Abstract
OBJECTIVES
Antegrade cerebral perfusion makes deep hypothermia non-essential for neuroprotection; therefore, there is a growing tendency to increase the body temperature during circulatory arrest with selective brain perfusion. However, very little is known about the clinical efficacy of mild-to-moderate hypothermia for ischemic organ protection during circulatory arrest. The aim of this study was to evaluate the safety and efficiency of mild-to-moderate hypothermia for lower-body protection during aortic arch surgery with circulatory arrest and antegrade cerebral perfusion.
METHODS
Between January 2005 and December 2009, a total of 347 patients underwent non-emergent arch surgery. In all patients, the systematic cooling was adapted to the expected time of circulatory arrest, and cerebral perfusion was performed at a constant blood temperature of 28 °C. There were 40 cardiac or aortic re-operations, 312 patients had concomitant aortic valve or root surgery, and 10 patients had replacement of the descending aorta. All examined data were collected prospectively.
RESULTS
The duration of circulatory arrest and the deepest rectal temperature were 18 ± 11 min (range, 6–70 min) and 31.5 ± 1.6 °C (range, 26.0–35.0 °C) for all 347 patients, and 34 ± 12 min (range, 17–70 min) and 29.9 ± 1.7 °C (range, 26.0–34.6 °C) for 77 patients having total/subtotal arch replacement. The maximum serum lactate level on the first postoperative day was, on average, 2.3 ± 1.2 mmol l−1. In the statistical analysis, no association between the duration of temperature-adapted circulatory arrest and lactate, creatinine, or lactate dehydrogenase levels after surgery could be demonstrated. The 30-day mortality was 0.9%. Permanent neurological deficit or temporary dysfunction occurred in three (0.9%) and eight (2.3%) patients, respectively. No paraplegia and no hepatic failure were reported; however, mesenteric ischemia occurred in one patient with severe stenosis of the celiac and upper mesenteric arteries. Temporary dialysis was necessary primarily after surgery in five patients. All of them underwent hemiarch replacement only, and four patients had an increased creatinine level before surgery.
CONCLUSION
Systemic mild-to-moderate hypothermia that is adapted to the duration of circulatory arrest is a simple, safe, and effective method of organ protection and can be recommended in routine aortic arch surgery with circulatory arrest and cerebral perfusion.
Keywords: Aortic arch, Circulatory arrest, Hypothermia, Organ protection
INTRODUCTION
The tolerance of cerebral ischemia is restricted under normothermia to very few minutes and, even under profound hypothermia, it can only be prolonged with limitation. Yet, hypothermia is associated with extended time of cardiopulmonary bypass (CPB) needed for cooling and rewarming and with serious negative side effects, such as coagulopathy or organ dysfunction. On the other hand, after the introduction of antegrade cerebral perfusion, deep hypothermia became non-essential for neuroprotection, which led to a growing interest in increasing the body temperature during circulatory arrest (CA). Even if the ischemic tolerance time of several organs, such as the liver, kidneys, or even the spinal cord, at normothermia is much longer than the brain, very little is known about the safety and clinical efficacy of mild-to-moderate hypothermia for organ protection during the average time of CA needed for aortic arch repair [1]. This study was conducted to evaluate the efficiency of mild-to-moderate hypothermia for organ protection in aortic arch surgery using CA with antegrade cerebral perfusion.
PATIENTS AND METHODS
Between January 2005 and December 2009, a total of 410 patients underwent aortic arch surgery at our institution using the defined protocol, presupposing that, during CA, antegrade cerebral perfusion at a constant blood temperature of 28 °C was used for cerebral protection, and systematic cooling for organ protection was adapted to the expected time of CA. Patients with acute dissection (n = 63) were excluded from this evaluation to eliminate a possible impact of a preexisting malperfusion on postoperative organ function. The study population included the 347 remaining patients, with an average age of 63 years. The main indication for surgery in this series was chronic degenerative (medial degeneration) aneurysm in 189 cases followed by an atherosclerotic or mixed form in 150 cases. Other pathologies, such as false aneurysm or infection, were the cause of surgery in the remaining eight patients. Detailed preoperative patient characteristics are presented in Table 1.
Table 1:
Preoperative patient characteristics
| Characteristics | No (%) or mean ± SD (range) |
|---|---|
| Sex male | 238 (69) |
| Age (Y) | 63 ± 13 |
| NYHA functional class III/IV | 138 (40) |
| Concomitant disease | |
| Aortic valve defect | 299 (86) |
| Insufficiency | 169 (49) |
| Stenosis | 43 (12) |
| Mixed | 87 (25) |
| Hypertension | 285 (82) |
| Coronary heart disease | 92 (27) |
| Diabetes | 26 (7) |
| COPD | 25 (7) |
| Impaired renal function | 16 (5) |
| Dialysis | 3 (1) |
| Previous neurologic events | |
| Permanent | 14 (4) |
| Transient | 16 (5) |
| Previous cardiac surgery | 40 (12) |
| Aortic valve | 34 (10) |
| Euro SCORE logistic (%) | 12.8 ± 9.9 (range, 3.3–59.2) |
NYHA = New York Heart Association; COPD = chronic obstructive pulmonary disease.
Surgical technique
According to the extent of surgery, the thoracic aorta was exposed via median sternotomy in 337 patients, including a left lateral extension in three patients. Posterolateral thoracotomy or clamshell thoracotomy was performed in two and five patients, respectively.
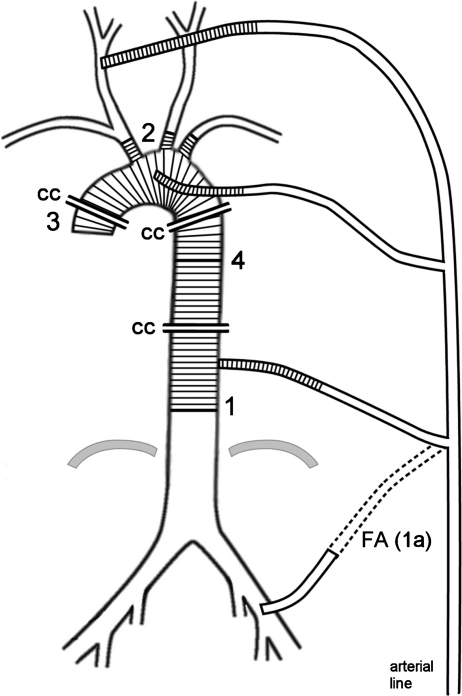
The arterial return for CPB was achieved by isolated cannulation of the right or left common carotid artery in 217 (62.5%) and 103 (29.5%) patients, respectively. The innominate artery was used for cannulation in 19 (5.5%) cases, one of which was combined with a cannulation of the left common carotid artery because of an occlusion of the right internal carotid artery, hypoplasia of the left vertebral artery, and multiple pathologies of the intracranial cerebral arteries. This was the only patient in the series in whom bilateral cerebral perfusion was performed during CA. Simultaneous carotid and femoral cannulation with a Y-shaped line was performed in eight (2.3%) patients. This approach was used in complex surgery of the thoracic aorta to limit an extended time of CA by re-establishing lower body perfusion after completing the distal anastomosis (Fig. 1). The decision for choosing the particular cannulation technique was made preoperatively on the basis of computed tomography (CT), which was performed on all patients. A neurovascular examination included Doppler ultrasonography of the extracranial vessels and, when feasible, transcranial Doppler ultrasonography. Cranial CT angiography was carried out to evaluate the circle of Willis at the beginning of the series; however, because we had not found a clinical relevance for this examination, it was abandoned in 2007 [2].
Figure 1:
Schematic illustration of complete arch and descending aorta replacement that demonstrates method for limiting duration of circulatory arrest. Arterial line is armed with 2 additional branches using Y-shaped connectors. The first line is placed in carotid artery (CA) for establishment of cardiopulmonary bypass and unilateral cerebral perfusion during circulatory arrest. After completing distal anastomosis (1), aortic graft is cannulated with the second branch of the arterial line (1a) and cross-clamped (CC) in order to re-establish perfusion of lower body. Alternatively the femoral artery can be cannulated with this branch primarily (FA). After completing anastomoses between aortic arch graft and supraaortic arteries (2), graft is cannulated with third branch of the arterial line and cross-clamped at both ends allowing re-establishment of full cerebral perfusion. The next step consists of proximal aortic and cardiac repairs, and re-establishment of myocardial perfusion (3), followed lastly by anastomosing arch graft with aorta descending prosthesis (4). For other details see text.
The cannulation technique has been described previously [3–5]. In short, the innominate artery or, in 13 cases, the left common carotid artery, was isolated within the chest. In 315 patients, the right (n = 220) or left (n = 96) common carotid artery was prepared through a separate approach in the neck along the medial margin of the sternocleidomastoid muscle. In all patients, after heparinization, the exposed segment of the artery was cross-clamped, a longitudinal incision was made, and an 8- or 10-mm vascular sealed polyester graft was anastomosed to the artery with a continuous 5/0 polypropylene suture. If additional cannulation of the femoral artery was considered necessary, it was done simultaneously in the usual manner, and both arterial lines were connected with a Y-shaped tube for arterial in-flow from one pump (Fig. 1).
After connecting the arterial line and cannulating the right atrium, CPB was started with a mean flow of 4.6 ± 0.5 l min−1 and range 3.0–6.0 l min−1 (2.2–2.4 l min−1 m−2 of body surface).
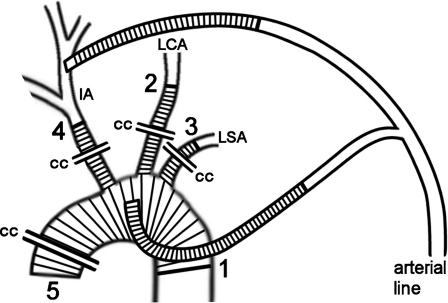
In all patients, CA was used for arch repair. Systematic cooling was performed using the α-stat method for acid–base management. The deepest temperature was determined by the surgeon in accordance with the expected time of CA, with a target rectal temperature adapted to the approximate scope of CA: about 32–34 °C for 15 min, 30–32 °C for 30 min, and 28–30 °C for 45 min or longer. However, the cerebral perfusion was always performed at an arterial blood temperature of 28 °C, which can be reached after just a few minutes of cooling, regardless of the systemic (rectal) temperature achieved. The technique of unilateral cerebral perfusion has been described previously [6]. In brief, the arch arteries were cross-clamped at the beginning of CA, and unilateral cerebral perfusion at a constant blood temperature of 28 °C was set up by simply reducing the arterial flow to a mean flow of 0.9 ± 0.2 l min−1 when the left common carotid artery was used for perfusion and, considering the flow to the right arm, to a mean flow of 1.5 ± 0.3 l min−1 when the innominate artery or the right common carotid artery was used. The arterial line included one or, occasionally, even two Y-shaped cannulas (Figs. 1 and 2).
Figure 2:
Schematic illustration of complete aortic arch replacement that demonstrates the method for limiting duration of circulatory arrest and unilateral cerebral perfusion. After completing distal anastomosis (1) and cross-clamping (CC) the aortic arch graft and its side branches, graft is cannulated through the 4th side branch and perfusion of lower body is re-established. Cerebral perfusion is re-established step-by-step after completing of each further anastomosis (2–4) starting with the left carotid artery (LCA), then followed by the left subclavian artery (LSA) then the innominate artery (IA). Thereafter the arterial line branch to the carotid artery is cross-clamped and arterial return is performed through aortic arch graft exclusively. Surgery is completed by proximal aortic and cardiac repairs and re-establishment of myocardial perfusion (5). For further details see text.
During cerebral perfusion, the Y-shaped line can be used to perfuse the other brain hemisphere for bilateral cerebral perfusion, if necessary. Nevertheless, only once did we have to make use of this additional cerebral perfusion for the patient in whom, as mentioned earlier, a bilateral cerebral perfusion was used primarily. During rewarming, the Y-shaped arterial line was used to switch the arterial perfusion from the cannulated artery to the aortic graft. In complex surgeries, these additional lines allow limiting the duration of CA and unilateral cerebral perfusion by re-establishing body and/or bilateral cerebral perfusion (Figs. 1 and 2). For this purpose, vascular grafts with one side branch or with four side branches (InterGard Hemabridge or InterGard Aortic Arch; InterVascular, La Ciotat, France) were used.
The surgical procedures and the operative data are shown in Table 2.
Table 2:
Surgical procedures and operative data
| Data | No (%) or mean ± SD (range) |
|---|---|
| Aortic arch replacement | 347 (100) |
| Partial replacement (hemiarch)a | 270 (78) |
| Total/subtotal replacementb | 77 (22) |
| Ascending aorta replacement | 343 (99) |
| Valve conduit | 121 (35) |
| Root repair | 117 (34) |
| Supra-coronary | 105 (30) |
| Aortic valve surgery | 312 (90) |
| Replacement | 177 (51) |
| Repair (valve and/or root) | 135 (39) |
| CABG | 73 (21) |
| Mitral valve surgery | 24 (7) |
| CPB duration (min) | 149 ± 45 |
| Aortic cross-clamp time (min)c | 97 ± 32 |
| Circulatory arrest time (min) | 18 ± 11 |
| Lowest rectal temp (°C) | 31 ± 1.8 |
aOblique anastomosis without reimplantation of aortic arch branches.
bIncluding elephant trunk technique (3 patients) or descending aortic replacement (10 patients).
cIncluding circulatory arrest; CABG = coronary artery bypass grafting; CPB = cardiopulmonary bypass.
Statistical analysis
All perioperative data were collected prospectively. Values in the tables and text are expressed as mean ± standard deviation, unless otherwise indicated. The statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) software (SPSS Inc., Chicago, IL, USA).
To test the hypothesis that lactate, creatinine, and/or lactate dehydrogenase (LDH) levels mirror the ischemic organ injury during CA, a generalized linear model was built in which the highest values of these biochemical parameters, determined during 48 h after surgery, were examined as dependent variables and the duration of CA and the duration of CPB as independent variables.
RESULTS
The duration of CA and the deepest rectal temperature were 18 ± 11 min (range, 6–70 min) and 31.5 ± 1.6 °C (range, 26.0–35.0 °C) for all 347 patients, and 34 ± 12 min (range, 17–70 min) and 29.9 ± 1.7 °C (range, 26.0–34.6 °C) for 77 patients having total (with reimplantation of all aortic arch branches, n = 44) or subtotal (without reimplantation of the left subclavian artery, n = 33) arch replacement. In a few patients, especially in those with a very low body mass, the rectal temperature was slightly lower than the target temperature. Only one patient (female, 59 kg) had a temperature of 26 °C.
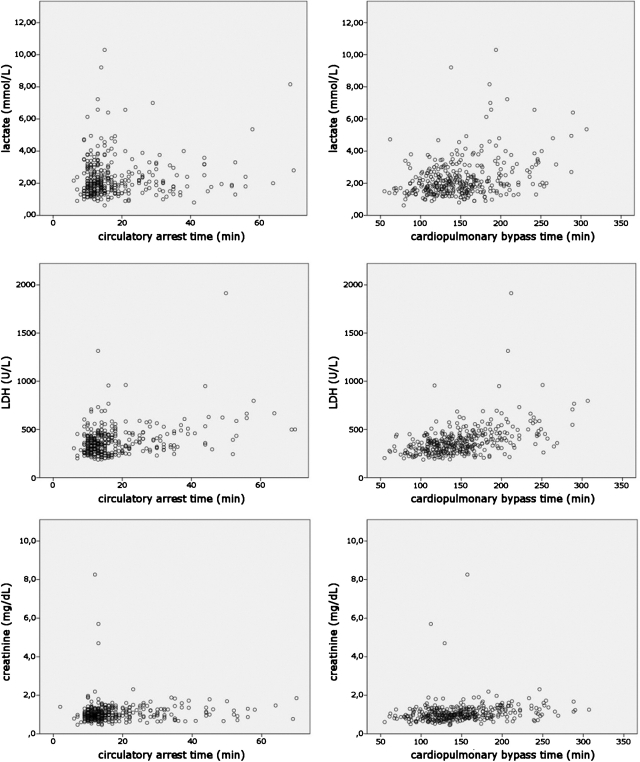
The maximum serum lactate level as measured directly after surgery and every 3 h until discharge from the intensive care unit was, on average, 2.3 ± 1.2 mmol l−1. Furthermore, in the statistical analysis, no association between the duration of CPB and the temperature-adapted CA, within the time range used in the examined population, and postoperative lactate, creatinine, or LDH levels could be demonstrated (Fig. 3).
Figure 3:
Scatter plots showing no association between lactate level (upper row), LDH level (mid row) and creatinine level (bottom row), and duration of CA and CPB.
No paraplegia and no hepatic failure were reported; however, mesenteric ischemia occurred in one patient with a porcelain aorta and severe stenosis of the celiac and upper mesenteric arteries.
The duration of CA and/or CPB was neither associated with the occurrence of neurological dysfunction nor with the necessity for postoperative dialysis.
Permanent neurological deficit or temporary dysfunction occurred in three (0.9%) and eight (2.3%) patients, respectively. Among the stroke patients, two had undergone hemiarch replacement and one patient subtotal arch replacement with a CA duration of 9, 18 and 20 min, respectively. All patients had presented with severe calcification of the aortic annulus, which had to be decalcified during the valve replacement. Accordingly, the CT scan of the neurocranium demonstrated the evidence of intracranial embolization in all these patients.
Postoperative temporary neurologic dysfunction, such as confusion, delirium, or agitation lasting more than 48 h but without focal deficit, occurred in eight patients, with a mean age of 72 ± 6 years. Hemiarch and arch replacement was performed in six and two of them, respectively. The median time of CA was 14 min (range, 10–35 min).
Temporary dialysis was necessary primarily after surgery in five patients. All of them had CA ≤ 30 min and four patients had an increased creatinine level before surgery.
There were three deaths (0.9%) during the 30 days after surgery. One patient (female, 71 years) suffering from severe aortic valve stenosis combined with porcelain aorta died of mesenteric ischemia as the result of severe stenosis of the celiac and upper mesenteric arteries.
Another patient (male, 68 years) with chronic aneurysm and atherosclerotic ulcer penetrating the left lung died after complete arch replacement due to severe pneumonia.
The third patient (female, 76 years) with thoracic mega-aortic aneurysm combined with coronary heart disease died after coronary artery bypass grafting and complete replacement of the entire thoracic aorta through clamshell thoracotomy due to postoperative myocardial infarction caused by a heparin-induced thrombocytopenia and coronary graft occlusion.
Seven patients required prolonged primary postoperative ventilation, and 12 patients had to be reintubated due to respiratory insufficiency, the most frequent postoperative complication. Twelve patients required tracheotomy (five after prolonged primary ventilation and seven after reintubation). The in-hospital mortality was 1.2% because, among the patients with pulmonary complications, another death occurred in-hospital, but after the 30-day time range. This patient underwent composite valve graft re-replacement and partial aortic arch replacement due to a false aneurysm and coronary bypass grafting due to three-vessel coronary heart disease. After surgery, he suffered from respiratory insufficiency and developed sepsis and multiple-organ failure during prolonged ventilation after reintubation and tracheotomy.
DISCUSSION
Deep hypothermia, which was described by Griepp et al. [7], improves the ischemic brain tolerance considerably and has therefore enabled the development of modern aortic arch surgery. Up to the present, it is the most tried-and-true method of cerebral protection and offers favorable results when CA duration is limited to 30 min or less [8]. However, the protection time has limits and the risk of stroke increases significantly when the duration of deep hypothermic CA exceeds 40 min [9]. The inevitable extension of CPB needed for cooling and rewarming had also been identified as an independent risk factor for an adverse outcome [10]. Moreover, deep hypothermia causes many negative side effects, such as coagulopathy and organ dysfunction, and, therefore, the attempts to avoid deep hypothermia are almost as old as its history [11]. To extend the safe period of cerebral protection, avoid deep hypothermia, and shorten the time of CPB, several techniques of antegrade cerebral perfusion have been proposed, which initially consisted of temporary or even permanent bypassing of the arch arteries [12]. The modern era of antegrade cerebral perfusion, pioneered by Bachet et al. [13] and Kazui et al. [14] brought enormous development of many cannulation and perfusion methods. Although antegrade cerebral perfusion was originally mostly used as an adjunct to deep hypothermia, systemic cooling became non-essential for neuroprotection. This led to a growing interest in increasing the body temperature during CA, which results in shortening the bypass time and limiting the negative side effects of hypothermia. However, despite favorable clinical results reported [6,15–17], there is a concern that this approach may be at the expense of an increased risk of ischemic organ injury. In the animal experimental study, recently published in this Journal, Etz et al. [18] demonstrated that paraplegia was probable after 90 min and inevitable after 120 min of cerebral perfusion and CA at 28 °C. Commenting on this article, Ranasinghe and Bonser [19] questioned the rationale of elevating the temperature during CA because it leads to reducing the safety margin in aortic arch surgery. Yet, do we not similarly do this in all other fields of cardiac surgery, for example, by use of minimally invasive approaches or off-pump surgeries? Why should the negative side effects of unphysiological cold perfusion, which relate to all aspects of cardiac surgery necessitating extracorporeal circulation, not be considered for surgery of the aortic arch? Lastly, does moderate hypothermia really lead to a reduction of the safety margin in modern aortic arch surgery?
Because multiple studies have demonstrated that CA, CPB, and surgery times are the clear predictors of an increased risk in cardiovascular surgery, the shortening of all these aspects can be the best way to improve surgical results. Admittedly, extensive diagnostics as well as surgical and perfusion planning are necessary, but they are critical to ensure that the surgeon remains in full control of each phase of surgery, especially during CA. This, in our opinion, should apply equally for elective as well emergent aortic arch surgery [20].
Using bifurcated arterial lines and branched vascular prostheses, as being practiced by us and others [21, 22], enables the gradual, here described in detail, re-establishment of perfusion after completing the particular anastomoses (Figs. 1 and 2). This gradual re-establishment of perfusion is a very important adjunct to refine the perfusion and surgical technique and shorten the CA time, especially in complex aortic pathologies or any situation in which the anastomosing time could be prolonged. However, because, in our experience, complete arch replacement seldom extends beyond 30 min in elective patients, we do not use gradual perfusion re-establishment routinely and, therefore, there is no distinct difference between the CA time and cerebral perfusion time in our entire population. Yet, in the few patients with complex aortic surgery, this difference averaged 15 min, as a result of limiting the time of CA (lower body ischemia) to nearly 30 min. Nevertheless, we apply the technique of gradual reperfusion very often in the replacement of an acute dissected aortic arch, in which anastomosing the dissected and fragile aortic wall is much more challenging and time-consuming. To enable the limitation of CA in appropriate cases, the strategy of using cannulation of a supraaortic artery and bifurcated arterial line has always been applied in general. It enables complete arch replacement without a considerably longer time of lower body ischemia, even in such situations when elective hemiarch replacement is planned and an unexpected situation (e.g., atherosclerotic ulcer within the arch) demands an extension of surgery. Otherwise, deep hypothermia would have had to be used in all patients categorically to ensure a safe, though not unlimited, time range for the extension of surgery.
It may be true that straight hypothermia does not increase the risk in routine aortic arch surgery with a short repair time [9]; however, especially the patients with complex pathologies can, in our opinion, benefit from avoiding deep hypothermia and shortening the CA, CPB, and surgical times. To avoid deep hypothermia in such patients, alternative methods consisting of bypassing the arch arteries and endografting the arch have been proposed recently, and their results are still compared with the ‘old-fashioned’ surgery. We do not doubt that endovascular techniques may be a practical enrichment of the surgical armamentarium; however, the refined technique of conventional aortic arch repair, although more invasive, provides definitive repair with excellent clinical results and the possibility to simultaneously repair concomitant cardiac pathologies.
The fact that the CA times used in this study were quite short is a limitation of the study. However, as an aortic referral center, we were able to present a large number of patients operated on using comparable conditions, namely cannulation and perfusion through a supraaortic artery, cerebral perfusion at the same blood temperature, and systemic mild-to-moderate hypothermia adapted to the duration of CA. On the other hand, the use of the surgical strategy described here ensures that CA can be limited to the duration of the one (distal) anastomosis, and, therefore, an extension of the CA time beyond the range reported is not necessary. Furthermore, the study presents everyday aortic arch surgery comprising, as demonstrated by the average logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation), a normal proportion of risk patients. Further, the 22% share of complete arch replacements matches the ratio in other large studies reporting arch surgery [23,24].
In conclusion, the study supports the safety and effectiveness of mild-to-moderate hypothermia for organ protection when the temperature is adapted to the duration of CA; we therefore recommend the use of antegrade brain perfusion with systemic mild-to-moderate hypothermia in routine aortic arch surgery.
ACKNOWLEDGEMENT
The authors would like to thank Ms. Melissa Lindner, Mrs. Alexandra Metz, and Mrs. Bianca Müller for the assistance in preparing this article.
Conflict of interest: P.P.U. discloses a financial relationship with InterVascular, Inc.
REFERENCES
- 1.Kirklin J, Barrat-Boyes B. Hypothermia, circulatory arrest, and cardiopulmonary bypass. In: Kirklin J, Barrat-Boyes, editors. Cardiac surgery. Philadelphia: Churchill-Livingstone; 2003. pp. 66–130. [Google Scholar]
- 2.Urbanski PP, Lenos A, Blume JC, Ziegler V, Griewing B, Schmitt R, Diegeler A, Dinkel M. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur J Cardiothorac Surg. 2008;33:402–8. doi: 10.1016/j.ejcts.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Urbanski PP. Cannulation of the left common carotid artery for proximal aortic repair. J Thorac Cardiovasc Surg. 2003;126:887–8. doi: 10.1016/s0022-5223(03)00705-0. [DOI] [PubMed] [Google Scholar]
- 4.Urbanski PP, Lenos A, Lindemann Y, Weigang E, Zacher M, Diegeler A. Carotid artery cannulation in aortic surgery. J Thorac Cardiovasc Surg. 2006;132:1398–403. doi: 10.1016/j.jtcvs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Urbanski PP, Lenos A, Lindemann Y, Zacher M, Frank S, Diegeler A. Use of a carotid artery for arterial cannulation: side-related differences. Thorac Cardiovasc Surg. 2010;58:276–9. doi: 10.1055/s-0029-1240979. [DOI] [PubMed] [Google Scholar]
- 6.Urbanski PP, Lenos A, Zacher M, Diegeler A. Unilateral cerebral perfusion: right versus left. Eur J Cardiothorac Surg. 2010;37:1332–7. doi: 10.1016/j.ejcts.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–63. [PubMed] [Google Scholar]
- 8.Kunihara T, Grün T, Aicher D, Langer F, Adam O, Wendler O, Saijo Y, Schäfers HJ. Hypothermic circulatory arrest is not a risk factor for neurologic morbidity in aortic surgery: a propensity score analysis. J Thorac Cardiovasc Surg. 2005;130:712–8. doi: 10.1016/j.jtcvs.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades A. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007;84:759–67. doi: 10.1016/j.athoracsur.2007.04.107. [DOI] [PubMed] [Google Scholar]
- 10.Kazui T, Washiyama N, Muhammad BAH, Terada H, Yamashita K, Takinami M. Improved results of atherosclerotic arch aneurysm operations with a refined technique. J Thorac Cardiovasc Surg. 2001;121:491–9. doi: 10.1067/mtc.2001.112469. [DOI] [PubMed] [Google Scholar]
- 11.Livesay JJ, Cooley DA, Duncan JM, Ott DA, Walker WE, Reul GJ. Open aortic anastomosis: improved results in the treatment of aneurysms of the aortic arch. Circulation. 1982;66(Pt 2):I122–I7. [PubMed] [Google Scholar]
- 12.Crawford ES, Saleh SA, Schuessler JS. Treatment of aneurysm of transverse aortic arch. J Thorac Cardiovasc Surg. 1979;78:383–93. [PubMed] [Google Scholar]
- 13.Bachet J, Guilmet D, Goudot B, Termignon JL, Teodori G, Dreyfus G, Brodaty D, Dubois C, Delentdecker P. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg. 1991;102:85–94. [PubMed] [Google Scholar]
- 14.Kazui T, Inoue N, Yamada O, Komatsu S. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg. 1992;53:109–14. doi: 10.1016/0003-4975(92)90767-x. [DOI] [PubMed] [Google Scholar]
- 15.Pacini D, Leone A, Di Marco L, Marsilli D, Sobaih F, Turci S, Masieri V, Di Bartolomeo R. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg. 2007;31:618–22. doi: 10.1016/j.ejcts.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya H, Hagl C, Kropivnitskaya I, Böthig D, Kallenbach K, Khaladj N, Martens A, Haverich A, Karck M. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg. 2007;133:501–9. doi: 10.1016/j.jtcvs.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Saritas A, Kervan U, Vural KM, Kucuker SA, Yavas S, Birincioglu LC. Visceral protection during moderately hypothermic selective antegrade cerebral perfusion through right brachial artery. Eur J Cardiothoracic Surg. 2010;37:669–76. doi: 10.1016/j.ejcts.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Etz CD, Luehr M, Kari FA, Lin HM, Kleinman G, Zoli S, Plestis KA, Griepp RB. Selective cerebral perfusion at 28 °C – is the spinal cord safe? Eur J Cardiothorac Surg. 2009;36:946–55. doi: 10.1016/j.ejcts.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 19.Ranasinghe AM, Bonser RS. The brain, the spinal cord, selective antegrade cerebral perfusion and corporeal arrest temperature – are we reducing the margin of patient safety in aortic arch surgery? Eur J Cardiothorac Surg. 2009;36:943–5. doi: 10.1016/j.ejcts.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Urbanski PP, Lenos A, Schmitt R, Diegeler A. Extended arch resection in acute type A aortic dissection: PRO. Cardiol Clin. 2010;28:335–42. doi: 10.1016/j.ccl.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Sun LZ, Qi RD, Chang Q, Zhu JM, Liu YM, Yu CT, Lv B, Zheng J, Tian LX, Lu JG. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg. 2009;138:1358–62. doi: 10.1016/j.jtcvs.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Kouchoukos NT, Masetti P. Total aortic arch replacement with a branched graft and limited circulatory arrest of the brain. J Thorac Cardiovasc Surg. 2004;128:233–7. doi: 10.1016/j.jtcvs.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Khaladj N, Shrestha M, Meck S, Peterss S, Kamiya H, Kallenbach K, Winterhalter M, Hoy L, Haverich A, Hagl Ch. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–14. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Hagl Ch, Ergin MA, Galla JD, Lansmann SL, McCullough JN, Spielvogel D, Sfeir P, Bodian CA, Griepp RB. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg. 2001;121:1107–21. doi: 10.1067/mtc.2001.113179. [DOI] [PubMed] [Google Scholar]