Abstract
Purpose
Release of inhibitory coregulatory proteins into the circulation may represent one mechanism by which tumors thwart immune responses. Our objective was to determine whether sB7-H1 levels in patients with clear cell renal cell carcinoma (ccRCC) are associated with pathologic features and patient outcome.
Experimental Design
We developed an ELISA for quantification of sB7-H1 in biologic fluids. Biochemical confirmation of the measured analyte as sB7-H1 was done by protein micro-sequencing using supernates from tumor cell lines. Biological activity of sB7-H1 was assessed in vitro utilizing T cell apoptosis assays. We tested sB7-H1 levels in the sera from 172 ccRCC patients and correlated sB7-H1 levels with pathologic features and patient outcome.
Results
sB7-H1 was detected in the cell-supernatants of some B7-H1-positive tumor cell lines. Protein sequencing established that the measured sB7-H1 retained its receptor-binding domain and could deliver pro-apoptotic signals to T cells. Higher preoperative sB7-H1 levels were associated with larger tumors (p<0.001), tumors of advanced stage (p=0.017) and grade (p=0.044), and tumors with necrosis (p=0.003). A doubling of sB7-H1 levels was associated with a 41% increased risk of death (p=0.010).
Conclusion
Our observations suggest that sB7-H1 may be detected in the sera of ccRCC patients and that sB7-H1 may systemically impair host immunity, thereby fostering cancer progression and subsequent poor clinical outcome.
Keywords: Costimulation, Neoplasm, T Lymphocyte, Immunosuppression, Immunotherapy and Immunity, Renal Cell Carcinoma
INTRODUCTION
Considerable uncertainty exists regarding the existence and molecular forms of circulating coregulatory molecules. Soluble forms of B7-family coregulatory proteins (including B7.1, B7.2, CD28, CTLA-4, and B7-H4) in sera of patients with malignancy, infection and autoimmune disorders have been claimed to be detected (1–5), invariably without accompanying biochemical proof. Whether soluble B7-H1 (sB7-H1) exists, too, remains an unresolved issue. Conflicting B7-H1 serological studies have led to a misperception that the existence of sB7-H1 is firmly established (6–8). Wan et al suggested that sB7-H1 is present and elevated among rheumatoid arthritis patients. However, those results, largely obtained with potentially cross-reacting polyclonal antibodies, were questioned (7) and not confirmed (8).
Prompted by these contradictory reports, and because membrane expression of B7-H1 among a small percentage of tumor cells in patients with clear cell renal cell carcinoma (ccRCC) affords a dismal prognosis (9, 10), we developed a sB7-H1 ELISA and biochemically confirmed the identity of the detected protein. We then measured levels of sB7-H1 in ccRCC patient and normal control sera and correlated sB7-H1 levels with pathologic features of ccRCC tumors and patient outcome.
MATERIALS AND METHODS
Development of antibodies against B7-H1
The detection antibody, 5H1-A3, was subcloned from the anti-B7-H1 producing 5H1 hybridoma (11). To generate the capture antibody, 2.2B, 624MEL cells were transfected with full-length human B7-H1 (11) and injected (5x106 cells/injection) intraperitoneally into Balb/c mice weekly for 6 weeks. Immune splenocytes were isolated and fused with A38 cells to form a hybridoma using standard techniques (12). 5H1-A3 and 2.2B hybridoma supernatants were screened by ELISA for reactivity against a recombinant human protein B7-H1-human IgG (R&D Systems) which only contains the extracellular domain of B7-H1 (amino acids 19 to 239) and for absence of cross-reactivity to an irrelevant recombinant protein P-Selectin-human IgG (BD Biosciences) or mouse immunoglobulins (Sigma).
Development of sandwich ELISA for sB7-H1
We developed a sandwich ELISA using paired mouse IgG1 monoclonal antibodies (2.2B and 5H1-A3) raised against the extracellular domain of human B7-H1. We validated the specificity of each individual antibody by immunohistochemistry, indirect ELISA (data not shown) and flow cytometry (Supplementary Figure S1A). Both antibodies bind to the extracellular domain of B7-H1 and bind to different sites on the B7-H1 molecule (Supplementary Figures S1B and S1C). The configuration of 2.2B (capture) and 5H1-A3 (detection) exhibits an optimal detection range (C2.5 to C97.5) between 0.086 and 3.67 ng/mL, with a coefficient of variation of 10% (Supplementary Figure S2). The assay is specific for B7-H1 and does not exhibit cross-reactivity to other B7-H homologues (B7-H2, B7-H3, B7-H4, B7.1 or PD-1, all from R&D Systems), immunoglobulin or third party recombinant protein (P-selectin, R&D Systems) expressing a shared Fc carrier element (Figure 1A). Binding of 2.2.B or 5H1-A3 to B7-H1 in the ELISA can be blocked by pre-incubating appropriate standards with antibody (data not shown).
Figure 1. Development and validation of a new B7-H1 ELISA and assessment of sB7-H1 in human serum samples and cell lines.
(A) The B7-H1-specific ELISA (red line) does not cross-react with other B7 family members (B7-H2, B7-H3, B7-H4, B7.1 and PD-1) or control proteins (P-Selectin and mouse IgG). The results of 3 experiments with 4–6 replicates/each are depicted. (B) Soluble B7-H1 is detected in the media of several membrane B7-H1-positive cell lines, but in none of the B7-H1-negative cells. Asterisks represent undetectable sB7-H1 levels. Error bars represent SEM. Data are representative of at least 3 individual measures per cell line.
2.2B was used as the plate-fixed capture antibody and biotinylated 5H1-A3 was used as the detection antibody. Biotinylation was performed using a solid-phase kit (Pierce). Individual ELISA steps involved three washes using a TBS + 0.05% Tween-20 buffer. High-binding polystyrene plates (Corning Life Sciences) were coated for 2h at 21°C with 0.2μg/well of 2.2B. Free binding sites were blocked with 200μL/well of Superblock (Pierce) 1h at 21°C. After washing, 50μL of sample were added to 50 μL of assay buffer (PBS + 1% BSA) and incubated overnight at 4°C. Biotinylated 5H1-A3 (100μL/well at 1μg/mL diluted in PBS + 0.1% BSA) was added and incubated 1h at 21°C. 100μL/well of horseradish peroxidase-conjugated streptavidin (BD Biosciences) diluted in PBS + 0.1% BSA was added and incubated 1h at 21°C. Plates were developed with TMB (Pierce), stopped using 0.5N H2SO4 and read at 450nm using a Benchmark Plus plate reader and associated software (Bio-Rad). For calibration, each plate was loaded with parallel dilutions of recombinant B7-H1 fusion protein (R&D Systems) ranging in concentration from 0.07 – 10 ng/mL.
Calibration of the B7-H1 ELISA against standard B7-H1 dilution curves was done by fitting a 4-parameter logistic regression model using the drc package for R (13, 14). A calibration plot with 95% confidence and prediction intervals was generated using 16 consecutive and independent assays using concentrations of B7-H1 fusion protein ranging from 10 μg/mL to 1.2 pg/mL. The calibration model revealed a coefficient of determination (R2) of 0.959, representing an excellent model fit and minimal inter-assay variability. For the specificity determinations, generalized additive regression models were constructed with the mgcv package for R (15), and smooth fits of the results for the non-specific proteins were plotted and overlaid.
Cancer cell lines
Human cancer cell lines 624MEL, 293T, Jurkat, 22RV1, DU145, PC3, Caki-2, J82, Karpas-299 and SUDHL-1 were purchased from ATCC and propagated in complete media (RPMI + 10% FBS, 20mM HEPES and Penicillin/Streptomycin). The 624MEL/B7-H1 cell line containing the human full-length B7-H1 sequence was generated as previously described (11). BT10B is a spontaneously immortalized bladder cancer cell line established in our laboratory from a cystectomy specimen classified as a high-grade urothelial carcinoma. BT10B expresses uroplakin III and cytokeratin-20, indicative of urothelial origin (16). To test for the presence of tumor derived sB7-H1, cells were cultured for 3-5 days. Supernates were collected, centrifuged at 2000g x 10 min to remove cellular debris and tested using the ELISA described above.
Protein purification and sequence identification by tandem mass spectrometry (MS/MS)
Non-adherent sB7-H1-positive cell lines Karpas-299 and SUDHL-1 were grown in roller bottles for 7-10 days in complete media. Supernatants were harvested before becoming acidic and while cell viability remained >98%. To eliminate cell debris, enriched media was centrifuged at 2000g x 20 min followed by clarification through 0.45μm filters. Processed supernates were passed through a 2.2B affinity column coupled to a BioLogic LP monitor (Bio-Rad). Following sB7-H1 capture, the column was thoroughly washed and 1mL fractions eluted with 0.1M glycine buffer pH 2.7. Collected fractions were tested by ELISA to identify sB7-H1-positive containing samples. Positive fractions were treated with cold acetone to precipitate sB7-H1 protein. Pellets were resuspended in reducing buffer, boiled at 95°C x 10 min and loaded onto duplicate polyacrylamide gels. Proteins from one gel were transferred to a PDVF membrane and blotted with 5H1-A3-biotin to localize sB7-H1 bands. The second gel was fixed, and proteins revealed by silver staining (17). B7-H1-positive bands from immunoblotting and silver gel staining were aligned and excised. The sB7-H1 material was digested in situ with trypsin and prepared for mass spectrometry analysis (18). Protein identification was performed by nano-flow liquid chromatography electrospray tandem mass spectrometry (nanoLC-ESI-MS/MS) using a LTQ Orbitrap Hybrid Mass Spectrometer (ThermoElectron Bremen) coupled to a nanoLC-2D HPLC system (Eksigent). Peptide sequences were analyzed against the Swissprot database. Confirmation of protein identity as B7-H1 was based on a >95% probability of sequence alignment for a minimum of three peptides.
Apoptosis assay for activated human T cells
Normal human PBMCs were isolated from leukoreduction filters (Pall) as previously described (19, 20). CD4 and CD8 T cells were obtained by negative magnetic isolation (Miltenyi Biotech). Cells were placed in 96-well plates pre-coated with 2 μg/mL of anti- human CD3 (clone UCHT1, BD Biosciences) and cultured 3 days in complete media. 10μg/mL recombinant human B7-H1/Fc or P-Selectin/Fc fusion protein (R&D Systems) were preincubated with 30 μg/mL of functional grade B7-H1 blocking antibody (clone MIH1) or an isotype control antibody (both from eBioscience) for 30 min at 4°C. The mixture was then added to cultures of activated T cells. Following an overnight incubation, T cells were harvested and stained for Annexin-V (BD Biosciences) and propidium iodide, PI (Sigma). Relative percentages of apoptotic (Annexin-V+PI−) CD4 and CD8 T cells were quantified by flow cytometry (FACSCalibur, BD) in combination with FlowJo analysis software (Tree Star). Paired t-tests were used to compare apoptosis among activated T cells.
ccRCC patient selection
We designed a pilot study to test if sB7-H1 was present and detectable in the sera of ccRCC patients. On approval from the Institutional Review Board, we reviewed the Mayo Clinic Nephrectomy Registry, which contains over 5,000 patients treated surgically for a solid renal mass since 1970. From this Registry, we identified a consecutive series of 650 patients treated surgically for unilateral, sporadic ccRCC between May 2003 and December 2007. Histological subtype and pathologic features were obtained by a review of all microscopic slides from the nephrectomy specimens by a urologic pathologist (J.C.C.) according to the Union Internationale Contre le Cancer, American Joint Committee on Cancer and Heidelberg guidelines (21, 22) without knowledge of patient outcome.
To ensure complete patient follow-up, a registered nurse abstractor assigned to the Registry reviews the medical records of all patients who are still alive annually. For patients who have died, the nurse abstractor reviews a number of sources to determine cause of death including death certificates, medical records, and correspondence with the patients’ families and local physicians. To date, <3% of patients in the Registry have been lost to follow-up.
Serum collection and sB7-H1 analyses
Biospecimen collection for patients in the Registry began in May 2003. Of the 650 ccRCC patients identified above, 172 consented to provide preoperative blood samples for our biospecimen repository and had archived sera available for study. There was not a statistically significant difference in overall survival between patients who did and did not consent to provide blood samples (p=0.38; log-rank test). Serum samples were stored at −80°C until used.
To perform the B7-H1 ELISA, serum samples were thawed at 4°C and tested in duplicate along with protein standards and controls. The technician performing the B7-H1 ELISA was blinded to the pathologic features and outcome of the ccRCC patients analyzed. Associations of the average of the duplicate sB7-H1 levels with clinical and pathologic features of the ccRCC patients were evaluated using Spearman rank correlation coefficients and Kruskal-Wallis and Wilcoxon rank sum tests. Associations of sB7-H1 levels with patient outcome following surgery were evaluated using Cox proportional hazards regression models, both univariately and after adjusting for the Stage, Size, Grade, and Necrosis (SSIGN) score (23) developed specifically for patients with ccRCC. For these analyses, sB7-H1 levels were transformed to the log(2) scale so that they were approximately normally distributed.
sB7-H1 in normal controls and ELISA reproducibility
To compare sB7-H1 levels between ccRCC patients and normal controls and to evaluate intra- and inter-assay reproducibility, we randomly selected 50 of the 172 ccRCC patients and identified 50 normal controls (mean age 52 years; 30 males and 20 females). Sera from the normal controls were obtained from healthy volunteers undergoing blood donation at the Mayo Clinic Department of Transfusion Medicine. sB7-H1 levels in these 100 specimens (50 ccRCC patients and 50 normal controls) were again tested in duplicate in two consecutive experiments. sB7-H1 levels were compared between ccRCC patients and normal controls univariately and after adjusting for age and gender using a Wilcoxon rank sum test and a linear model of the log(2) transformed sB7-H1. The intra- and inter-assay reproducibility of the B7-H1 ELISA were assessed using Lin's concordance correlation coefficient (24). This measure ranges from 0.0 to 1.0, with higher values indicating a greater level of agreement or reproducibility.
RESULTS
ELISA measurement of sB7-H1 in the culture supernatants of human cancer cell lines
To determine if tumor cell lines release sB7-H1 in vitro, the culture supernatants from membrane B7-H1-positive and -negative (Supplementary Figure 3) cancer cell lines were tested using the B7-H1 ELISA (Figure 1B). sB7-H1 was detected in the supernates of membrane B7-H1-positive cell lines BT10B (bladder cancer), DU145 (prostate cancer), 624MEL/B7-H1 (B7-H1-transfected) and Karpas-299 and SUDHL-1 (both lymphomas). In contrast, sB7-H1 was not detected among supernates from membrane B7-H1-negative cell lines (Jurkat, 293T, 624MEL and 22RV1). Cell lines expressing RT-PCR B7-H1 mRNA (Supplementary Figure 4) were positive for sB7-H1 release.
Purification of sB7-H1 from tumor cell supernatants and sB7-H1 protein sequencing
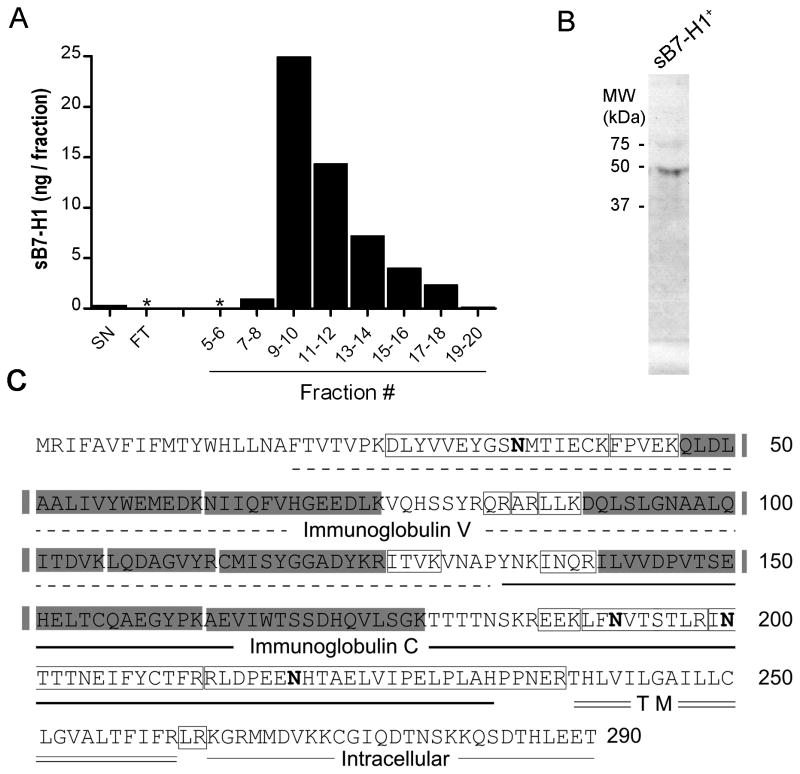
To study the putative protein sequence of sB7-H1 we performed affinity chromatography using a 2.2B (capture mAb)-coupled column, using cell culture supernatant from sB7-H1-positive SUDHL-1 cells (Figure 2A). sB7-H1 ELISA-positive soluble protein factions were pooled, subjected to protein electrophoresis and immunoblotted with 5H1-A3 (detection)-biotin labeled mAb. A band (Figure 2B) was detected corresponding to a molecular weight of ~ 45kDa, consistent with reported molecular weight of B7-H1 (25–27). Protein sequencing of the 45KDa band determined that it corresponded to the N-terminus of B7-H1 and contained the Ig-V ligand-binding domain required for interaction with the cognate receptor for B7-H1, PD-1 (Figure 2C). Similar results were obtained with supernates from other sB7-H1-positive cells including Karpas-299 and BT10B (data not shown). No detectable sB7-H1 material was collected from 2.2B affinity columns using supernates from membrane B7-H1-negative tumor cell lines (data not shown).
Figure 2. Soluble B7-H1 purification and sequencing.
(A) Supernatants from the sB7-H1-producing cell line SUDHL-1 were purified by a 2.2B affinity chromatography and the eluted fractions were tested by ELISA. Media before (SN) and flow-through (FT) after chromatography is shown as a control. Supernatants from sB7-H1-negative cell lines yielded undetectable levels of sB7-H1 and are therefore not depicted. (B) Immunoblotting the fraction with highest sB7-H1 levels revealed a positive band (arrow). For full blot refer to Supplementary Figure S5. (C) Peptides identified by MS/MS (shaded areas) confirmed that sB7-H1 retains the ligand-binding domain. Peptides that were excluded from the analysis because contained N-glycosylations (bold N) or were too small to be detected are shown in open boxes.
Effect of sB7-H1 on activated CD4 and CD8 T cell apoptosis
Protein sequencing of the 45KDa bands indicated retention of PD-1 binding domain by sB7-H1 molecules, suggesting that sB7-H1 protein may be biologically active and capable of triggering apoptotic signals in target T cells. Bead-purified human CD4 or CD8 T cells were activated with anti-CD3 for 3 days. Activation was confirmed by increased expression of PD-1 (Figure 3A). Activated CD4 or CD8 T cells were co-cultured with soluble recombinant B7-H1/Fc (or control fusion protein), pre-adsorbed with either B7-H1 blocking (MIH1) or isotype control antibody. After 16 hours, cells were harvested and analyzed for apoptotic activity. Exposure to soluble B7-H1/Fc increased apoptosis of activated CD4 T cells (p=0.031; Figure 3B, left panel) to a greater extent than CD8 T cells (p=0.276; Figure 3B, right panel). Apoptosis of CD4 T cells was mitigated by pre-incubation with specific blocking antibody (p=0.037). Control soluble P-Selectin/Fc failed to alter frequencies of apoptotic CD4 or CD8 T cells (Figure 3B). Therefore, sB7-H1 which retains the PD-1 binding domain can deliver immunosuppressive signals to T cells.
Figure 3. Effect of sB7-H1 on T cells.
(A) Expression of PD-1 on Activated T Cells. Purified human CD4 (left panels) and CD8 (right panels) T cells were analyzed for PD-1 expression immediately (upper panels) or after 3 days with anti-CD3 activation (lower panels). Percentages of positive cells were obtained after subtracting the isotype background (filled histograms). (B) Exposure of activated CD4 T cells (left panel) to solubilized rB7-H1/Fc (solid line) increases apoptosis versus control protein (shaded area; p=0.031). Specific blocking antibody reverses the effect (dashed line; p=0.037). Activated CD8 T cells (right panel) were not affected by solubilized hB7-H1/Fc (p=0.276). Figures are representative of 13 independent samples.
ELISA measurement of sB7-H1 in the sera of ccRCC patients
Clinical and pathologic features for the 172 ccRCC patients studied are summarized in Table 1. Mean age was 62 years (median 62; range 27–90) and mean SSIGN score was 3.8 (median 2; range 0–15). Among the 24 patients classified as M1 at nephrectomy, 5 were treated preoperatively with Sunitinib, 1 with Sorafenib, 1 with interferon alpha, and 1 with low-dose interleukin-2. Mean sB7-H1 level in the 172 ccRCC patient sera studied was 0.34 ng/mL (median 0.23; range 0–4.40); 7 patients (4%) had undetectable sB7-H1 levels. Associations of preoperative sB7-H1 levels with clinical and pathologic features are summarized in Table 1 and some selected associations are depicted in Figure 4. Preoperative sB7-H1 levels tended to increase as tumor extent and aggressiveness increased. There were statistically significant positive correlations between sB7-H1 levels and tumor size (correlation coefficient 0.26; p<0.001) and SSIGN score (correlation coefficient 0.24; p=0.001). Patients having tumors extending beyond the confines of the kidney (i.e., pT3a, pT3b, pT3c, or pT4) had significantly higher sB7-H1 levels compared with patients with localized tumors (p=0.017). There was also a trend for higher sB7-H1 levels among patients with distant metastases at surgery (p=0.076). Patients with high-grade or necrotic tumors had significantly higher levels of sB7-H1 compared with patients with low-grade (p=0.044) or non-necrotic tumors (p=0.003).
Table 1.
Associations of preoperative sB7-H1 levels (ng/mL) with clinical and pathologic features for 172 ccRCC patients
| Feature | N (%) | Mean (Median; Range) sB7-H1 | P-value |
|---|---|---|---|
| Age at Surgery (years) | |||
| <65 | 96 (56) | 0.32 (0.22; 0 – 3.00) | 0.308 |
| ≥65 | 76 (44) | 0.36 (0.26; 0 – 4.40) | |
| Gender | |||
| Female | 45 (26) | 0.40 (0.24; 0 – 4.40) | 0.438 |
| Male | 127 (74) | 0.32 (0.22; 0 – 3.00) | |
| Tumor Size (cm) | |||
| <5 | 75 (44) | 0.28 (0.21; 0 – 2.25) | 0.063 |
| 5 to <7 | 37 (22) | 0.32 (0.24; 0.02 – 3.00) | |
| 7 to <10 | 32 (19) | 0.49 (0.26; 0 – 4.40) | |
| ≥10 | 28 (16) | 0.37 (0.29; 0 – 1.25) | |
| 2002 Primary Tumor Classification | |||
| pT1a, pT1b | 102 (59) | 0.29 (0.22; 0 – 3.00) | 0.017 |
| pT2 | 17 (10) | 0.39 (0.21; 0 – 1.37) | |
| pT3a, pT3b, pT3c, pT4 | 53 (31) | 0.42 (0.29; 0 – 4.40) | |
| Regional Lymph Node Involvement | |||
| pNX/pN0 | 162 (94) | 0.34 (0.23; 0 – 4.40) | 0.232 |
| pN1/pN2 | 10 (6) | 0.34 (0.32; 0.14 – 0.86) | |
| Distant Metastases | |||
| M0 | 148 (86) | 0.34 (0.23; 0 – 4.40) | 0.076 |
| M1 | 24 (14) | 0.34 (0.30; 0.13 – 0.86) | |
| 2002 TNM Stage Groupings | |||
| I | 100 (58) | 0.30 (0.22; 0 – 3.00) | 0.079 |
| II | 14 (8) | 0.39 (0.20; 0 – 1.37) | |
| III | 33 (19) | 0.47 (0.26; 0 – 4.40) | |
| IV | 25 (15) | 0.33 (0.29; 0.13 – 0.86) | |
| Tumor Thrombus | |||
| None | 136 (79) | 0.31 (0.22; 0 – 3.00) | 0.086 |
| Level 0 | 21 (12) | 0.40 (0.32; 0 – 1.63) | |
| Level I – IV | 15 (9) | 0.55 (0.27; 0 – 4.40) | |
| Nuclear Grade | |||
| 1, 2 | 72 (42) | 0.26 (0.20; 0 – 1.66) | 0.044 |
| 3, 4 | 100 (58) | 0.40 (0.24; 0 – 4.40) | |
| Coagulative Tumor Necrosis | |||
| Absent | 126 (73) | 0.30 (0.22; 0 – 3.00) | 0.003 |
| Present | 46 (27) | 0.46 (0.30; 0 – 4.40) | |
Figure 4. Significant associations of preoperative sB7-H1 levels with pathologic features.
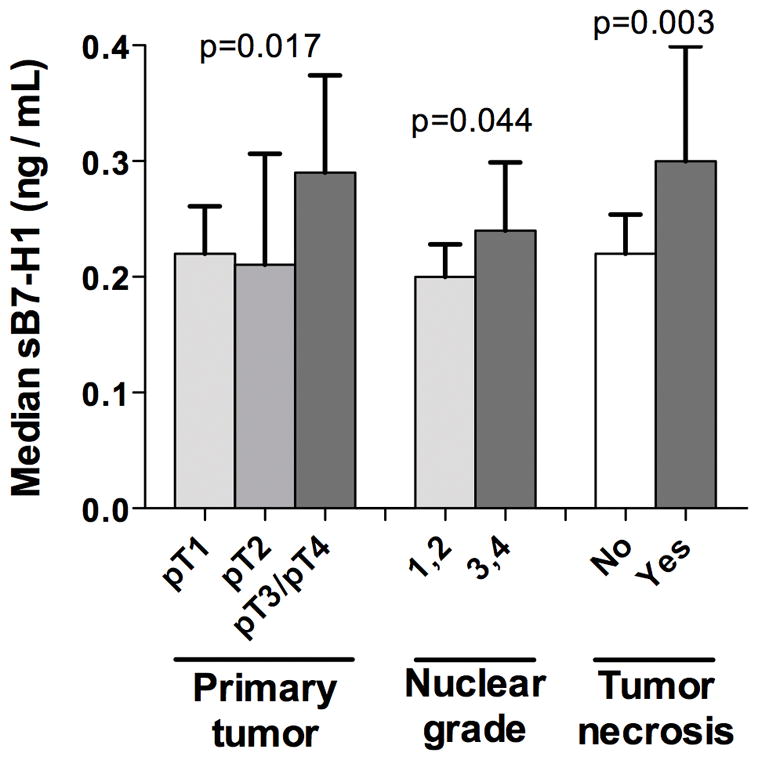
Error bars represent standard error of the mean (SEM).
At last follow-up, 39 of the 172 patients under study had died at a mean of 2.3 years following surgery (median 2.0; range 0.1–6.2), including 23 who died from RCC. Among the 133 patients still alive at last follow-up, the mean time from surgery to last follow-up was 3.8 years (median 3.6; range 0.1–7.3); only 4 (3%) of these patients had fewer than 2 years of follow-up. The hazard ratio for the univariate association of the log(2) transformed sB7-H1 with death from any cause was 1.41 (95% CI 1.08–1.83; p=0.010), indicating that a doubling of sB7-H1 was associated with a 41% increase in the risk of death. The hazard ratio for the association of log(2) sB7-H1 with death after adjusting for the SSIGN score was 1.29 (95% CI 0.95–1.76; p=0.11).
sB7-H1 in normal controls and ELISA reproducibility
sB7-H1 levels were significantly elevated for the 50 randomly selected ccRCC cases compared with normal controls (p<0.001), even after adjusting for age and gender (p=0.016). Mean sB7-H1 level for the 50 ccRCC patients was 0.42 ng/mL (median 0.25; range 0–6.21) compared with 0.20 ng/mL (median 0.21; range 0.13 – 0.57) for the 50 normal controls. Lin's concordance correlation coefficient for the duplicate sB7-H1 levels measured during the first experiment was 0.99 (p<0.001), indicating nearly perfect intra-assay reproducibility. Lin’s concordance correlation coefficient for the average of the duplicate sB7-H1 levels evaluated in two consecutive experiments was 0.74 (p<0.001), indicating substantial inter-assay reproducibility.
DISCUSSION
B7-H1, an immune inhibitory molecule expressed on the surface of activated T cells and macrophage-lineage cells (28), is aberrantly expressed on the membrane of many human tumors including kidney (9), bladder (29), ovary (30), breast (31), stomach (32) and lymphoma (33). We have reported that patients with ccRCC expressing even low amounts of membrane B7-H1-positive cells in their primary tumors or their metastases exhibit an increased risk of progression and cancer-specific death (10, 34). We hypothesized that tumor-derived B7-H1 may locally inactivate immune cells via B7-H1:PD-1 signaling, but systemic effects either by recirculation of immune cells through B7-H1-positive tumor sites or by the release of biologically active soluble forms of B7-H1 into the circulation cannot be excluded. Both scenarios can contribute to global immunosuppression.
In contradistinction to studies reporting detection of sB7-H1 (1, 3, 4, 6, 8), our study included biochemical confirmation of our analyte as B7-H1. Utilizing micro-sequencing of sB7-H1 isolated by affinity chromatography using 2.2B ELISA capture antibody we have determined that sB7-H1 derived from B7-H1 tumor cell lines retains the extracellular PD-1 binding domain. Because of limited individual patient volumes and technical concerns over pooling a large number of patient samples, sB7-H1 biochemical characterization necessitated the use of tumor cell culture supernates. In vitro tumor-derived sB7-H1 retained the signaling domain necessary for interacting with PD-1 on T cells and delivering immunoinhibitory signals. sB7-H1 may be a contributing factor in compromising anti-tumoral immune responses.
Whether sB7-H1 molecules comprise a population of full length and truncated or membrane clipped forms remains an unresolved question. Thus far, we have not detected the presence of intracellular B7-H1 sequences from affinity-purified preparations. It has been proposed that metaloproteinases may generate truncated soluble forms of coregulatory molecules (35). The intracellular region of B7-H1 may be more susceptible to serum proteases and rapidly degraded. Whether in vivo sB7-H1 is actively shed from tumor cells or is released by dying cells cannot be gleaned from the current data, although retention of the extracellular PD-1 binding domains suggests the presence of sB7-H1 may be one means by which tumors compromise immune responses.
This study distinguishes itself from past reports of other soluble B7-homologues - sB7-H4 in the sera of ovarian cancer (3) and RCC (5) patients and sB7-H3 in the sera of lung cancer patients (36) - because biochemical proof for the presence of the purported molecule was provided; a larger sample size was studied; and a statistically significant correlation between sB7-H1 and the pathologic features of tumor size, primary tumor classification, nuclear grade and tumor necrosis was established. Our study is the first to report an association of a sB7-H ligand with long-term patient outcome. Increased levels of sB7-H1 resulted in an elevated risk of death. The association of sB7-H1 levels with death was attenuated after adjusting for the SSIGN score, however, indicating that an even larger study will be needed to establish the utility of sB7-H1 as an independent prognostic biomarker (37). Nevertheless, we believe that our results provide potential insight into the mechanism by which a ccRCC tumor becomes clinically advanced.
These findings are consistent with our previous immunohistochemical studies wherein it was determined that expression of B7-H1 and B7-H4 correlated with different ccRCC pathologic features. Distinct immunohistochemical expression patterns of membrane B7-H1 (tumor cells) and membrane B7-H4 (tumor vasculature) may account for these varying associations. It is possible that simultaneously determining sB7-H1 and sB7-H4 levels might have more prognostic value than either soluble marker alone and studies are currently underway to test this possibility.
These results warrant further investigation. In this cohort, 8 patients (5%) were treated preoperatively for metastatic disease, which was not sufficient to evaluate the influence of various systemic treatments on preoperative sB7-H1 levels. Although we identified elevated preoperative sB7-H1 levels among patients with more aggressive tumors, it is not known if sB7-H1 levels fluctuate during tumor progression or remission. Likewise, although we determined that sB7-H1 levels in ccRCC patients were significantly higher than in normal controls, this study was not designed to establish sB7-H1 as screening tool. Rather, the goal of this study was to investigate associations of preoperative sB7-H1 levels with pathologic features and patient outcome for patients with confirmed ccRCC.
Treatments to inactivate or remove sB7-H1 molecules may be clinically beneficial. Surgical excision of the primary ccRCC tumor could remove a major reservoir of sB7-H1, resulting in a precipitous decline in post-surgery sB7-H1 levels and opening a window of opportunity for augmenting immune reactivity to residual tumor cells. However, circulating sB7-H1 may be replenished by B7-H1-expressing metastatic tumors. It is conceivable that both non-surgical treatments and anti-tumor responses themselves could inadvertently exacerbate sB7-H1 levels by inducing the release of active sB7-H1 by dying ccRCC cells. As phase I clinical trials begin, novel antibody capture therapies might offer a means to inactivate or remove noxious immunosuppressive sB7-H1 molecules and may have clinical benefit.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Expression of membrane B7-H1 immune inhibitory molecules by clear cell renal cell carcinoma (ccRCC) tumors is associated with poor patient outcome. Mechanisms by which membrane B7-H1-positive tumors cells, detected immunohistochemically, thwart anti-tumoral responses are largely conjectural. Using a B7-H1-specific ELISA we determined that higher preoperative levels of circulating soluble B7-H1 (sB7-H1) molecules in ccRCC patients were associated with aggressive pathologic features and an increased risk of death. Release of sB7-H1 molecules may be a tumor mechanism for impairing anti-tumoral responses systemically. Treatments to inactivate or remove serum sB7-H1 molecules may be clinically beneficial.
Acknowledgments
We thank Carol Preissner, Diana Gil-Pages, Adam Schrum and the Mayo Proteomics Research Center for technical assistance with our studies. We thank Tom Beito at the Mayo Antibody Hybridoma Core Facility for producing 5H1-A3 and 2.2B monoclonal antibody hybridomas. We thank Allan Dietz, Michael Gustafson and Mayo Clinic Department of Transfusion Medicine personnel for help obtaining normal control samples. Support for this work was provided by NIH/NCI R01 grant CA134345 as well as generous support from The Richard M. Schulze Family Foundation and the Mayo Foundation Career Development Award (H.D.).
Footnotes
DISCLOSURES: Both E.D. Kwon and the Mayo Clinic have received royalties greater than the federal threshold for significant financial interest from the licensing to Medarex of technology related to B7-H1. Additionally, E.D. Kwon and the Mayo Clinic have contractual rights to receive future royalties from the licensing of this technology. In addition, some of the authors above (E.D. Kwon, X. Frigola and H. Dong) have filed patents for potential use of B7-H1, B7-H3 and B7-H4 as prognostic markers for assessment of cancer.
BIBLIOGRAPHY
- 1.Hebbar M, Jeannin P, Magistrelli G, et al. Detection of circulating soluble CD28 in patients with systemic lupus erythematosus, primary Sjogren's syndrome and systemic sclerosis. Clin Exp Immunol. 2004;136:388–92. doi: 10.1111/j.1365-2249.2004.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeannin P, Magistrelli G, Aubry JP, et al. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity. 2000;13:303–12. doi: 10.1016/s1074-7613(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 3.Simon I, Zhuo S, Corral L, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer research. 2006;66:1570–5. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 4.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:989–94. doi: 10.1093/rheumatology/keh663. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RH, Zang X, Lohse CM, et al. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer research. 2008;68:6054–8. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan B, Nie H, Liu A, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–50. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen C, Barington T, Hansen S, Lillevang ST. Comment on "Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2007;178:4708. doi: 10.4049/jimmunol.178.8.4708. author reply 9. [DOI] [PubMed] [Google Scholar]
- 8.Her M, Kim D, Oh M, Jeong H, Choi I. Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus. 2009;18:501–7. doi: 10.1177/0961203308099176. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer research. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.de StGroth SF, Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35:1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- 13.Ritz C, Streibig J. Bioassay Analysis using R. J Statist Software. 2005;12:22. [Google Scholar]
- 14.Robison-Cox JF. Multiple estimation of concentrations in immunoassay using logistic models. J Immunol Methods. 1995;186:79–88. doi: 10.1016/0022-1759(95)00137-y. [DOI] [PubMed] [Google Scholar]
- 15.Wood S. Generalized Additive Models: An Introduction with R. Chapman & Hall/CRC; 2006. [Google Scholar]
- 16.Parker DC, Folpe AL, Bell J, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol. 2003;27:1–10. doi: 10.1097/00000478-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–9. [Google Scholar]
- 18.Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20:601–5. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Inman BA, Frigola X, Harris KJ, et al. Questionable Relevance of {gamma}{delta} T Lymphocytes in Renal Cell Carcinoma. J Immunol. 2008;180:3578–84. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietz AB, Bulur PA, Emery RL, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–9. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. The Journal of pathology. 1997;183:131–3. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. The Journal of urology. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 24.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 25.Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol. 2006;176:3000–9. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
- 26.Holets LM, Hunt JS, Petroff MG. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biology of reproduction. 2006;74:352–8. doi: 10.1095/biolreprod.105.046581. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 29.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 30.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–8. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–58. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–91. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 35.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–46. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang G, Xu Y, Lu X, et al. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. Journal of the National Cancer Institute. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.