Abstract
Background
The effect of bariatric surgery on health care utilization and costs among individuals with type 2 diabetes remains unclear.
Objective
To examine healthcare utilization and costs in an insured cohort of individuals with type 2 diabetes after bariatric surgery.
Research Design
Cohort study derived from administrative data from 2002–2008 from 7 Blue Cross Blue Shield Plans.
Subjects
7,806 individuals with type 2 diabetes who had bariatric surgery
Measures
Cost (inpatient, outpatient, pharmacy, other) and utilization (number of inpatient days, outpatient visits, specialist visits).
Results
Compared to pre-surgical costs, the ratio of hospital costs (excluding the initial surgery), among beneficiaries who had any hospital costs, was higher in years 2 through 6 of the post-surgery period and increased over time [post 1: OR = 0.58 (95% CI: 0.50, 0.67); post 6: OR = 3.43 (95% CI: 2.60, 4.53)]. In comparison to the pre-surgical period, the odds of having any healthcare costs was lower in the post-surgery period and remained relatively flat over time. Among those with hospitalizations, the adjusted ratio of inpatient days was higher after surgery [post 1: OR = 1.05 (95% CI: 0.94, 1.16); post 6: OR = 2.77 (95% CI: 1.57, 4.90)]. Among those with primary care visits, the adjusted odds ratio was lower after surgery [post 1: OR = 0.80 (95% CI: 0.78, 0.82); post 6: OR = 0.66 (95% CI: 0.57, 0.76)].
Conclusion
In the six years following surgery, individuals with type 2 diabetes did not have lower healthcare costs than before surgery.
Obesity affects one-third of U.S. adults (1) and results in $147 billion of medical spending annually (2). Excess body weight is the single greatest predictor of developing diabetes (3), which is estimated to cost $153 billion in medical costs annually (4).
Bariatric surgery is an obesity intervention which produces sustained weight loss (5) and improvement in many obesity-related conditions, including diabetes (6). While there is a reduction in medical costs and prescription drug use after bariatric surgery, (7–9) (10) as well as sustained reductions in HbA1c (11), there is little information about utilization and cost outcomes in patients with type 2 diabetes after bariatric surgery. Understanding costs and utilization in this population is important given that one-third of all patients undergoing bariatric surgery have type 2 diabetes (12). If bariatric surgery substantially reduces use of health care resources and overall medical costs in patients with type 2 diabetes, then bariatric surgery may prove to be cost-saving.
The objective of this study was to examine the healthcare utilization and costs, in an insured cohort of individuals with type 2 diabetes after bariatric surgery, and compare this to utilization and costs before surgery. We hypothesized that total costs, pharmacy costs, hospitalizations and inpatient days, inpatient costs, specialist visits (e.g., endocrinologists, cardiologists) and primary care visits would be persistently lower by the end of the first year after surgery and in subsequent years due to improvements in heath status. We also hypothesized that outpatient costs would be higher in the first year after surgery due to surgery-related follow-up visits and then lower by the end of the 2nd and 3rd post-surgical years due to improvements in heath status and that hospital costs would be higher in the first year after surgery due to possible complications and then decline due to improvements in heath status. We tested these hypotheses using a large dataset of claims from beneficiaries insured by Blue Cross Blue Shield (BCBS).
METHODS
Design
This was a retrospective cohort study of individuals with type 2 diabetes who received bariatric surgery. In this study we compared the post-surgical costs and utilization to the pre-surgical costs and utilization with the assumption that the pre-surgical measures reflect the experience of individuals with diabetes who do not undergo surgery.
Data
We obtained claims data from 2002 to 2008 from commercially-insured individuals from seven Blue Cross Blue Shield (BCBS) health plans providing coverage across the United States: BCBS of Tennessee, BCBS of Hawaii, BCBS of Michigan, BCBS of North Carolina, Highmark, Inc. (of Pennsylvania), Independence Blue Cross (of Pennsylvania), Wellmark BCBS of Iowa, and Wellmark BCBS of South Dakota. Data from 2007 and 2008 were exclusively from Michigan and Highmark. The data included enrollment files; benefits information to determine medical and pharmacy coverage; and inpatient, outpatient, and pharmacy claims records containing International Classification of Disease-9 Clinical Modification (ICD-9-CM) codes, Common Procedural Terminology (CPT) codes, prescription National Drug Codes (NDC), and costs and charges (submitted, allowed, and paid).
The data were de-identified in accordance with the Health Insurance Portability and Accountability Act’s (HIPAA) definition of a limited data set. The Johns Hopkins University Office of Research Subjects deemed the study to be exempt from federal regulations because the research activities were considered to be of minimal risk to subjects.
Inclusion criteria
Analytic cohort
BCBS beneficiaries were included in the analytic cohort if they had 6 months of continuous coverage in each of the one-year intervals, including pharmacy coverage, prior to the date of bariatric surgery; were between 18 years and 64 years of age, inclusive; did not have an ICD-9 code for cancer of the esophagus (ICD-9 150–150.9), stomach (ICD-9 151–151.9), small intestine (ICD-9 152–152.9) or pancreas (ICD-9 157–157.9), or malignant neoplasm without specification of site (ICD-9 199–199.2).
Individuals with bariatric surgery
BCBS beneficiaries were classified as having bariatric surgery if they had any of the ICD-9 procedure codes or CPT codes for bariatric surgery as shown in Appendix A. For four of the ICD-9 codes, we required that a code for morbid obesity (ICD-9-287.1) be present concurrent with the procedure code. If a relevant surgery was coded but the primary diagnosis code indicated ulcer disease (Appendix A), we excluded the individuals as the surgery was unlikely to have been primarily for treatment of obesity. We excluded individuals if they had a code indicating a revision procedure prior to the bariatric procedure of interest, suggesting that there had been an earlier bariatric surgery (Appendix A).
Individuals with type 2 diabetes
BCBS beneficiaries were classified as having type 2 diabetes if they had one relevant inpatient code or two outpatient codes separated by at least 30 days (relevant codes: 250.xx, 648.0 diabetes mellitus with pregnancy; 362.0: diabetic retinopathy, or 266.41: diabetic cataract). Individuals were also classified as having diabetes if they filled a prescription for a medication for treatment of hyperglycemia anytime preceding surgery (Appendix B). If the prescription was for metformin alone, the individual was also required to have an ICD-9 code for diabetes for inclusion in this group. Individuals who only had codes for type 1 diabetes (250.x3) or gestational diabetes (648.83) were excluded.
Outcomes
The utilization variables included the number of inpatient days, outpatient visits, and outpatient subspecialist visits; hereafter referred to as specialist visits (e.g., endocrinologists, cardiologists). We also examined total annualized costs as well as several sub-categories–inpatient costs, outpatient costs, pharmacy costs and other costs (professional office and professional other) – which we standardized to adjust for differences in reimbursement between plans and for inflation over time. Professional office services refer to services rendered in an office and include things such as visit costs, laboratory costs, and diagnostic imaging. Professional other services refer to all other charges and include things such as diagnostic lab facilities, imaging facilities, home health care, or nursing facility. Emergency costs and emergency visits were excluded from the analyses because most beneficiaries included in our sample (96%) had none. This did not change our results.
Inpatient costs were standardized using the Diagnosis Related Groups (DRG) and the Centers for Medicare and Medicaid Services (CMS) national average inpatient cost per DRG. Professional services costs were standardized using the procedure code and the CMS Resource-Based Relative Value Scale (RBRVS) unit for the procedure. All other costs were calculated using the total amount paid as submitted on the claim (total amount paid = amount paid by plan + amount paid out-of-pocket by patient including co-pay, deductible and coinsurance + amount paid for coordination of benefits). If an inpatient cost or professional services cost could not be standardized due to an invalid DRG or invalid procedure code, respectively, it was standardized using the total amount paid. The standardized costs were trimmed at the 99th percentile for each of the years following surgery.
Post-surgery outcomes were calculated beginning on the day of discharge and did not include the direct costs or health care utilization associated with the hospitalization for the bariatric surgery.
Analyses
To test for differences in the pre- and post-surgery periods, we compared post-surgical costs and utilization to pre-surgical costs and utilization. All outcomes post-surgery were measured in one-year intervals. Cost and utilization variables were annualized for each individual based on at least six months of data.
To test for differences in the pre- and post-surgery periods for the utilization outcomes, we used zero-inflated negative binomial regression to examine differences in inpatient days, primary care visits and specialist visits. Due to the repeated measurements among individuals, we used bootstrap methods in which resampling was done on the cluster level in order to estimate the variance of the parameters in the models (13). To examine differences in costs, we used a two-part model to account for the skewed nature of the data where 1) logistic regression was used to estimate differences in the probability of any costs (reported as odds ratios, OR) and 2) a generalized linear model with a log link function and a gamma distribution of the error term was used to estimate differences in costs between the pre- and post-surgery intervals among those who had any costs (reported as cost ratios, CR). The first part of the model predicted the probability of an individual having any costs, and the second part predicted costs or utilization conditional on the individual having positive costs. Costs analyses were conducted using raw and truncated (at 99%) costs. We report the results from the analyses using raw costs since the difference between the two results was very small.
In all models, we adjusted for age, sex, health plan, and propensity to be obese – a measure validated in our claims data (14). We also adjusted for diabetes severity at baseline measured by the Diabetes Complications Severity Index (DCSI) which had been previously validated as predictive of hospitalizations and death (15). We validated the DCSI in our own data, which lacked the two laboratory measurements that are in the initial scale. As in the original study, we found that this index is predictive of hospitalizations. To examine and control for comorbidities other than those that are diabetes-related, we used the Johns Hopkins University Adjusted Clinical Groups®(ACG) Case-Mix System (16). ACGs are mutually exclusive health status categories defined by morbidity pattern, age, and sex. The comorbidity index is predictive of resources to be consumed in the next year. A higher comorbidity value indicates a higher morbidity burden.
Analyses were performed using (SAS, version 9.1; SAS Institute Inc, Cary, North Carolina).
RESULTS
Our study sample included 31,940 individuals who received bariatric surgery; 21,898 individuals were excluded for not having diabetes, missing data on sex or for being outside of the age range; 2236 individuals were excluded for lacking at least one medical claim in the first six months of enrollment. Our final analytic sample included 7806 beneficiaries of which 6,376 had six plus months of enrollment in pre-surgery period.
Table 1 presents the characteristics of the study sample in the pre-surgery period and during the six post-surgical years. Approximately one quarter of the sample was male with a mean age of 47 years in the pre-surgical period. The distribution of comorbidities and diabetes severity were similar during the pre- and post-surgical periods.
Table 1.
Characteristics of the Cohort of Individuals with Type 2 Diabetes Before and After Bariatric Surgery
| Pre-Surgery | Post Year 1 | Post Year 2 | Post Year 3 | Post Year 4 | Post Year 5 | Post Year 6 | ||
|---|---|---|---|---|---|---|---|---|
| N | 6376 | 5623 | 4057 | 3113 | 2017 | 1109 | 502 | |
| Sex | Male | 24.3% | 24.0% | 24.5% | 23.0% | 22.4% | 22.1% | 21.5% |
| Age (years) | Mean (SD) | 47.1 (0.1) | 47.2 (0.1) | 47.7 (0.2) | 47.8 (0.2) | 47.6 (0.2) | 47.3 (0.3) | 47.0 (0.4) |
| Comorbidity Level | 0 (most healthy) | 21.6% | 21.5% | 22.0% | 21.8% | 22.5% | 23.7% | 21.9% |
| 1 | 20.2% | 19.9% | 19.0% | 18.7% | 18.7% | 18.4% | 18.5% | |
| 2 | 20.5% | 20.0% | 20.2% | 19.7% | 18.4% | 16.8% | 16.3% | |
| 3 | 21.1% | 21.0% | 21.3% | 21.6% | 21.2% | 21.7% | 22.5% | |
| 4 (least healthy) | 16.5% | 17.7% | 17.5% | 18.3% | 19.1% | 19.4% | 20.7% | |
| Diabetes Complications Severity Index | 0 | 88.7% | 88.8% | 88.4% | 88.4% | 88.6% | 88.0% | 87.9% |
| 1 | 10.7% | 10.7% | 11.0% | 11.1% | 11.1% | 11.6% | 11.6% | |
| 2 or more | 0.5% | 0.6% | 0.6% | 0.6% | 0.4% | 0.4% | 0.6% | |
Table 2 presents the mean and median cost and utilization outcomes. The raw data demonstrate an increase in total healthcare costs in the post-surgical period, which was primarily due to higher inpatient and outpatient costs. We also observed an increase in inpatient days, and a decrease in primary care and specialist visits in the post-surgery periods compared to the pre-surgery period. Appendix D presents mean and median costs for laparoscopic bariatric surgery and open bariatric surgery separately. Total pre-surgical costs were lower among individuals receiving laparoscopic surgery. Like the overall sample, the increase in total healthcare costs post-surgery for both groups was primarily driven by higher inpatient and outpatient costs.
Table 2.
Mean and Medians for Costs and Utilization Measures for Individuals with Diabetes Before and After Bariatric Surgery
| Pre-Surgery | Post Year 1 | Post Year 2 | Post Year 3 | Post Year 4 | Post Year 5 | Post Year 6 | ||
|---|---|---|---|---|---|---|---|---|
| Costs | ||||||||
| Mean (SD) dollars/person | Total | $9,326 ($17,258) | $13,400 ($22,368) | $14,240 ($24,913) | $13,109 ($20,870) | $12,918 ($24,605) | $13,408 ($26068) | $13,664 ($28,703) |
| Inpatient | $2,101 ($9,819) | $5,213 ($16,595) | $5,695 ($18,191) | $4,772 ($14,024) | $4,603 ($18,143) | $4,437 ($15,355) | $5,185 ($10,248) | |
| Outpatient | $3,149 ($8,290) | $3,796 ($6,683) | $4,437 ($8,917) | $4,398 ($8,479) | $4,393 ($8,467) | $5,103 ($14,114) | $4,750 ($10,248) | |
| Other† | $2,274 ($3,787) | $2,526 ($4,918) | $2,476 ($5,330) | $2,287 ($3,778) | $2,334 ($4,686) | $2,261 ($3,029) | $2,168 ($3,639) | |
| Pharmacy | $1,781 ($3,579) | $1,825 ($3,558) | $1,607 ($3,229) | $1,637 ($3,621) | $1,584 ($3,066) | $1,607 ($3,317) | $1,561 ($2,744) | |
| Median (IQR) dollars/person | Total | $4,674 ($7,437) | $6,250 ($11,736) | $5,743 ($14,729) | $5,246 ($14,134) | $5,096 ($12,484) | $5,399 ($13,640) | $4,811 ($13,060) |
| Inpatient | $0 ($0) | $76 ($413) | $0 ($0) | $0 ($0) | $0 ($0) | $0 ($0) | $0 ($0) | |
| Outpatient | $1,232 ($2,718) | $1,477 ($4,077) | $1,265 ($4,874) | $1,228 ($4,949) | $1,258 ($5,055) | $1,501 ($5,288) | $1,074 ($5,090) | |
| Other‡ | $1,231 ($1,792) | $1,514 ($2,307) | $1,344 ($2,571) | $1,273 ($2,515) | $1,298 ($2,478) | $1,275 ($2,857) | $1,205 ($2,488) | |
| Pharmacy | $712 ($2,049) | $775 ($2,304) | $384 ($1,906) | $313 ($1,979) | $305 ($1,965) | $235 ($1,905) | $242 ($2,051) | |
| Utilization | ||||||||
| Mean (SD) days/person visits/person | Inpatient days | 0.9 (3.4) | 5.0 (11.0) | 1.4 (5.5) | 1.2 (4.8) | 1.2 (5.4) | 1.0 (3.2) | 1.8 (12.2) |
| Primary care visits | 5.2 (5.0) | 4.1 (4.8) | 3.2 (4.4) | 2.8 (3.9) | 2.9 (4.1) | 2.8 (3.9) | 2.6 (4.1) | |
| Specialist visits | 6.1 (5.7) | 4.8 (6.1) | 4.3 (6.1) | 3.7 (5.2) | 3.7 (5.6) | 3.8 (5.0) | 3.6 (5.0) | |
| Median (IQR) days/person visits/person | Inpatient days | 0.0 (0.0) | 3.0 (3.0) | 0.0 (0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Primary care visits | 4.0 (7.0) | 3.0 (6.0) | 2.0 (5.0) | 1.5 (4.0) | 1.5 (4.0) | 1 (4.0) | 1.2 (3.6) | |
| Specialist visits | 5.0 (6.6) | 3.0 (5.9) | 3.0 (5.0) | 2.0 (5.0) | 2.0 (5.0) | 2.0 (5.0) | 2.0 (5.3) |
Includes professional office and professional other costs such as visit costs, lab, and diagnostic imaging.
Notes: Each post year refers to time after surgery. For example, “Post 1” is equivalent to the first year after surgery. SD = Standard deviation; IQR = Interquartile range
Change in costs post-surgery
Total healthcare costs
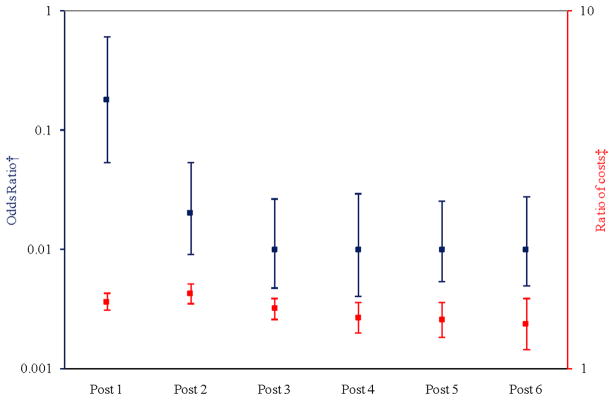
Figure 1 illustrates the adjusted odds of having any healthcare costs (left axis, blue bars) and the adjusted ratio of total mean costs in the post-surgical period as compared to the pre-surgical period (right axis, red bars). In comparison to the pre-surgical period, the odds of having any healthcare costs were much lower in the post-surgery period and remained relatively flat over time. However, the absolute numbers of beneficiaries having no healthcare costs were low in the pre-surgical (0.05%) and post-surgical periods [post year 1: (0.3%), post year 2: (2.6%), post year 3: (4.8%), post year 4: (3.9%), post year 5: (4.1%), post year 6: (4.0%)]. The ratios of total healthcare costs among beneficiaries with any costs, however, were higher in the post-surgical period compared to the pre-surgical period but declined overtime. In short, most beneficiaries had some healthcare costs after surgery, and among those who had costs, their healthcare expenditures were higher after surgery than before surgery. Point estimates are in Appendix C.
Figure 1.
Adjusted Odds of Any Costs and Adjusted Ratio of Mean Total Costs in Post-surgical Periods compared to Pre-surgical Period
† Probability of having any positive costs (
 )
)
‡ Cost ratio conditional on having any positive costs (
 )
)
Notes: Each post year refers to time after surgery. For example, “Post 1” is equivalent to the first year after surgery. The squares represent the point estimate for each year along with the corresponding confidence interval. The model controlled for morbidity level, Diabetes Complications Severity Index (DCSI), sex, health plan site, linear age, and obesity propensity. Y-axes are presented on logarithmic scales.
Inpatient costs
In contrast to the total cost results, the odds that beneficiaries would have any inpatient costs increased in the three years immediately following surgery, particularly the first post-surgery year, and then declined in the subsequent three years [post 1: OR = 12.28 (95% CI: 11.18, 13.48); post 2: OR = 1.41 (95% CI: 1.27, 1.57); post 3: OR = 1.17 (95% CI: 1.04, 1.31); post 4: OR = 0.95 (95% CI: 0.82, 1.09); post 5: OR = 0.92 (95% CI: 0.77, 1.11); post 6: OR = 0.69 (95% CI: 0.52, 10.91)]. The ratios of hospital costs, among those beneficiaries who had any hospital costs, were higher in years two through six of the post-surgery period and increased overtime [post 1: cost-ratio (CR) = 0.58 (95% CI: 0.50, 0.67); post 2: CR = 2.26 (95% CI: 1.97, 2.58); post 3: CR = 2.26 (95% CI: 1.96, 2.61); post 4: CR = 2.60 (95% CI: 2.12, 3.18); post 5: CR = 2.52 (95% CI: 2.07, 3.06); post 6: CR = 3.43 (95% CI: 2.60, 4.53)].
Outpatient costs
The odds that beneficiaries would have any outpatient costs were lower in the post-surgery period compared to the pre-surgery period (post 1: OR = 0.50 (95% CI: 0.44, 0.57); post 2: OR = 0.25 (95% CI: 0.22, 0.29); post 3: OR = 0.17 (95% CI: 0.15, 0.20); post 4: OR = 0.16 (95% CI: 0.14, 0.19); post 5: OR = 0.15 (95% CI: 0.13, 0.18); post 6: OR = 0.13 (95% CI: 0.10, 0.16). However, among those beneficiaries who had any outpatient costs, the ratios of costs were higher in all post-surgery periods (post 1: CR = 1.34 (95% CI: 1.25, 1.43); post 2: CR = 1.55 (95% CI: 1.43, 1.69); post 3: CR = 1.49 (95% CI: 1.36, 1.63); post 4: CR = 1.38 (95% CI: 1.24, 1.52); post 5: CR = 1.44 (95% CI: 1.24, 1.69); post 6: CR = 1.44 (95% CI: 1.18, 1.75).
Pharmacy costs
The odds of having any pharmacy costs were lower in the post-surgery period as compared to the pre-surgery period (post 1: OR = 0.98 (95% CI: 0.93, 1.03); post 2: OR = 0.68 (95% CI: 0.63, 0.74); post 3: OR = 0.57 (95% CI: 0.52, 0.62); post 4: OR = 0.56 (95% CI: 0.50, 0.62); post 5: OR = 0.51 (95% CI: 0.44, 0.59); post 6: OR = 0.46 (95% CI: 0.37, 0.58). However, among those beneficiaries who had any pharmacy costs, the ratios of costs were higher in the post-surgery periods compared to the pre-surgery period (post 1: CR = 1.09 (95% CI: 1.04, 1.14); post 2: CR = 1.04 (95% CI: 0.97, 1.11); post 3: CR = 1.08 (95% CI: 1.00, 1.16); post 4: CR = 1.10 (95% CI: 0.99, 1.21); post 5: CR = 1.18 (95% CI: 1.03, 1.35); post 6: CR = 1.20 (95% CI: 1.03, 1.41).
Other costs
As compared to the pre-surgery time, the odds of having any other costs (professional office and professional other such as visit costs, lab, and diagnostic imaging) were substantially lower in the post-surgery periods [post 1: OR = 0.12 (95% CI: 0.07, 0.21); post 2: OR = 0.03 (95% CI: 0.02, 0.06); post 3: OR = 0.02 (95% CI: 0.01, 0.03); post 4: OR = 0.02 (95% CI: 0.01, 0.03); post 5: OR = 0.01 (95% CI: 0.01, 0.02); post 6: OR = 0.01 (95% CI: 0.01, 0.02)]. However, the ratios of other costs, among those beneficiaries who had any other costs, were higher in the post-surgery periods [post 1: CR = 1.13 (95% CI: 1.07, 1.19); post 2: CR = 1.13 (95% CI: 1.06, 1.21); post 3: CR = 1.08 (95% CI: 1.01, 1.16); post 4: CR = 1.07 (95% CI: 0.98, 1.16); post 5: CR = 1.07 (95% CI: 0.98, 1.17); post 6: CR = 1.01 (95% CI: 0.87, 1.19)].
Laparoscopic bariatric surgery vs open bariatric surgery
We additionally conducted post-hoc, exploratory stratified analyses to examine costs for laparoscopic bariatric surgery and open bariatric surgery separately (see Appendix E). We found that among beneficiaries in the laparoscopic and open surgical groups who had any costs; total costs, inpatient costs and outpatient costs were higher after surgery than before surgery, as was seen in the combined results. However, among those who had any costs; pharmacy costs and other costs were higher post-surgery in the laparoscopic group (as in the combined results), but were lower post-surgery in the open surgical group (in contrast to the original results). As in the combined analyses, we observed similar patterns among both groups in the odds that beneficiaries would have any costs post-surgery and the ratio of costs among those beneficiaries who had any costs in the six years post-surgery.
Change in utilization post-surgery
Inpatient days
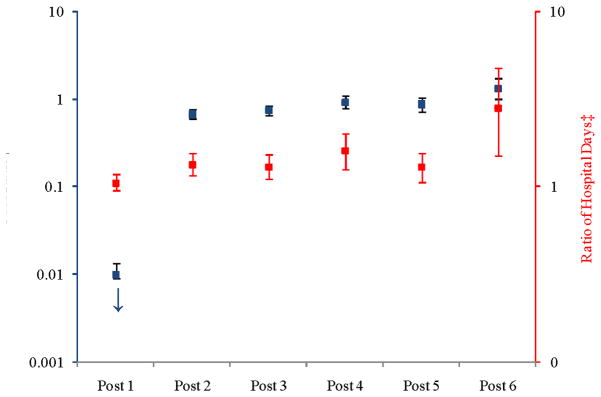
Figure 2 illustrates the adjusted odds of having no hospitalizations (left axis, blue bars) and the adjusted ratio of hospitalizations in the post-surgery period compared to the pre-surgery period (right axis, red bars). In comparison to the pre-surgical period, the odds of beneficiaries having no hospitalization were lower in the first three years post-surgery, almost the same in the 4th and 5th year post-surgery and higher in the 6th year post surgery. Among those beneficiaries who had hospitalizations, the adjusted ratio of inpatient days was higher in the post-surgery period. Point estimates are in Appendix C.
Figure 2.
Adjusted Odds of a Hospitalization and Adjusted Ratio of Counts of Hospitalization in Post-surgical Periods compared to Pre-surgical Period
† Probability of having no hospitalizations (
 )
)
‡ Count ratio conditional on having any hospitalization (
 )
)
Notes: Each post year refers to time after surgery. For example, “Post 1” is equivalent to the first year after surgery. The squares represent the point estimate for each year along with the corresponding confidence interval. The model controlled for morbidity level, Diabetes Complications Severity Index (DCSI), sex, health plan site, linear age, and obesity propensity. We used the biased corrected percentile confidence intervals (rather than the normal theory confidence intervals) to account for the skewed nature of the data. Y-axes are presented on logarithmic scales. The odds ratio for post-year 1 is OR = 3.27 × 10−11 (95% CI: 6.24 × 10−16, 1.72 × 10−6).
Specialist visits
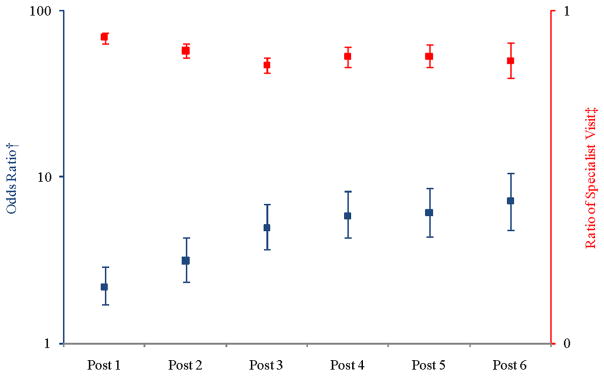
Figure 3 illustrates the adjusted odds of having no specialist visits (left axis, blue bars) and the adjusted ratio of specialist visits in the post-surgery period compared to the pre-surgery period (right axis, red bars). The odds of beneficiaries having no specialist visits were higher in the post-surgery period as compared to the pre-surgery period. Among those beneficiaries who had specialist visits, the adjusted ratio of specialist visits was lower in the post-surgery period. Point estimates are in Appendix C.
Figure 3.
Adjusted Ratio of Counts of Specialist Visits in Post-surgical Periods compared to Pre-surgical Period
† Probability of having no specialist visits (
 )
)
‡ Count ratio conditional on having any specialist visits (
 )
)
Notes: Each post year refers to time after surgery. For example, “Post 1” is equivalent to the first year after surgery. The squares represent the point estimate for each year along with the corresponding confidence interval. The model controlled for morbidity level, diabetes severity index, sex, health plan site, linear age, and obesity propensity. We used the biased corrected percentile confidence intervals (rather than the normal theory confidence intervals) to account for the skewed nature of the data. Y-axes are presented on logarithmic scales.
Primary care visits
Figure 4 illustrates the adjusted odds of having no primary care visits (left axis, blue bars) and the adjusted ratio of primary care visits in the post-surgery period compared to the pre-surgery period (right axis, red bars). The odds of beneficiaries having no primary care visits were higher in the post-surgery period as compared to the pre-surgery period. Among those beneficiaries who had primary care visits, the adjusted ratio of primary care visits was lower in the post-surgery period. Point estimates are in Appendix C.
Figure 4.
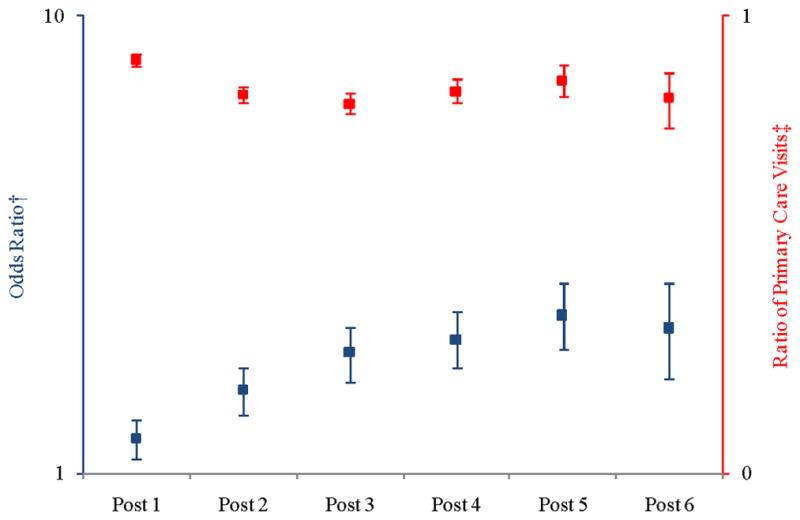
Adjusted Ratio of Counts of Primary Care Visits in Post-surgical Periods compared to Pre-surgical Period
† Probability of having no primary care visits (
 )
)
‡ Count ratio conditional on having any primary care visits (
 )
)
Notes: Each post year refers to time after surgery. For example, “Post 1” is equivalent to the first year after surgery. The squares represent the point estimate for each year along with the corresponding confidence interval. The model controlled for morbidity level, diabetes severity index, sex, health plan site, linear age, and obesity propensity. We used the biased corrected percentile confidence intervals (rather than the normal theory confidence intervals) to account for the skewed nature of the data. Y-axes are presented on logarithmic scales.
DISCUSSION
This paper examined the impact of bariatric surgery on healthcare costs and utilization among adults with type 2 diabetes. For up to six years after having bariatric surgery, individuals with type 2 diabetes are more likely to have higher healthcare expenditures (e.g., total mean costs were $9,326 pre-surgery, $13,400 one year after surgery and $13,664 six years after surgery), are more likely to have hospitalizations, but are less likely to have primary care and specialist visits compared to their respective costs and utilization prior to surgery. With the exception of our finding that specialist and primary care visits were lower after surgery, these results are contrary to what we hypothesized. Lower pharmacy costs in the post-surgery period among beneficiaries who received open bariatric surgery might reflect individuals in this group coming off their medications more quickly than individuals who received laparoscopic bariatric surgery. While the change in pharmacy costs from pre-surgery to post-surgery was greater among the group receiving open bariatric surgery, absolute costs were lower in the laparoscopic group compared to the open group in both the pre- and post-surgery periods.
This study contradicts previous research showing cost savings after bariatric surgery in a general population (17–18) and among individuals with type 2 diabetes (10, 19) as well as research showing that bariatric surgery is cost-effective among the severely obese with diabetes (20). It is consistent with recent research showing higher health care expenditures after bariatric surgery (21) and higher hospital use after Roux-en-Y gastric bypass (22). Differences between our results and earlier studies could be due to the relatively large number of laparoscopic surgeries in our cohort (~40%), our larger sample size or our considerably longer length of follow-up.
One possible explanation for our finding of higher costs and utilization after surgery could be complications following bariatric surgery, which have been documented in the literature (23), particularly in the first post-operative year (22). In our data, we found that individuals were much more likely to have costs in the first year after surgery than in the pre-surgical time.
While these results do not support bariatric surgery as a cost savings procedure among individuals with diabetes, there is recent research which suggests that bariatric surgery is safe in obese, diabetic patients (24) and has clinical benefits in this patient population (25). Our finding that specialist and primary care visits declined after surgery deserves future study. On one hand, it is expected that individuals should have continued interactions with health care providers for preventative services and health maintenance for other comorbid conditions. On the other, benefits from surgery, such as weight loss, may have resolved diabetes in some patients and reduced their need for frequent clinical encounters.
In addition to the costs identified in this study, there is also lost productivity, including presenteeism and absenteeism, which may affect employers’ decisions regarding the coverage of bariatric surgery. Recent research suggests that the annual cost attributable to obesity among full-time employees is $73.1 billion [3]. Individuals with a body mass index greater than 35 kg/m2 represent 37% of the obese population but are responsible for 61% of excess costs. Our finding that bariatric surgery is not cost-saving among adults with diabetes may discourage employer interest in providing this coverage. However, a reduction or elimination of weight-related comorbidities, improved quality of life and improved mobility as a result of surgery may make this a valuable procedure in this population of patients.
This study was restricted to adults between the ages of 18 and 64 years. The Medicare program, for adults ages 65 and older, covers bariatric surgery for patients with co-existing conditions such as diabetes (26). Given the rising prevalence of severe obesity in older adults (27) and the fact that the rate of increase of bariatric surgery is highest among the near elderly (ages 55–64) (28), an increased number of Medicare beneficiaries are likely to become eligible for this procedure. Therefore, more research is needed to understand cost and utilization post-surgery among older patients with diabetes. While we unfortunately lack race/ethnicity data on our study sample, previous research suggests that bariatric surgery is primarily utilized by privately insured, middle-aged, white women (29–30), so our sample may not be representative of all individuals with type 2 diabetes who are eligible for bariatric surgery. Additional research is also needed to understand differences in cost and utilization by demographic characteristics.
There are some limitations to this study which suggest that it may be prudent to focus on the observed trends of utilization across time rather than the absolute numbers. Rules for billing patients immediately after surgery, particularly in the first 30 days, may differ by health plan. We, therefore, caution against the over-interpretation of costs in the first post-surgical year; for some patients in our cohort, there is utilization but no cost claims during that period. Because there was not a perfect overlap between costs and utilization (e.g., not all outpatient costs were represented in outpatient utilization) we focused mostly on total costs. Small differences in how professional claims were standardized may reduce comparability of cost estimates across health plans. For all other claims (hospital, facility outpatient, pharmacy), costs were standardized in a consistent manner across plans. Only a portion of the sample (8%) had data out to six years. Therefore, the trends observed in this analysis may be biased by left censoring over time (not drop outs) because most patients in the reference group (pre-surgery) did not contribute to the later years of the analysis. We lack a control group of individuals who did not receive bariatric surgery. Without this comparison group, we cannot prove that the change in costs after surgery was due to the procedure. We assumed that severely obese individuals with diabetes would not have a spontaneous decrease in costs and utilization; it is more likely that their costs and utilization are stable or increase over time. Given the limitations of creating a control group from observational data – namely confounding by indication – we opted to not include one in this analysis.
We did not study individuals receiving usual diabetes care as the control group for two reasons. First, diabetics who receive bariatric surgery may have different preferences for medical care than diabetics who receive usual care. In particular, surgery recipients may be higher utilizers of all health care services. Second, diabetics who receive bariatric surgery may have higher health care costs – due to a greater frequency of interactions with the health care system – to maintain the same clinical level as diabetics receiving usual care. In our dataset, individuals with diabetes who received usual care were clinically comparable to individuals with diabetes who received bariatric surgery (data not shown). Clinically similar patients may have different utilization patterns due to patient preferences for care or provider preferences or different requirements of services to maintain this clinical similarity. Finally, we lack beneficiary information on race/ethnicity, so we are unable to examine patterns of cost and utilization among those sub-populations.
To conclude, this study uses a large and dataset to examine the health care costs and utilization among adults with diabetes before and after bariatric surgery. We found that in the six years following surgery, bariatric surgery is not associated with lower costs among individuals with diabetes. We suggest, however, that even if bariatric surgery is not cost-saving among adults with diabetes, a reduction or elimination of weight-related comorbidities, improved quality of life and improved mobility may make this a valuable procedure in this population of patients. More research is needed to understand whether the higher costs and utilization we observed persist among this population in the long-term or reverse; to understand what clinical conditions and services drive this utilization; to understand whether an increase in elective procedures (e.g., knee replacement, plastic surgery), not available to patients prior to surgery, partially explain increased cost and utilization post-surgery; and to understand clinical determinants of the post-operative costs of bariatric surgery among adults with diabetes.
Supplementary Material
Acknowledgments
This research was conducted by the Johns Hopkins University DEcIDE Center under contract to the Agency for Healthcare Research and Quality (Contract # HHSA290-2005-0034-I-TO4-WA1, Project I.D. # 35-EHC), Rockville, MD. The dataset used in this current study was originally created for a different research project on patterns of obesity care within selected Blue Cross/Blue Shield (BCBS) plans. The previous research project (but not the current study) was funded by unrestricted research grants from Ethicon Endo-Surgery, Inc. (a Johnson & Johnson company); Pfizer, Inc; and GlaxoSmithKline. The data and database development support and guidance were provided by the BCBS Association, BCBS of Tennessee, BCBS of Hawaii, BCBS of Michigan, BCBS of North Carolina, Highmark, Inc. (of Pennsylvania), Independence Blue Cross (of Pennsylvania), Wellmark BCBS of Iowa, and Wellmark BCBS of South Dakota. All investigators had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All listed authors contributed to the design, analysis or writing of this study and none has conflicts of interest. The authors of this abstract are responsible for its content. No statement may be construed as the official position of the Agency for Healthcare Research and Quality of the U. S. Department of Health and Human Services. We also wish to acknowledge Dr. Christine Weston for her valuable contributions. This research was also supposed by a grant from the National Institute of Allergy and Infectious Diseases (K01AI071754).
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual Medical Spending Attributable To Obesity: Payer- And Service-Specific Estimates. Health Aff (Millwood) 2009 Jul 27; doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Klein Woolthuis EP, de Grauw WJ, van Gerwen WH, et al. Yield of opportunistic targeted screening for type 2 diabetes in primary care: the diabscreen study. Ann Fam Med. 2009 Sep-Oct;7(5):422–30. doi: 10.1370/afm.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall TM, Zhang Y, Chen YJ, et al. The economic burden of diabetes. Health Aff (Millwood) 2010 Feb;29(2):297–303. doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- 5.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003 Dec 2;139(11):933–49. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004 Oct 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 7.Hodo DM, Waller JL, Martindale RG, et al. Medication use after bariatric surgery in a managed care cohort. Surg Obes Relat Dis. 2008 Sep-Oct;4(5):601–7. doi: 10.1016/j.soard.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Raebel MA, Malone DC, Conner DA, et al. Health services use and health care costs of obese and nonobese individuals. Arch Intern Med. 2004 Oct 25;164(19):2135–40. doi: 10.1001/archinte.164.19.2135. [DOI] [PubMed] [Google Scholar]
- 9.Segal JB, Clark JM, Shore AD, et al. Prompt reduction in use of medications for comorbid conditions after bariatric surgery. Obes Surg. 2009 Dec;19(12):1646–56. doi: 10.1007/s11695-009-9960-1. [DOI] [PubMed] [Google Scholar]
- 10.Makary MA, Clarke JM, Shore AD, et al. Medication use and healthcare costs after bariatric surgery in patients with type 2 diabetes mellitus. Annals of Surgery. 2010;145(8):726–31. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 11.Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001 Jan 10;285(2):182–9. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 12.Residori L, Garcia-Lorda P, Flancbaum L, et al. Prevalence of co-morbidities in obese patients before bariatric surgery: effect of race. Obes Surg. 2003 Jun;13(3):333–40. doi: 10.1381/096089203765887615. [DOI] [PubMed] [Google Scholar]
- 13.Efron B. The Jackknife, the Bootstrap, and Other Resampling plans. Philadelphia: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- 14.Clark JM, Chang HY, Bolen SD, et al. Development of a Claims-Based Risk Score to Identify Obese Individuals. Popul Health Manag. 2010 May 5; doi: 10.1089/pop.2009.0051. [DOI] [PubMed] [Google Scholar]
- 15.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008 Jan;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 16.Johns Hopkins Bloomberg School of Public Health. The Johns Hopkins ACG Case-Mix System. Version 7.0. Release. 2005. [Google Scholar]
- 17.Gorman RS, Stern DL, Inclan DV, et al. Outcomes, health status, and medical resource utilization after bariatric surgery. Compr Ther. 2006 Spring;32(1):34–8. doi: 10.1385/comp:32:1:34. [DOI] [PubMed] [Google Scholar]
- 18.Cremieux PY, Buchwald H, Shikora SA, et al. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008 Sep;14(9):589–96. [PubMed] [Google Scholar]
- 19.Klein S, Ghosh A, Cremieux PY, et al. Economic Impact of the Clinical Benefits of Bariatric Surgery in Diabetes Patients With BMI >/=35 kg/m (2) Obesity (Silver Spring) 2010 Sep 9; doi: 10.1038/oby.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoerger TJ, Zhang P, Segel JE, et al. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010 Sep;33(9):1933–9. doi: 10.2337/dc10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciejewski ML, Smith VA, Livingston EH, et al. Health care utilization and expenditure changes associated with bariatric surgery. Med Care. 2010 Nov;48(11):989–98. doi: 10.1097/MLR.0b013e3181ef9cf7. [DOI] [PubMed] [Google Scholar]
- 22.Zingmond DS, McGory ML, Ko CY. Hospitalization before and after gastric bypass surgery. JAMA. 2005 Oct 19;294(15):1918–24. doi: 10.1001/jama.294.15.1918. [DOI] [PubMed] [Google Scholar]
- 23.Encinosa WE, Bernard DM, Chen CC, et al. Healthcare utilization and outcomes after bariatric surgery. Med Care. 2006 Aug;44(8):706–12. doi: 10.1097/01.mlr.0000220833.89050.ed. [DOI] [PubMed] [Google Scholar]
- 24.Steele KE, Prokopowicz GP, Chang HY, et al. Comparable Complication Rates after Bariatric Surgery Among Individuals With and Without Type 2 Diabetes Mellitus. Surgery for Obesity and Related Diseases. doi: 10.1016/j.soard.2011.05.018. In press. [DOI] [PubMed] [Google Scholar]
- 25.Sjostrom CD, Peltonen M, Wedel H, et al. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000 Jul;36(1):20–5. doi: 10.1161/01.hyp.36.1.20. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. CMS Manual System, Pub. 100-03 Medicare National Coverage Determinations, Treatment of Obesity. 2004 October 1; http://obesity.procon.org/sourcefiles/CMSMedicareNatlCoverage2004.pdf.
- 27.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010 Jan 20;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 28.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005 Oct 19;294(15):1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 29.Santry HP, Lauderdale DS, Cagney KA, et al. Predictors of patient selection in bariatric surgery. Ann Surg. 2007 Jan;245(1):59–67. doi: 10.1097/01.sla.0000232551.55712.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinogle JA, Owings MF, Kozak LJ. Gastric bypass as treatment for obesity: trends, characteristics, and complications. Obes Res. 2005 Dec;13(12):2202–9. doi: 10.1038/oby.2005.273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.