Abstract
OBJECTIVES
Video-assisted thoracoscopic surgery (VATS) and median sternotomy (MS) are two approaches in lung-volume reduction surgery (LVRS). This study focused on the two surgical approaches with regard to postoperative pain.
METHODS
In this prospective, non-randomized study, pain was measured preoperatively and postoperatively using the visual analog scale (VAS) and the brief pain inventory (BPI). Incentive spirometry (IS) assessed restriction of the thoracic cage due to pain. Factors associated with treatment complications, medication usage, hospital stay, operating times, and chest-tube duration differences were examined between groups.
RESULTS
Of 85 patients undergoing LVRS, 23 patients underwent reduction via MS and 62 patients via bilateral VATS. VAS scores revealed no difference in postoperative pain except for VAS scores on days 6 (PM) and 7 (PM). BPI scores yielded higher scores in the VATS group on postoperative day (POD) 1 in the reactive dimension, but no other overall differences. MS patients receiving tramadol consumed a higher mean amount than VATS patients on POD 5 and POD 6. IS change from baseline to postoperative were similar between groups, and increased pain correlated with decreased IS scores on POD 1. Chest-tube duration, complications, and pain medication were similar between groups.
CONCLUSIONS
Bilateral VATS and MS offer similar outcomes with regard to postoperative pain and complications. These results suggest that the choice of LVRS operative approach should be dependent on disease presentation, surgeon expertise, and patient preference, not based upon differences in perceived postoperative pain between MS and bilateral VATS.
Keywords: Median sternotomy, Bilateral thoracoscopy, Pain, Lung-volume reduction surgery, Visual analog scale, Brief pain inventory
INTRODUCTION
Video-assisted thoracoscopic surgery (VATS) is widely accepted for pulmonary procedures. Although research suggests its superiority to thoracotomy in terms of pain, morbidity, hospital stay, and costs [1–9], use of VATS usually depends on disease presentation and surgeon preference.
Median sternotomy (MS) can also be used in many of the same procedures as bilateral VATS. Like VATS, MS has been suggested as superior to thoracotomy in terms of pain, morbidity, length of hospital stay, and cost [5,10]. Comparison between the two approaches reveals similar outcomes [11–17]. Few, if any, trials have been conducted to determine if one approach is superior with respect to pain.
We investigated pain experienced with MS and bilateral VATS for lung-volume reduction surgery (LVRS). This technique presents a unique opportunity to study pain of two approaches for the same procedure. The potential for differences in pain is important as VATS evolves in modern cardiothoracic surgery.
MATERIALS AND METHODS
This prospective, non-randomized study was conducted at a university medical school associated with a Midwestern hospital. The Springfield Committee for Research Involving Human Subjects approved the study (IRB# 98-036). Subjects were adults scheduled for bilateral LVRS via MS or VATS between May 1998 and May 2010. Any patient with previous thoracic surgery was excluded due to potential adhesions. Additional exclusion criteria included severe complications interfering with data collection, that is, prolonged intubation. Patients who used a patient-controlled analgesic (PCA) for pain control postoperatively were excluded (n = 5), as this is not routine in our LVRS.
Of the 85 subjects, 55 were male and 30 female. VATS was the approach for 62 patients and 23 underwent MS. When optional, the choice of approach was made by the patient. Patients were placed in the MS group, if they were believed to have extensive adhesions or needed concomitant resection of a lung nodule more amenable to MS. Of the 85 patients, 22 were placed in the MS group due to their disease presentation and only one of the remaining 63 chose MS over VATS.
Pain
Pain was evaluated using the visual analog scale (VAS), the brief pain inventory (BPI), incentive spirometry (IS), and pain-medication usage. The VAS is a numeric scale with zero representing no pain and 10 representing worst possible pain. It was administered preoperatively and twice daily postoperatively in the morning and evening from the first through the fifteenth day (or until discharge) and at 1 month, 3 months, and 1 year.
The BPI is a tool of the Pain Research Group. Permission to use the copyrighted BPI was granted by Charles S. Cleeland, Ph.D., Director Pain Research Group, Texas Medical Center, Houston, TX. The BPI measures two parameters of pain: the intensity (sensory dimension), and how this interferes with the capacity to function (the reactive dimension). The BPI questions are presented on a numeric scale ranging from 0 to 10, with 10 being the worst possible pain. BPI scores were recorded every other day through postoperative day 15 (POD) (or until discharge) and at 1 month, 3 months, and 1 year.
Incentive spirometry
IS results were obtained with the Voldyne Volumetric Exerciser (Teleflex Medical, Research Triangle Park, NC, USA). Pain may instigate decreased respiratory effort following thoracic surgery. Patients were taught to use the IS preoperatively and proper technique was assured. Patients were encouraged to use IS every hour while awake. We investigated the potential for a relationship between IS and pain. IS values were recorded every other day through POD 15 (or until discharge) and at 1 month, 3 months, and 1 year.
Additional data included complications, operative time, duration of chest tube, and hospital stay.
Surgical techniques
A single board-certified thoracic surgeon was the operating physician. Under MS, with the patient under general endotracheal anesthesia, a sternotomy incision was made. Single-lung ventilation was instituted with a double-lumen endotracheal tube. The sternum was opened, one side was approached, and the pleura opened. The emphysematous bullous disease was identified, grasped, and excised with the endoscopic stapler (US Surgical, Norwalk, CT, UK), and buttressed with bovine pericardium (Synovis Surgical Innovations, St. Paul, MN, USA). After resection, the lung was re-expanded and a subxiphoid 28-French chest tube placed. The lung was examined for any significant air leaks and the pleura closed. Single-lung ventilation was switched to the opposite side and the procedure repeated. For incidental pulmonary nodules, the nodule was excised using an endoscopic stapler (US Surgical, Norwalk, CT, USA).
Thoracoscopy: A double-lumen endotracheal tube was placed, and, under general endotracheal anesthesia, the patient placed in a full lateral position. Single-lung ventilation was instituted. Three 10-mm access ports were used. Lung resection and removal of any nodules were performed in the same way for MS and VATS. The trocar sites were injected with 0.25% Marcaine. The first side was closed with an intercostal 28-French chest tube in place, and the procedure was repeated on the opposite side.
Pain management
We documented the amount of pain medication received in 24-h increments. Dosages were recorded until POD 15 (or until discharge).
Statistical analysis
Descriptive statistics including means, medians, standard deviations, ranges, and frequency distributions were used to examine and summarize data. Comparisons between the groups were made with parametric and non-parametric tests. Independent group t-tests compared groups on continuous measures whose distributions did not excessively depart from normality. Wilcoxon rank-sum tests were used to compare groups on continuous measures whose distributions were more skewed. Chi-square tests of independence, or exact tests as appropriate, were used to compare groups on categorical outcomes. Results were considered statistically significant for p < 0.05.
RESULTS
Of the 85 bilateral LVRS patients, 62 had VATS and 23 MS. The two groups were similar except for a greater proportion of males in the MS group (p = 0.035) (Table 1). Twenty (24%) underwent concurrent wedge resection for a nodule (12 VATS and eight MS (p = 0.136). Operating times (incision to closure) were longer for VATS (109 ± 40 vs 82 ± 32 min, p = 0.023).
Table 1:
Preoperative demographics
| VATS (n = 52) | MS (n = 21) | p-Value | |
|---|---|---|---|
| Age (years) | 65 ± 6.26 | 62 ± 7.24 | .085 |
| Male/female | 28/24 | 17/4 | .031 |
| FEV1 (ml) | .762 ±.428 | .755 ± .232 | .930 |
| FEV% predicted | 27.39 ± 11.54 | 24.48 ± 6.91 | .191 |
FEV, = forced expiratory volume in 1 second; FEV = forced expiratory volume; ml = milliliters.
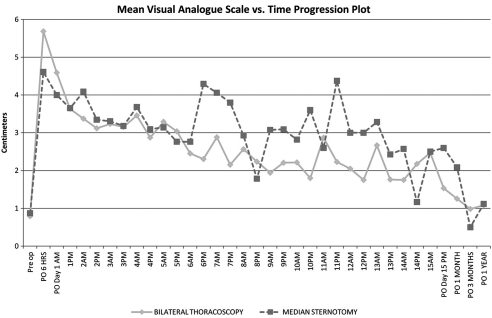
The VAS (Fig. 1) revealed no difference in pain between groups, with the exception of PODs 6 (PM) and 7 (PM). The differences in mean VAS scores between groups reached statistical significance (POD 6 (PM) VATS 2.3 vs MS 4.3 (p = 0.007), POD 7 (PM) VATS 2.2 vs MS 3.8 (p = 0.032)), and seemed to suggest more pain in MS patients. As PODs increased, the number of VAS scores recorded per group decreased due to hospital discharge.
Figure 1:
PO = postoperative; PRE OP = preoperative.
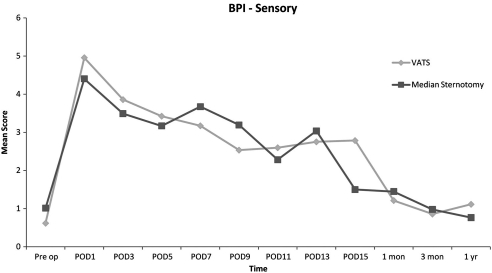
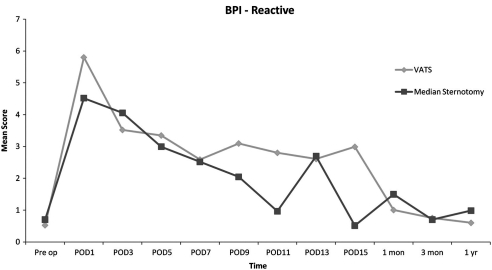
The BPI results yielded no overall statistically significant differences between the two approaches except for day 1. On POD 1, the VATS group indicated an increase in overall interference in the reactive dimension (p = 0.036). No other significant differences were identified in the sensory or reactive dimensions for the remaining time periods (Figs. 2 and 3). In examining differences in the individual BPI items, only six of 144 statistical comparisons reached significance. As postoperative hospital days increased, the number of BPI scores recorded per group decreased due to hospital discharges.
Figure 2:
Overall BPI scores – sensory.
Figure 3:
Overall BPI scores – reactive.
Mean baseline IS did not differ between groups, with VATS patients having 1593.88 ± 468.33 cm3 and MS patients having 1465.00 ± 685.39 cm3 (p = 0.448). When changes from baseline IS were measured postoperatively, there was no significant difference between groups at any time (Table 2).
Table 2:
Postoperative changes in incentive spirometry from baseline
| IS | VATS | MS | p-Value |
|---|---|---|---|
| Day 1 | −532.8 ± 547.8 (n = 56) | −420.8 ±618.9 (n = 20) | .451 |
| Day 3 | −435.9 ±430.8 (n = 56) | −265.9 ±618.6 (n = 22) | .247 |
| Day 5 | −264.9 ±478.8 (n = 51) | −142.5 ±624.1 (n = 20) | .378 |
| Day 7 | −235.7 ±435.1 (n = 43) | −215.6 ±785.4 (n = 16) | .924 |
| Day 9 | −189.4 ±531.3 (n = 33) | −142.3 ±642.9 (n = 13) | .800 |
| 1 Month | 258.4 ± 569.4 (n = 52) | 331.0 ± 681.3 (n = 21) | .643 |
| 3 Month | 410.8 ± 548.9 (n = 50) | 539.5 ±523.0 (n = 19) | .382 |
| 1 Year | 477.6 ± 679.4 (n = 40) | 592.3 ±550.3 (n = 13) | .584 |
IS = incentive spirometry; MS = median sternotomy; (n) = number of patients included for that interval; VATS = video-assisted thoracic surgery.
There was a significant correlation between VAS and overall BPI pain scores that ranged from r = 0.52 preoperatively at day 1 to 0.78–0.82 at 1 month, 3 months and 1 year. On POD 1, there was a significant inverse correlation between BPI and IS for pain at least (r = −0.34, p = 0.0022) and pain on average (r = −0.23, p = 0.0442). There was also a significant inverse correlation between IS and interference of pain with walking ability (r = −0.34, p = 0.0022). At 1 month, an inverse relationship was seen between BPI and IS for pain at worst (r = −0.31, p = 0.0065), pain on average (r = −0.30, p = 0.0094), and pain right now (r = −0.32, p = 0.0048). At 1 month, there existed an inverse correlation between BPI and IS for pain interference on: general activity (r = −0.27, p = 0.0196), mood (r = −0.26, p = 0.0228), walking ability (r = −0.30, p = 0.0038), sleep (r = −0.35, p = 0.0021), and enjoyment of life (r = −0.38, p = 0.0007). By postoperative month 3, all significant correlations between IS and VAS or BPI disappeared.
With the exception of days 5 and 6, no significant difference was seen between the groups’ pain medication from PODs 1 to 15. During day 5, MS patients receiving tramadol consumed a higher mean amount than VATS patients (291.7 ± 111.4 mg vs 144.7 ± 84.8 mg, p = 0.002). On POD 7, MS patients receiving tramadol also consumed a higher mean amount than their VATS counterparts (301.7 ± 119.2 mg vs 196.67 ± 177.8 mg, p = 0.012).
Chest-tube duration (192 h VATS vs 168 h MS), was not statistically different (p = 0.567). Hospital stay (10.5 days MS vs 9 days VATS) was not statistically significant (p = 0.823). In-hospital mortality occurred among one of 62 VATS patients (1.6%) and none of the MS patients (p = 1.0). The resulting overall in-hospital mortality rate was 1.2%.
Pulmonary complications did not differ between groups (p = 0.815). As many as 26 of 62 VATS patients (42%) and nine of 23 MS patients (39%) experienced one or more pulmonary complications. Non-pulmonary complications did not occur to any notable degree in either group (Table 3).
Table 3:
Postoperative complications
| VATS (n=52 (%)) | MS (n=21 (%)) | |
|---|---|---|
| None | 33 (63.5) | 13 (61.9) |
| Chest tube reinsertion | 7 (13.5) | 2 (9.5) |
| Pneumonia | 6 (11.5) | 3 (14.2) |
| Pleural effusion | 1 (1.9) | 1 (4.8) |
| Ventilator dependence | 2 (3.8) | 0 (0) |
| Seroma | 0 (0) | 1 (4.8) |
| Gastrointestinal | 6 (11.5) | 2 (9.5) |
| Wound infection | 0 (0) | 2 (9.5) |
MS=median sternotomy; VATS; = video-assisted thoracic surgery.
DISCUSSION
Postoperative pain is a key consideration in surgical approach selection for a thoracic patient. Pain is associated with postoperative morbidity [18] and it detracts from health [19]. Posterolateral thoracotomy is the most widely used approach, but is associated with a great deal of pain and complications [5,19,20]. VATS has become more common and is less painful than thoracotomy [1–9]. Another alternative is MS, also less painful than thoracotomy [5,10]. Virtually no research has examined whether VATS or MS is less painful. This study focused on the differences in pain associated with MS and bilateral VATS in patients undergoing LVRS.
Literature is inconclusive about the superiority of one approach over the other with regard to LVRS mortality. A comparison of two large bilateral VATS studies in LVRS reveals similar mortality rates. McKenna et al. [21] reported a rate of 4.5% for VATS patients and Cooper et al. [22] reported a rate of 4% for MS patients. Wisser et al. [23] and the National Emphysema Treatment Trial (NETT) [24] found comparable mortality between the two approaches. However, Kotloff et al. [16] found VATS to be associated with lower mortality than MS. Although not the focus of this study, the in-hospital mortality was one of 62 (1.6%) in VATS patients and none of 23 in MS patients, for an overall mortality of 1.2%. One VATS patient was withdrawn from the study due to inability to complete the questionnaires in the immediate postoperative period. Our in-hospital mortality rate for patients undergoing LVRS is 20 of 418 (4.8%) since 1994 and is two of 81 (2.5%) since 2000.
Similarly to the NETT [24], there was no statistical difference between the two approaches in terms of morbidity. LVRS using bilateral thoracoscopy takes longer to perform than the MS approach. Similar studies revealed results, which align with this study [14,15,24]. The excess time of VATS is attributed to the repositioning of the patient. Despite a longer operation, VATS and MS patients demonstrated similar lengths of hospital stay. Kotloff et al. [25] and Roberts et al. [14] also found the length of stay to be similar between groups. By contrast, the NETT studies have documented that VATS patients have shorter hospital stays after LVRS [15,25]. Regardless of length of hospital stay, both approaches offer equivalent results in pulmonary-function improvement, similar to what has been reported in the literature [14–16,23,25].
Pain studies have limitations based on difficulty quantifying pain. An individual’s perception of pain is subjective and relative. By using BPI and VAS scales, we measured various aspects of pain. The scales were administered preoperatively so that each patient could serve as their own control. The consistent correlations between VAS and BPI suggest the accuracy of these tools. The correlation between the quantity of pain (shown in the VAS) and the impact of pain (shown in the BPI) highlights the relationship between the sensory and reactive dimensions. The inverse correlation between IS and pain (VAS and BPI) substantiates our assumption that IS is effected by patient pain.
Our study was limited by the lack of standardized dosages of pain medication. The amount of medication given was determined on an individual basis. Some patients requested enough medication to merely reduce pain, whereas others may have sought to eliminate it altogether. Nevertheless, overall pain medication intake was similar between groups. The direct relationship between elevated tramadol consumption on days 5 and 6, and higher VAS scores on days 6 and 7 among MS patients should be noted, as the increase in pain could have caused the increase in tramadol consumption.
Interestingly, a significantly greater proportion of male patients underwent MS. The NETT did not note a disparity in males and females undergoing MS [25]. Hazelrigg et al. [12], Roberts et al. [14] and Kotloff et al. [16] did not find a difference in the proportion of males that undergoes MS. Unless dictated by lung nodules or potential adhesions, patients participated in the decision about surgical approach. Twenty-two of 23 MS patients had MS due to their pathology and only one of 63 remaining patients chose MS over VATS. This may support the thought that the public perceives VATS to be less painful and superior to MS. We assume patients are attracted to the ‘minimally invasive’ aspects of VATS, including smaller incisions/scars, minimal postoperative lifting restrictions, and avoidance of sternotomy.
A number of factors may account for similarities in pain between MS and VATS. Though smaller incisions are made for VATS, total incision length is comparable (six 2 cm incisions vs one 15 cm incision). The potential for intercostal nerve pain is greater with VATS due to the presence of intercostal incisions and trochars intra-operatively and bilateral intercostal chest tubes postoperatively, as opposed to the subxiphoid chest tubes of MS. As with other minimally invasive surgeries, patients undergoing VATS may have a perception of postoperative pain that is significantly less than the actual pain experienced. Patients undergoing MS assume a significant level of pain will be present postoperatively. Expectations for less pain with VATS may effect how patients perceive postoperative pain.
CONCLUSION
The surgical approaches for LVRS of MS and bilateral VATS are similar with regard to pain. Using measurements of VAS, BPI, and narcotic usage, we found little difference. Our data confirm an inverse relationship between IS and pain. This suggests the importance of minimizing postoperative pain to reduce pulmonary complications. Our findings are similar to previous studies suggesting there is a lack of data favoring one approach over the other with regard to complications.
The choice of surgical approach for LVRS should be dependent on disease presentation (i.e., nodules, perceived adhesions, and so on) surgeon expertise, and patient preference, and not upon perceived differences in postoperative pain between MS and bilateral VATS.
Funding
This work was supported by the Southern Illinois University School of Medicine, Division of Cardiothoracic Surgery.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors wish to thank Jean Long for assistance with article submission.
REFERENCES
- 1.Demmy TL, Nwogu CM. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thoracic Surg. 2008;85:s719–28. doi: 10.1016/j.athoracsur.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Roviaro G, Varoli F, Vergani C, Maciocco M, Nucca O, Pagano C. Video-assisted thoracoscopic major pulmonary resections: technical aspects, personal series of 259 patients, and review of the literature. Surg Endosc. 2004;18:1551–8. doi: 10.1007/s00464-004-6006-6. [DOI] [PubMed] [Google Scholar]
- 3.Landreneau RJ, Mack MJ, Hazelrigg SR, Naunhein K, Dowling RD, Ritter P, Magee MJ, Nunchuck S, Keenan RJ, Ferson PF. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted surgery. J Thorac Cardiovasc Surg. 1994;107:1079–86. doi: 10.1097/00132586-199412000-00051. [DOI] [PubMed] [Google Scholar]
- 4.Landreneau RJ. Role of thoracoscopy in thoracic surgical practice. West J Med. 1997;166:59–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Hazelrigg SR, Cetindag IB, Fullerton J. Acute and chronic pain syndromes after thoracic surgery. Surg Clin North Am. 2002;82:849–65. doi: 10.1016/s0039-6109(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 6.Landreneau RJ, Wiechmann RJ, Hazelrigg SR, Mack MJ, Keenan RJ, Ferson PF. Effect of minimally invasive thoracic surgical approaches on acute and chronic postoperative pain. Chest Surg Clin North Am. 1998;8:891–904. [PubMed] [Google Scholar]
- 7.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–84. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 8.Tschernko EM, Hofer S, Bieglmayer C, Wisser W, Haider W. Early postoperative stress: video-assisted wedge resection/lobectomy vs conventional axillary thoracotomy. Chest. 1996;109:1636–42. doi: 10.1378/chest.109.6.1636. [DOI] [PubMed] [Google Scholar]
- 9.Landreneau RJ, Hazlerigg SR, Mack MJ, Dowling RD, Burke D, Gavlick J, Perrino MK, Ritter PS, Bowers CM, Defino J, Nunchuck SK, Freeman J, Keenan RJ, Ferson RJ, Naunheim KS, Mathisen DJ, Warren WH. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–9. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 10.Asaph JW, Handy JR, Grunkemeier GL, Douville EC, Tsen AC, Rogers RC, Keppel JF. Median sternotomy versus thoracotomy to resect primary lung cancer: analysis of 815 cases. Ann Thorac Surg. 2000;70:373–9. doi: 10.1016/s0003-4975(00)01364-3. [DOI] [PubMed] [Google Scholar]
- 11.Fischel RM, McKenna RM. Video-Assisted thoracic surgery for lung volume reduction surgery. Chest Surg Clin North Am. 1998;8:789–807. [PubMed] [Google Scholar]
- 12.Hazelrigg SR, Boley TM, Magee MJ, Lawyer CH, Henkle JQ. Comparison of staged thoracoscopy and median sternotomy for lung volume reduction. Ann Thorac Surg. 1998;66:1134–9. doi: 10.1016/s0003-4975(98)00801-7. [DOI] [PubMed] [Google Scholar]
- 13.Hazelrigg SR, Boley TM, Grasch A, Shawgo T. Surgical strategy for lung volume reduction surgery. Eur J Cardiothorac Surg. 1999;16:57–60. doi: 10.1016/s1010-7940(99)00188-8. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JR, Bavaria JE, Wahl P, Wurster A, Friedberg JS, Kaiser LR. Comparison of open and thoracoscopic bilateral volume reduction surgery: complications analysis. Ann Thorac Surg. 1998;66:1759–65. doi: 10.1016/s0003-4975(98)00938-2. [DOI] [PubMed] [Google Scholar]
- 15.Ko CY, Waters PF. Lung volume reduction surgery: a cost and outcomes comparison of sternotomy versus thoracoscopy. Am Surg. 1998;64:1011–1013. [PubMed] [Google Scholar]
- 16.Kotloff RM, Tino G, Bavaria JE, Palevsky HI, Hansen-Flaschen J, Wahl PM, Kaiser LR. Bilateral lung volume reduction surgery for advanced emphysema: a comparison of median sternotomy and thoracoscopic approaches. Chest. 1996;110:1399–406. doi: 10.1378/chest.110.6.1399. [DOI] [PubMed] [Google Scholar]
- 17.Teschler H, Thompson AB, Stamatis G. Short- and long-term functional results after lung volume reduction surgery for severe emphysema. Eur Respir J. 1999;13:1170–6. doi: 10.1034/j.1399-3003.1999.13e38.x. [DOI] [PubMed] [Google Scholar]
- 18.Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin. 2005;15:105–21. doi: 10.1016/j.thorsurg.2004.08.004. [Abstract] [DOI] [PubMed] [Google Scholar]
- 19.Passlick B, Born C, Sienel W, Thetter O. Incidence of chronic pain after minimal invasive surgery for spontaneous pneumothorax. Eur J Cardiothorac Surg. 2001;19:355–9. doi: 10.1016/s1010-7940(01)00568-1. [DOI] [PubMed] [Google Scholar]
- 20.Lemmer JH, Jr, Gomez MN, Symreng T, Ross AF, Rossi NP. Limited lateral thoracotomy: improved postoperative pulmonary function. Arch Surg. 1990;125:873–7. doi: 10.1001/archsurg.1990.01410190071011. [DOI] [PubMed] [Google Scholar]
- 21.McKenna RJ, Brenner M, Fischel RJ, Singh N, Yoong B, Gelb AF, Osann KE. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg. 1997;114:957–67. doi: 10.1016/S0022-5223(97)70010-2. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JD, Patterson AG, Sundaresan SR, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112:1319–30. doi: 10.1016/S0022-5223(96)70147-2. [DOI] [PubMed] [Google Scholar]
- 23.Wisser W, Tschernko E, Senbaklavaci O, Kontrus M, Wanke T, Wolner E, Klepetko W. Functional improvement after volume reduction: sternotomy versus videoendoscopic approach. Ann Thorac Surg. 1997;63:822–827. doi: 10.1016/s0003-4975(96)01259-3. [DOI] [PubMed] [Google Scholar]
- 24.National Emphysema Treatment Trial Research Group Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg. 2004;127:1350–60. doi: 10.1016/j.jtcvs.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff RM, Tino G, Bavaria JE, et al. Bilateral lung volume reduction surgery for advanced emphysema. Chest. 1996;110:1399–404. doi: 10.1378/chest.110.6.1399. [DOI] [PubMed] [Google Scholar]