Abstract
Objectives
To study the association of microinfarcts (MBI) to ante-mortem global cognitive function (CF), and to investigate whether brain weight (BW), Alzheimer’s lesions (neurofibrillary tangles (NFT) or neuritic plaques (NP) mediate the association.
Methods
Subjects are 437 well-characterized male decedents from the Honolulu Asia Aging Autopsy Study. Brain pathology was ascertained with standardized methods, CF was measured by the Cognitive Abilities Screening Instrument (CASI)and data were analyzed using formal mediation analyses, adjusted for age at death, time between last CF measure and death, education, and head size. Based on ante-mortem diagnoses, demented and non-demented subjects were examined together and separately.
Results
In those with no dementia, MBI were strongly associated with the last ante-mortem CF score; this was significantly mediated by BW, and not NFT or NP. In contrast, among those with an ante-mortem diagnosis of dementia, NFT had the strongest associations with BW and with CF, and MIB were modestly associated with CF.
Interpretation
This suggests microinfarct pathology is a significant and independent factor contributing to brain atrophy and cognitive impairment, particularly before dementia is clinically evident. The role of vascular damage as initiator, stimulator, or additive contributor to neurodegeneration may differ depending on when in the trajectory towards dementia the lesions develop.
Dementia is a devastating syndrome for which there are many questions about relevant pathogenic processes and their determinants. The global cerebral atrophy commonly observed in late-age dementia is generally interpreted as evidence of widespread neurodegeneration attributed to Alzheimer processes(1). However, existing data also suggests ischemic vascular disease may contribute additively or synergistically to the development of cognitive impairment or dementia(2).
The most frequently studied dementia-related cerebrovascular substrates are large and small focal ischemic lesions and white matter hyperintensities seen on neuroimaging (2). More recently several autopsy studies have identified microbrain infarcts [MBI] as a pervasive and very prevalent pathology that may contribute importantly to the pathophysiology or clinical presentation of dementia in an important sub-set of late-life cases(3–6). These lesions are different from larger infarcts in size and spatial distribution, are below the limits of resolution of today’s clinical neuroimaging methods and can only be seen on microscopic examination of brain tissue. Often, the lesions are widely distributed across brain regions, reflecting more global and diffuse ischemic processes. Due to the limited accessibility for study, little is known about the association of these MBI to dementia, or their possible role in processes leading to brain atrophy.
The Honolulu Asia Aging Study(HAAS)provides the opportunity to examine these MBI in a well characterized sub-cohort of autopsied decedent participants. Here we test the hypothesis that MBI are associated with brain atrophy and to ante-mortem cognitive impairment. To give us insight into relationships of these vascular lesions to neurodegenerative changes and cognitive function, we also examine whether AD lesions mediate the association of MBI to brain atrophy and to ante-mortem cognitive function.
Methods
Data are from the HAAS autopsy study, described in detail elsewhere (4,7). Briefly, the HAAS began in 1991 as a continuation of the Honolulu Heart Program (HHP). The HHP cohort was comprised of Japanese-American men born between 1900 and 1920 and living on Oahu during the baseline examination in 1965 (8). Evaluation for dementia began at the 1991–1993 examination when the men were aged 73–94 (mean age 78 years). The autopsy sub-study was initiated in 1992. All HAAS participants were eligible for inclusion into the autopsy sample with special targeting of men diagnosed with dementia. As reported previously demented decedents in the autopsy study did not differ from demented decedents not in the autopsy study; this was similar when comparing non demented decedents who did and did not participate in the autopsy study. Informed consent for all aspects of the research was obtained from participants or from a legally authorized caretaker if the subject was not competent or able to provide his own consent. Consents for autopsy were provided by a next-of-kin family member or a legally authorized alternative. All aspects of the study were approved by the Kuakini Hospital Institutional Review Board.
Assessment of cognitive function and dementia
Cognitive function and presence of dementia in the total cohort (n=3734), including the autopsied sample, were assessed during four exam cycles (1991–1993, 1994–1996, 1997–1999, and 2000–2002)by means of a multi-step procedure described elsewhere (8). Briefly, the entire cohort was screened with the 100-point Cognitive Abilities Screening Instrument (CASI), a cross-culturally validated test of global cognitive function designed for use in comparative studies of dementia in the United States and Japan(9). Screen positive individuals were further evaluated for dementia with neuropsychological testing, a clinical exam by a neurologist or specially trained geriatrician, an interview administered to a proxy informant, blood tests, and a brain scan. A consensus diagnosis was reached by the study neurologist and at least two other physicians with expertise in geriatrics and dementia. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (10).
Autopsy sub-study
This current analysis is based on autopsies obtained between April, 1992 and October, 2001 and includes 16% of the total cohort that died in that interval, of whom approximately 32% had a study diagnosis of dementia. Autopsied decedents did not differ significantly from non-autopsied decedents in age at death, education level, head circumference, apolipoprotein E ε4 allele type, cigarette smoking and alcohol intake history, and major chronic diseases.
Lesion assessment
Microscopic neuropathologic assessments were performed by one of three senior neuropathologists who had trained together for standardization. Several days of training took place at the University of Kentucky Center of Aging (Director W, Markesbery) where readers discussed, defined and standardized the definitions of the neuropathologic lesions. No formal intra/inter reader reliability statistics were calculated. The neuropathologist who carried out the assessment was blind to clinical features, including ante mortem cognitive function and dementia diagnoses. Assessment and definitions of individual lesions are described in previously published studies (4, 7), and briefly below.
Cerebrovascular lesions
One-centimeter-thick coronal sections of formalin-fixed cerebral hemispheres and 3–5 mm transverse sections of brainstem and cerebellum were examined for gross vascular lesions. Lacunar infarcts were defined as circumscribed, cavitary lesions not exceeding 1 cm in longest dimension on the cut surface of sections. Infarcts whose longest dimension exceeded 1 cm were classified as large infarcts. Hemorrhages were similarly defined as large or small. MBI were counted on microscopic examination of hematoxylin and eosin–stained sections from 26 specified brain regions. MBI were defined as focal lesions judged to be older than 1 month, not seen on gross exam. A MBI was neuropathologically described as a focus of neuronal loss or demyelination, with an associated gliosis. Most lesions were approximately round or elliptical, with somewhat irregular and indistinct margins. The greatest dimension (diameter or length) ranged between 50 and 400 microns. About 5% of such lesions had an irregular cystic center and were classified as microlacunes, but counted as microinfarcts. The microinfarct counts used for these analyses were the total number identified on sections from the isocortex (8 sections), caudate (2), putamen (2), globus pallidus (2), thalamus (2), hippocampus (2), nucleus basal is (2), amygdala (2), brainstem (1), pons (1), and cerebellum (2). Because the gross brain examination involved exhaustive inspection of 0.5–1.0 cm coronal sections of both hemispheres and all midline structures to the cord, it is likely only very few lacunar and small infarcts were not recognized.
Alzheimer’s type lesions
To count neocortical neuritic plaques (NP)and neurofibrillary tangles (NFT)a modified Bielschowsky method was used to stain one 8 μm section each from the left middle frontal gyrus, middle temporal gyrus, inferior parietal lobule, and occipital association cortex contiguous with the calcarine sulcus. NPs were defined as amyloid plaques containing dystrophic silver-positive neuritis. Those without recognizable neurites were counted as diffuse plaques. NPs and NFTs were counted in five fields in each of the four neocortical regions, at magnifications of 100 μ for NP and 200 μ for NFT. Fields were selected from areas observed to have high lesion densities on preliminary scanning. Analyses utilized average counts across 20 neocortical fields – 5 each from the neocortical regions mentioned. All NP and NFT counts were standardized to 1 mm areas, taking microscope-specific field areas into account. There was no upper limit to the number of NFTs counted. Counts of NP counts were truncated at 17/mm2 because of plaque coalescence at higher densities.
Head size
Head circumference measured at the 1994–1996 exam (approximately 70% of the HAAS baseline participants)was used as the primary measure of intracranial volume. If head circumference was missing, we estimated intracranial volume in from measured maximum intracranial bitemporal width obtained at autopsy and height obtained in midlife. The measures were combined using an algorithm based on anthropometric data and manually outlined intracranial volumes measured on MR brain scans acquired on the subset of HAAS participants (11).
Brain weight[BW]
Brains were removed from the calvarium using a standardized method that included removal of the dura, cutting cranial nerves at standard points, and separating the brain from the cord with a standard transection between the medulla and cervical cord. Brains were weighed immediately after removal, and again after approximately one month of fixation in neutral formalin, in both cases after allowing liquid to drain prior to weighing. Pre-and postfixation weights were very similar. In these analyses brain weight is used as an indicator of brain atrophy.
Statistical methods
We tested two hypotheses: 1) MBI are associated with brain weight independent of Alzheimer ’s disease lesions [neuritic plaques and neurofibrillary tangles]; 2) MBI are associated with ante-mortem cognitive status, brain weight is on the pathway [i.e., mediates] between MBI and brain weight[see Figure 1], and these associations are independent of AD lesions. Corollary to these main hypotheses are tests of whether AD lesions are directly related to MBI, brain weight or to ante-mortem cognitive function and whether they are significant mediators of the association MBI to brain weight and to ante-mortem cognition. Age at death, time between last CASI exam and death, education, and head size were included in all models to reduce their possible confounding effects on the analyses.
FIGURE 1.
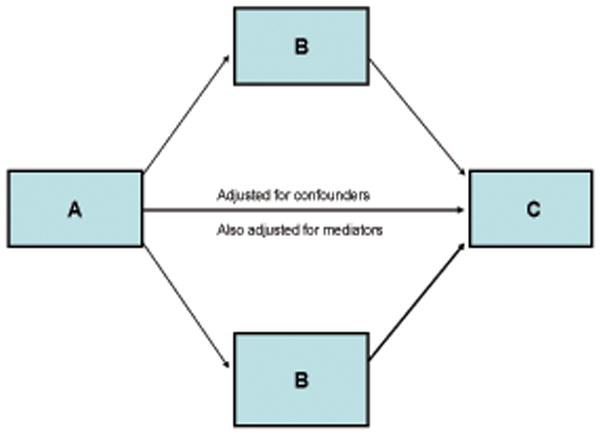
Model to test the mediating effect of variables (B)on the association of independent (A)and dependent (C)variables
Mediators and confounders act in a similar way in a statistical model – for a mediating or a confounding variable to be judged as significant, it should be associated with both the independent and dependent variables. However, conceptually a mediating variable is postulated to be a variable of interest in the research question, and a confounder is regarded as a ‘nuisance’ variable that can distort the inferences made from an analysis. Mediation should be tested in a model that also controls for confounding. The test of mediation is usually qualitative and accepted if the significance of a hypothesized association between variable A to C is reduced when a third variable is entered into the model that also includes confounding variables. Here we take a quantitative approach to assessing mediation (12, 13; Figure 1). With this approach we tested in one model each our two main hypotheses as well as the corollary hypotheses. The model quantifies the degree to which a variable (B)statistically mediates, i.e., changes (most often, but not always, in the direction of attenuation) the association of an dependent variable (A)with an independent variable(C)(Figure 1). Specifically, it generates estimates of the association of the dependent (A)to the independent variables (C), the association of the independent variable (C)to the mediator(s) (B), and the mediator(s) (B)to the dependent variable (A). The proportion of the variance of A to C that is attributed to B is calculated, and the A to C association is recalculated for the fully adjusted model. There is also a formal test of whether B is a significant mediator of the association of A to C ((c) on figure 1). Thus, this approach provides information on the mediation of B, as well as the direct independent association of B to A and to C. A boot strapping method was used to calculate a 90% Confidence Interval for the mediation effect (12). Analyses were conducted with statistical software, SAS and R (13, 14).
Analyses were conducted for the total autopsy sample(n=436), and separately for those who ante-mortem had a study identified clinically evident dementia (demented, D, n=144) and those who did not(non-demented, ND, 292). Thirty-eight percent of the ND group were mildly impaired (CDR scores = 0.5 or a last CASI score between 65 –74), or scored poorly on their last CASI (<65) but a possible or probable diagnosis of dementia could not be made with available data.
In preliminary analyses, we examined the association of brain weight to macroscopic vascular lesions (lacunar and large infarcts or hemorrhages), cortical Lewy Bodies, and hippocampal sclerosis. None of these lesions contributed significantly to brain weight after head size, MBI, AD lesions, and age at death were taken into account, so the analyses proceeded based on the core variables. Further, initial analyses confirmed prior observations that while infarcts seen on gross exam and MBI are significantly correlated, and each is individually correlated with cognitive impairment, the number of microinfarcts is significantly more robustly associated with impairment than other lesions. In addition, correlations between MBI and cognitive function do not substantially change after adjusting for the numbers and locations of lacunar or large infarcts, or hemorrhages.
Results
Men were mean age 85.9 (SD 5.25)yrs at death (Table 1). One third of the autopsied decedents had an ante-mortem diagnosis of dementia. MBI were identified in 64% of the brains examined. Demented and non-demented decedents differed, as expected, such that those with dementia were older, had lower CASI scores, lower brain weight, more NP and NFT, as well as more MBI.
Table 1.
Description of the sample by ante-mortem clinical diagnosis of dementia: HAAS autopsy study.
| Total Sample 436* |
Demented N=144 |
Non-demented N=292 |
|
|---|---|---|---|
| Age (yrs; Mean, SD) | 85.9 (5.25) | 87.1 (5.03) | 85.3 (5.26) |
| Education (% 9 yrs or less) | 47.6 | 52.1 | 45.2 |
| Last ante-mortem CASI (Median, 25th, 75th percentiles) | 70.7 (44.9,84.3) | 31.0 (10.7,56.1) | 80.9 (70.3,87.3) |
| Days between last CASI and death (Median, 25th, 75th percentiles) | 592 (315, 931) | 498 (285. 821) | 644 (347, 1002) |
| Estimated intracranial volume (ml3) | 1485 (60.1) | 1478 (58.8) | 1488 (60.5) |
| Brain weight (gms) | 1228 (121.20) | 1181.9 (110.2) | 1250.6 (120.0) |
| % with microinfarcts | 64.45 | 71.5 | 61 |
| Number of infarcts (Median, 25, 75th percentile; range) | 1 (0,3; range 0–43) | 2 (0,5; range 0–43) | 1 (0,3; range 0–15) |
| Isocortical Neurofibrillary tangles (av #/mm2) (Median, 25, 75th percentile) | 0.26 (0.0,1.28) | 0.77 (0.13,5.77) | 0.14 (0,0.61) |
| Isocortical Neuritic Plaques (av #/mm2) (Median, 25, 75th percentiles) | 0.20 (0.0,2.56) | 1.23 (0,4.53) | 0.04 (0,1.53) |
In the total sample(Table 2), MBI were significantly associated with brain weight (β= −2.83; p=0.014; line 1; eFigure 1). The association was especially strong in those decedents who were not demented at death (ND β=−5.04; p=0.017; eFigure 2). The addition to the models of NP and NFT, as possible mediators, changed the association only marginally (β=−2.76; p<0.02; line 2; eFigure 1). NFT were correlated to brain weight(β=−2.85; p=0.0012; efigure 1)in the total sample, a relation reflecting the strong association of NFT to brain weight in men with dementia (D β=−1.91, p=0.05, eFigure 3; ND β=−0.36; p=0.91; eFigure 2). NP were not associated with brain weight in the total, demented or non-demented groups. Thus, among men who did not have dementia at death, the strongest correlate of brain weight was MBI; in those who were demented at death, NFT, and not MBI, were most strongly associated with brain weight.
Table 2.
Association of microinfarcts and brain weight, mediated by AD lesions: the HAAS
| Microinfarcts | Total | Non Demented | Demented | |||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
| Direct effects | ||||||
| 1. No mediators* | −2.83 | 0.014 | −5.04 | 0.017 | 0.10 | 0.94 |
| 2. Adjusted for mediators† | −2.76 | 0.016 | −5.03 | 0.018 | −0.21 | 0.88 |
| Proposed Mediators – Indirect effects | ||||||
| β | 90% CI | β | 90 % CI | β | 90% CI | |
| 3. NP | −0.003 | (−0.11, 0.12) | −0.018 | (−0.24, 0.20) | −0.043 | (−0.28, 0.14) |
| 4. NFT | −0.069 | (−0.34, 0.22) | 0.0002 | (−0.12, 0.30) | 0.36 | (0.007, 0.86) |
| 5. Total mediating effect | −0.072 | (−0.35, 0.24) | −0.0178 | (−0.21, 0.33) | 0.315 | (−0.03, 0.79) |
Adjusted for time from test to death, age at death, education, and, to control for total intracranial volume we adjusted for head circumference or estimated intracranial volume.
Mediators include NP and NFT
MBI were significantly associated with CASI (β=−1.64; p<0. 0001; eFigure 4), especially in the non demented group (D β=−0.56, p=0.104; ND β= −1.03 p=0.003; table 3; eFigures 5–6). This association was significantly mediated by brain weight(β=−0.1851(−0.30, −0.08)), but not NP or NFT (Table 3, lines 3 and 4). However, the association in the total sample remained significant (β= −1.39, p<0.0001)even after including in the model brain weight, NFT and NP, suggesting other pathways through which MBI may affect ante-mortem cognition. Further, in this model, NFT were independently and significantly related to CASI score in the total sample (β= −1.4; p<0.0001; eFigure 4).
Table 3.
Association* of microinfarcts and last ante-mortem measure of cognitive function, mediated by brain weight and AD lesions: the HAAS
| Microinfarcts | Total | Non Demented | Demented | |||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
| Direct effects | ||||||
| 1. No mediators | −1.64 | <0.0001 | −1.03 | 0.003 | −0.56 | 0.10 |
| 2. Adjusted for mediators | −1.39 | <0.0001 | −0.85 | 0.01 | −0.70 | 0.03 |
| Proposed Mediators – Indirect effects | ||||||
| β | 90% CI | β | 90% CI | β | 90% CI | |
| 3. NP | −0.02 | (−0.08, 0.01) | −0.02 | (−0.10,0.05) | −0.002 | (−0.06,0.040) |
| 4. NFT | −0.03 | (−0.18, 0.10) | −0.0002 | (−0.04,0.05) | 0.13 | (0.015,0.30) |
| 5. Brain weight | −0.19 | (−0.30, −0.08) | −0.17 | (−0.30, −0.05) | 0.006 | (−0.11,0.15) |
| 6. Total Indirect effect | −0.24 | (−0.45, −0.05) | −0.19 | (−0.33, −0.03) | 0.14 | (−0.06,0.40) |
Adjusted for time from test to death, age at death, education, and, to control for total intracranial volume we adjusted for head circumference or estimated intracranial volume.
Results by dementia status differed: In the demented group (D), MBI were more strongly associated with CASI after controlling for NFT(Table 3 line 1; eFigure 6). In this group, NFT were a significant mediator of the association(line 4, table 3]. NFT were also directly associated with the last CASI score obtained prior to death (D β= −0.6919, =0.0046; eFigure 6). In contrast, among the ND (eFigure 5), the association of MBI to CASI was significant (ND β= −0.103, p=0.003), and remained significant after the potential mediators were entered into the model (line 2). Brain weight was a significant mediator in this group. NFT were not associated with last CASI score, and did not mediate the association of MBI to CASI.
Discussion
We found a significant association of higher numbers of MBI to lower brain weight and to poorer ante-mortem global cognitive performance measured by the CASI. The association with lower CASI score was partially mediated through lower brain weight. While NP were not significant correlates or mediators of BW, NFT were associated with BW, especially in demented decedents. In models containing MBI, NFT and NP, only NFT and MBI were significantly and independently correlated with CASI score.
There were differences in the magnitude of these associations between those who had vs. those who did not have an ante-mortem diagnosis of dementia. Compared to the D group, in the ND group, MBI were more strongly associated with brain weight. Consistent with these observations, we also found MBI were more strongly associated with a lower CASI score in ND. In contrast, among those who were diagnosed with dementia, more NFT were significantly associated with lower BW and CASI score in models that included both the cerebrovascular and Alzheimer lesions. NFT also marginally influenced the association of MBI to lower brain weight and to lower CASI scores(efigure 6).
Autopsy studies are by definition cross-sectional and cannot provide information on the temporal changes in the brain. Never-the-less, as other autopsy studies (1), our analyses may provide clues to the staging of lesions linked to dementia and to what is often considered normal aging-related declines in cognitive function. In this context, our data are consistent with the emerging hypothesis-generating model of late onset Alzheimer’s disease (16) published by Jack and colleagues. In this model brain changes evolve progressively from accumulating NP, NFT and decline in neuronal and synaptic reserves, evident as loss of brain tissue. Our findings that NFT are strongly related to brain weight and ante-mortem cognitive function in those with a dementia diagnosis are consistent with the proposal that NFT formation occurs downstream in the cascade, and is therefore the most strongly related to ante-mortem dementia.
We found MBI pathology is a significant and independent factor contributing to low brain weight, particularly in those who did not have an ante-mortem diagnosis of dementia. This suggests consequent vascular changes may occur relatively earlier in the trajectory to dementia. However, our non-demented group performed from good to poor on the CASI and may include subjects with insufficient data to support a diagnosis of dementia. Results for the demented group do suggest MBI are modestly associated with cognitive function, particularly when the models are adjusted for the variation in NFT. This increase in the statistical significance of MBI to the CASI score may reflect several different pathologic profiles. For instance, there is a wide distribution of NFT across subjects, so when we control for that variation, other factors contributing to cognitive function can be detected. There may also be a sub-group of subjects with a high MBI and low NFT load in the demented group.
One question that is currently debated is whether vascular damage initiates the cascade of neurodegeneration, plays a role in the progression of neurodegeneration, or adds to the pathology underlying cognitive impairment and dementia. The widely distributed vascular damage captured in MBI count could lead to ischemia, with attendant inflammation, oxidative stress, energy imbalance, glucocorticoid-mediated effects(17–19). It has been proposed, for instance, that APP is produced, or deposited, as an acute response to damage, which may be vascular (20). However, other data suggest vascular lesions contribute additively to the clinical presentation of dementia(6). Our findings suggest there may be multiple roles for vascular damage depending on the stage of disease. The role and position in the trajectory of dementia of vascular damage is critical to understand from both pathophysiologic and prevention perspectives. Studying the in vivo trajectory to dementia, although greatly aided by the imaging of amyloid deposition, remains difficult in the absence of bio-imaging biomarkers of microvascular disease, NFT and the other pathologies contributing to late-life dementia. However, these data suggest interventions to reduce vascular damage at an earlier, and possibly also a later stage of dementia may be beneficial to the clinical presentation.
The generalizability of these results should be taken into account. Our sample is based on Japanese American men. Possibly the results are modified by factors such as the type of education, cognitive reserve, the extent of unmeasured pathology in the brain or life style factors specific to this group. Possibly associations in women compared to men may differ for reasons that are under study. Second, we only examined a global measure of cognitive function in relation to a global measure of lesion load. More targeted examination of specific cognitive tests in relation to specific brain areas would be important to our understanding of how vascular lesions influence cognitive function.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health:National Institute on Aging, Grant Numbers 1 U01 AG19349 and 5 R01 AG017155 and the NIA Intramural Research Program.
Footnotes
Conflict of Interest: None of the contributing authors have any conflict of interest to disclose.
Author’s contribution: LRW designed the autopsy study and analysis; TMH analyzed data, LJL designed the analysis, supervised the project and wrote the first draft of the manuscript. The funding agency did not have any role in the preparation of this manuscript.
References
- 1.Braak H, Braak E. Neuropathologic staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Gold G. Defining the neuropathological background of vascular and mixed dementia and comparison with magnetic resonance imaging findings. Front Neurol Neurosci. 2009;24:86–94. doi: 10.1159/000197887. [DOI] [PubMed] [Google Scholar]
- 3.Fernando MS, Ince PC MRC Cognitive Function and Ageing Neuropthology Study Group. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;235:226, 13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.White L. Brain Lesions at Autopsy in Older Japanese-American Men as Related to Cognitive Impairment and Dementia in the Final Years of Life: A Summary Report from the Honolulu-Asia Aging Study. J Alzheimer’s Dis. 2009;18:713–25. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 5.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–4. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider JA, Aggarwal NT, Barnes LL, Boyle PD, Bennett DA. The neuropathology of older persons with and without dementia from community vs. clinic cohorts. Journal of Alzheimer’s Disease. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 8.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men living in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–60. [PubMed] [Google Scholar]
- 9.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- 11.Hartley SW, Scher AI, Korf ES, White LR, Launer LJ. Analysis and validation of automated skull stripping tools: a validation study based on 296 MR images from the Honolulu Asia aging study. Neuroimage. 2006;30:1179–86. doi: 10.1016/j.neuroimage.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 13.Taborga DP, Cheong JW, MacKinnion . Technical Assistance Report: Mediation Analysis. Research in Prevention Laboratory, NIDA Grant 5 R01 DA09757–04. Oct 18, 2000. [Google Scholar]
- 14.SAS Institute Inc. SAS v 9.0. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 15.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- 16.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer S. Is sporadic Alzheimer’s disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm. 1998;105:415–22. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 18.Baumbach GL. Changes in the cerebral circulation in chronic hypertension. In: Bevan RD, Bevan JA, editors. The human brain circulation. Humana Press; 1994. pp. 421–31. [Google Scholar]
- 19.Martins IJ, Hone E, Foster JK, Sünram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 20.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


