce proliferation, terminal differentiation, and senescence in systems where mTOR signaling is dominant (31, 32). Similar to what is observed in stem cell biology, we found that activation of Wnt signaling in CD8+ T cells through pharmacologic inhibition of GSK-3β facilitated the formation of self-renewing memory T cell subsets, including highly functional TSCMs, by limiting proliferation and effector differentiation (6). These findings would suggest that GSK-3β inhibition in naïve CD8+ T cells preferentially enhances β-catenin signaling over its effect on mTORC1. How Wnt/β-catenin arrests effector differentiation and enhances CD8+ TSCM formation remains to be completely elucidated, but TCF1 appears to play an important role because its overexpression alone can inhibit the acquisition of effector molecules such as interferon-γ (33).
TRANSLATING CIMMS INTO THE CLINIC
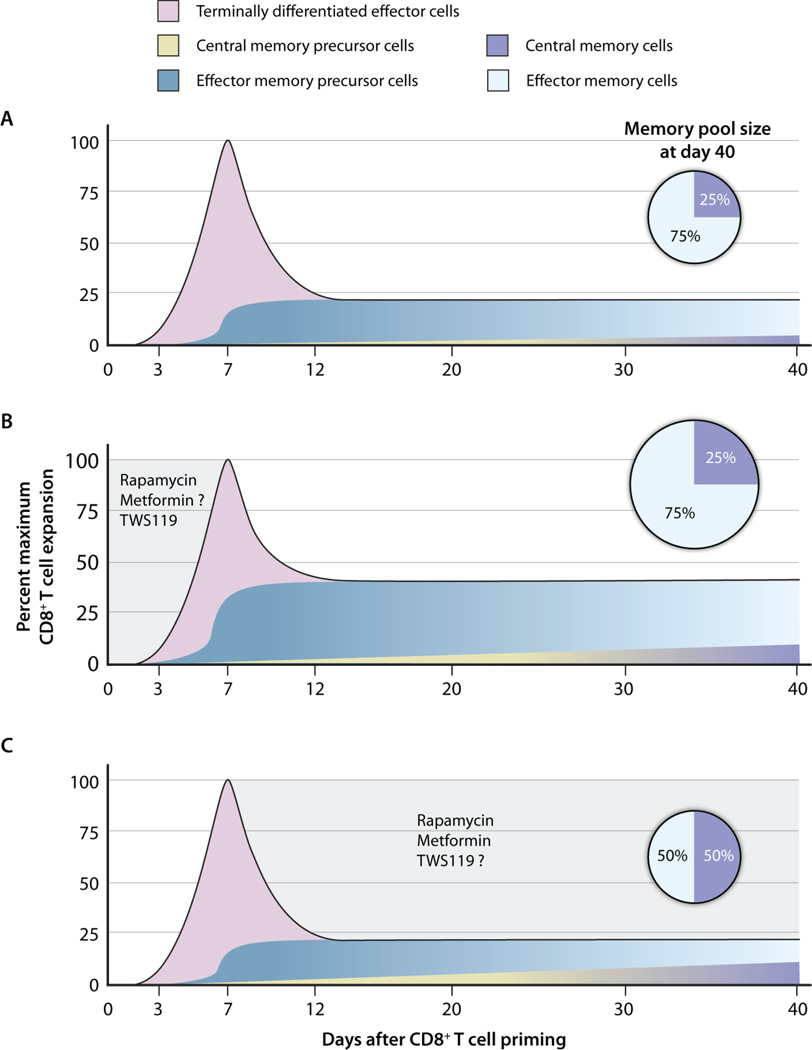
There is a critical need for reagents capable of enhancing preventative and therapeutic vaccines against human immunodeficiency virus, tuberculosis, viral hepatitis C, and cancer. Presently, alum is the only adjuvant approved by the U.S. Food and Drug Administration and it may be insufficient to trigger optimal T cell memory formation (34). Thus, CIMMs could rapidly increase the armamentarium of reagents capable of enhancing vaccines, because many candidate molecules are already approved or in clinical evaluation for other indications (Table 1). Some candidate CIMMs have established or putative immunosuppressive and antiproliferative activity, necessitating a careful evaluation of the dose. Araki and colleagues have demonstrated that, although low doses of rapamycin can potentiate CD8+ T cell memory formation, high doses can abrogate immune responses altogether (4). On the basis of mouse and nonhuman primate studies, we envision three modes of scheduling for CIMMs, correlating with diff erent phases of the immune response (Fig. 2). Th e administration of CIMMs during the priming and expansion phase could inhibit terminal effector differentiation, resulting in increased frequencies of memory precursors, leading to greater absolute numbers of memory T cells (4, 6). Conversely, administration during the contraction phase minimally affects the absolute number of memory T cells but might cause an enrichment in the highly functional TCM population (4, 5). The provision of CIMMs continuously during the immune response could have an additive effect and result in increased absolute numbers of memory T cells and TCMs (4). The choice of the dosing schedule should be dictated by the safety profile of the CIMM intended for use. For example, administration of inhibitors of GSK-3β might be limited to the priming and expansion phase to avoid the oncogenic potential of chronic activation of the Wnt/β-catenin pathway (29). Additionally, coadministration of different classes of CIMMs might be advantageous under certain circumstances. Because blockade of GSK-3 might have the undesirable downstream effect of promoting terminal differentiation by activating mTORC1, GSK-3 inhibitors might be more effectively used in combination with mTOR inhibitors such as rapamycin (32). Finally, we believe that proof-of-concept clinical trials should be performed in cancer patients with a high risk of recurrence, where a risk-to-benefit ratio is more favorable than in otherwise healthy patients. CIMMs might also be used in vitro to arrest terminal effector differentiation and generate memory-like CD8+ T cells, which have been shown to be the optimal population for cell-based therapies in animal models and early clinical trials (10, 35, 36). In summary, the modulation of key metabolic and developmental pathways by CIMMs appears to be a practical and translatable approach for the induction of enhanced CD8+ T cell memory.
Table 1. Clinical development and current indications of candidate CIMMs.
Data were obtained from ongoing or completed human clinical trials listed at http://www.clinicaltrials.gov.
| Metabolic pathway | Molecular target | Stage of clinical development; indication |
|---|---|---|
| mTOR | ||
| Rapamycin | mTORC1 | Approved; prophylaxis of organ rejection |
| Temsirolimus | mTORC1 | Approved; advanced renal cell carcinoma |
| Everolimus | mTORC1 | Approved; advanced renal cell carcinoma |
| Ridaforolimus | mTORC1 | Phase II; advanced solid and hematologic malignancies |
| OSI-027 | mTORC1 and mTORC2 | Phase I; advanced solid and hematologic malignancies |
| Oleandrin | p70S6K | Phase I; advanced solid tumors |
| AMPK | ||
| Metformin | AMPK | Approved; type II diabetes |
| AICAR | AMPK | Phase II; type II diabetes, Lesch-Nyhan syndrome |
| GW501516 | AMPK | Phase II; dyslipidemia |
| PI3K | ||
| SF1126 (LY294002 pro-drug) | PI3K | Phase I; advanced solid tumors |
| CAL-101 | PI3K (p110δ) | Phase I; hematologic malignancies |
| PX-866 | PI3K (p110α) | Phase I; advanced solid tumors |
| MK-2206 | AKT | Phase I; advanced solid tumors |
| Oleandrin | AKT | Phase I; advanced solid tumors |
| Wnt/β-catenin | ||
| Lithium | GSK-3α and -β | Approved; manic-depressive disorder |
| Valproic acid | GSK-3β | Approved; seizure disorder |
| Flavopiridol | GSK-3α and -β | Phase II; advanced solid and hematologic malignancies |
| Staurosporine | GSK-3α and -β | Phase II; advanced solid and hematologic malignancies |
| NP031112 | GSK-3β | Phase I/II; Alzheimer’s disease |
| TWS119 | GSK-3β | Preclinical |
Fig. 2. The timing of CIMM administration affects both the phenotype and numbers of CD8+ memory T cells.
(A) Kinetics of a prototypical primary T cell immune response. After antigenic stimulation, naïve CD8+ T cells undergo a multi-log clonal expansion, peaking during the first week. The majority of CD8+ T cells become terminally differentiated effector cells that rapidly undergo apoptosis. A minority of responding T cells (TCM and TEM precursors) will persist as long-lived memory T cells. (B) Eff ect of CIMM administration during the priming and expansion phase of an immune response. Provision of CIMM during the first week of a primary immune response minimally affects the total number of responding T cells at the peak of the response but yields greater numbers of memory T cell precursors, leading to increased numbers of memory T cells without altering the relative distribution of TCMs and TEMs. To date, the effect of metformin has yet to be directly tested during this phase. (C) Effect of CIMM administration during the contraction phase of an immune response. The provision of CIMMs after the peak of an immune response results in a more rapid acquisition of the phenotypic and functional attributes associated with central memory T cells, without changing the absolute number of persisting memory T cells. To date, the GSK-3β inhibitor TWS119 has not been tested during this phase. Curves are extrapolated from Araki et al. (4).
Acknowledgments
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Cancer Institute, Center for Cancer Research.
REFERENCES
- 1.Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 4.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 8.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 10.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 12.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: Models and controversies. Nat. Rev. Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 14.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 16.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 17.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfi eld KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 18.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 20.Manders PM, Hunter PJ, Telaranta AI, Carr JM, Marshall JL, Carrasco M, Murakami Y, Palmowski MJ, Cerundolo V, Kaech SM, Ahmed R, Fearon DT. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis R, Geesaman BJ, DiStefano PS. Ageing and metabolism: Drug discovery opportunities. Nat. Rev. Drug Discov. 2005;4:569–580. doi: 10.1038/nrd1777. [DOI] [PubMed] [Google Scholar]
- 23.Jones RG, Thompson CB. Revving the engine: Signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat. Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 29.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 30.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Zhang Y, Bersenev A, O’Brien WT, Tong W, Emerson SG, Klein PS. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 2009;119:3519–3529. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, Sen JM. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat. Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert C. Boosting our best shot. Nat. Med. 2009;15:984–988. doi: 10.1038/nm0909-984. [DOI] [PubMed] [Google Scholar]
- 35.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]