Abstract
OBJECTIVE
Acute renal failure (ARF) frequently complicates lung transplantation. This study determined the prevalence, predictive factors, and consequences of ARF on long-term renal function and survival.
METHODS
One hundred and seventy-four lung transplantation recipients were divided into two groups based on the presence or absence of ARF defined as a 50% decrease in creatinine clearance from baseline (group I: 67 patients with ARF; group II: 107 patients without ARF). Multivariate analysis compared pre-operative, operative, and post-operative risk factors to assess predictive factors. Renal function over time was assessed by two-way repeated measures analysis of variance (ANOVA).
RESULTS
ARF developed in 67 (39%) of patients. Multivariate analysis identified aprotinin (OR 2.20 (1.11; 4.36), p = 0.02) and double lung transplantation (OR 2.61 (1.32; 5.15), p = 0.006) as risk factors for post-operative renal failure. At 5 years following transplant, creatinine clearance was similar between the two groups (group I CrCl: 73 ml s−1; group II CrCl: 53 ml s−1; p = 0.54). Survival at 5 years was the same in the two groups. Multivariate analysis associated age at the time of transplantation (HR 1.030 (1.004; 1.057), p = 0.02) and intensive care unit (ICU) length of stay (HR 1.029 (1.008; 1.051), p = 0.007) with decreased survival.
CONCLUSIONS
The use of aprotinin and double lung transplantation are associated with ARF following lung transplantation. Age at the time of transplantation and a longer intensive care stay predict decreased survival. ARF after lung transplantation is not predictive of late renal dysfunction or decreased long-term survival.
Keywords: Lung transplantation, Acute renal failure, Aprotinin
INTRODUCTION
Acute renal failure (ARF) is a frequent and important complication following lung transplantation [1, 2]. Data on predisposing factors and long-term consequences of this complication are still scarce [3, 4]. Occurrence of ARF in the peri-operative period is an important predictive factor for subsequent chronic renal impairment in intensive care unit (ICU) patients [5, 6]. A decline in renal function in the first 6 months after heart or lung transplantation progressively worsens in subsequent years [2]. Furthermore, up to 5% of patients eventually progress to end-stage renal disease (ESRD) requiring permanent renal replacement therapy [7–9]. The International Society of Heart and Lung Transplantation (ISHLT) Registry reports a prevalence of renal failure following lung transplant of 26% and 38%, at 1 and 5 years, respectively [10]. However, little is known about the prevalence of ARF in the immediate post-operative period following lung transplantation and the long-term consequences of peri-operative ARF.
The objectives of the present study were to determine the prevalence of ARF in lung transplant recipients, to identify pre-operative and intra-operative predictive factors for ARF, and to assess the effects of ARF on long-term renal function and survival.
MATERIALS AND METHODS
Patient population
From 1 July 1997 to 31 December 2004, 174 patients (mean age: 46 ± 13 years, 83 men, 91 women) underwent lung transplantation at the Centre Hospitalier de l’Université de Montréal, Montréal, Québec, Canada. All patients were included except for intra-operative deaths, heart–lung transplantations, and for retransplantations. The study group included 89 single lung transplants (SLTs) and 85 double lung transplants (DLTs). Approval for the study, as well as a patient consent waiver, was obtained from the hospital’s institutional review board. Comprehensive data were prospectively gathered for all patients and entered into a database. Six patients who received heart–lung transplantations during the study period were excluded from the analysis. Follow-up was complete in all cases.
Data regarding renal function included baseline serum creatinine levels and estimated creatinine clearance (CrCl) at discharge, 30 days, 6 months, and every year subsequently. ARF was defined as a 50% decrease in glomerular filtration rate occurring within the first 30 days after surgery as estimated by the modified Cockroft–Gault formula compared to baseline, in concordance with the RIFLE-Injury criteria (RIFLE: risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage kidney disease) for ARF [11, 12]. The RIFLE criteria have been defined by the Acute Dialysis Quality Initiative Group to promote the use of a uniform definition of renal dysfunction.
Surgical technique
In all patients undergoing lung transplantation, the pulmonary artery pressure, cardiac output, and arterial blood gases were closely monitored intra-operatively. Transesophageal echocardiography was used in patients with pulmonary hypertension, right heart failure, or hemodynamic instability. Cardiopulmonary bypass was used electively in patients with severe pulmonary hypertension (mean pulmonary artery pressure ≥45 mmHg) and/or right ventricular dysfunction, and selectively in patients who became unstable during the transplant procedure. Aprotinin (Bayer HealthCare, Toronto, ON, Canada) was used intra-operatively since the start of the study in all patients requiring cardiopulmonary bypass, in patients presenting severe pulmonary hypertension, in patients with previous thoracic surgery, and selectively in patients considered at high risk for bleeding. Aprotinin administration consisted of 2 million kallikrein inhibiting units (KIU) as a loading dose, followed by 500 000 KIU h−1 constant infusion for the duration of surgery. An additional 2 million KIU were added to the cardiopulmonary bypass priming solution. Hemodynamic monitoring was used post-operatively in the intensive care unit. Aggressive diuresis was favored to maintain a negative fluid balance. Prophylactic antibiotics were used peri-operatively and appropriate cultures were obtained from the donor lung and recipient airway.
Immunosuppression protocol
All patients received intra-operative induction therapy with intravenous methylprednisolone (500 mg) administered immediately before reperfusion of each graft. Post-operatively, patients received a 6-day steroid taper of intravenous methylprednisolone followed by oral prednisone 0.5 mg kg−1 tapered to 0.1 mg kg−1 day−1 over 6 months. Patients with cystic fibrosis or bronchiectasis received tacrolimus (Prograf, Astellas Pharma Canada, Markham, ON, Canada), azathioprine, and prednisolone without induction therapy. Patients without septic lung disease received induction therapy followed by cyclosporine (Neoral, Novartis Pharma, Dorval, QC, Canada), azathioprine, and prednisolone. Fifty-one patients received rabbit antithymocyte immunoglobulin as induction therapy (3 doses of 1.5 mg kg−1) (RATG, Pasteur Mérieux, Lyon, France 1997–2003 and Genzyme, Mississauga, ON, Canada 2003–2004) intravenously during the first post-operative week. In 18 patients, Basiliximab (Simulect, Novartis Pharma, Dorval, QC, Canada) 20 mg intravenously was used as induction therapy on post-op days 1 and 4 instead of RATG. In 1999, mycophenolate mofetil (Cellcept, Hoffman-La Roche, Mississauga, ON, Canada) replaced azathioprine in the baseline regimen for all patients.
Azathioprine or mycophenolate mofetil dosages were adjusted to maintain a WBC count >4000 μl−1. Trough whole blood levels for cyclosporine of 300–400 ng ml−1 were targeted in the first 3 months post-operatively, 200–300 ng ml−1 between 3 and 6 months, and 100–200 ng ml−1 1 beyond 6 months. For tacrolimus, trough whole blood levels of 12–15 ng ml−1 were targeted in the first 3 months post-operatively, 10–12 ng ml−1 between 3 and 6 months, and 8–10 ng ml−1 beyond 6 months. Biopsy-proven acute cellular rejection episodes were treated with methylprednisolone 1000 mg daily for 3 days or intravenous thymoglobulin for refractory rejection [13–15].
Antifungal prophylaxis consisted of inhaled amphotericin B (Astellas Pharma, Markham, ON, Canada) at dose of 15 mg twice a day for 2 weeks, and then itraconazole (Janssen-Ortho Inc., Toronto, ON, Canada) at a dose of 200 mg twice a day for 3–6 months depending on culture results.
Statistical analysis
The results are presented as mean ± standard deviation or median (min, max) for continuous variables depending on variable distribution. Categorical variables are presented as frequency (%). In Tables 1 and 2, the Pearson chi-square test (categorical variables) and the Student’s t-test or Wilcoxon test (continuous variables) are used to compare patients with and without renal dysfunction. To identify predictive factors for a 50% decrease in CrCl after lung transplantation, potential predictors with a p ≤ 0.20 in univariate analysis were included in a multiple logistic regression analysis. The Student’s t-test was also used to compare changes in CrCl over time in patients whether or not they were treated with cyclosporine. A two-way repeated measures analysis of variance (ANOVA) was used to compare the evolution of renal function over time for patients in both groups. Since the interaction between time and group was significant, post hoc tests were performed to study difference between groups at each time point. Survival analysis was performed using the Kaplan–Meier method with the appropriate associated log rank. Univariate and multivariate Cox regression models were used to determine predictive factors for mortality. A p-value of less than 0.05 was considered statistically significant. All analyses were performed using SAS® software (release 8.02, SAS Institute Inc., Cary, NC, USA).
Table 1:
Baseline characteristics of patients
| GROUP I Renal dysfunction (n = 67) | GROUP II No renal dysfunction (n = 107) | p-value | |
|---|---|---|---|
| Demographic data | |||
| Recipient data | |||
| Men/women | 28 (42)/39 (58) | 55 (51)/52 (49) | 0.22 |
| Age (year) | 46 ± 13 | 46 ± 14 | 0.98 |
| BMI (kg m−2) | 23 ± 4 | 23 ± 5 | 0.53 |
| Donor data | |||
| Age (year) | 35 ± 14 | 35 ± 14 | 0.97 |
| Men/women | 32 (48)/35 (52) | 70 (65)/36 (35) | 0.02 |
| Microbiology (recipients +) | |||
| CMV (+) | 28 (42) | 39 (36) | 0.48 |
| PPD (+) | 11 (16) | 12 (11) | 0.32 |
| Associated medical condition | |||
| Systemic | |||
| Vasculitis | 1 (1) | 1 (1) | 0.74 |
| Sclerodermic | 2 (3) | 2 (2) | 0.63 |
| Previous chest surgery | 5 (7) | 14 (13) | 0.25 |
| Cardiovascular | |||
| Diabetes | 8 (12) | 15 (14) | 0.68 |
| Dyslipidemia | 8 (12) | 11 (10) | 0.73 |
| CAD | 14 (21) | 14 (13) | 0.17 |
| Hypertension | 7 (10) | 15 (14) | 0.49 |
| Anticoag Rx | 7 (10) | 5 (5) | 0.14 |
| NYHA | |||
| II | 5 (7) | 10 (9) | |
| III | 34 (51) | 61 (57) | |
| IV | 26 (39) | 35 (3) | 0.64 |
| Underlying lung disease | |||
| Emphysema | 20 (30) | 44 (41) | |
| Cystic fibrosis | 18 (27) | 26 (24) | |
| Idiopathic pulmonary fibrosis | 13 (19) | 11 (10) | |
| Bronchiectasis | 8 (13) | 3 (3) | |
| Alpha-1-anti-trrysine deficiency | 1 (1) | 9 (8) | |
| Primary pulmonary hypertension | 2 (3) | 1 (1) | |
| Others | 5 (5) | 13 (12) | 0.09 |
Data are presented as number (%) or mean ± standard deviation.
BMI: body mass index; CMV: cytomegalovirus; PPD: purified protein derivative (for tuberculosis); CAD: coronary artery disease; and NYHA: New York Heart Association Functional Class.
Table 2:
Operative and post-operative data
| Group 1 ARF (n = 67) | Group II No ARF (n = 107) | p-value | |
|---|---|---|---|
| Intra-operative | |||
| Use CPB | 12 (18) | 10 (9) | 0.10 |
| CPB duration (min) | 175 ± 58 | 180 ± 63 | 0.83 |
| Mean arterial pressure | 81 ± 14 | 81 ± 13 | 0.99 |
| (mmHg) | |||
| Intra-op inotropes use | 48 (45) | 40 (60) | 0.06 |
| SLT | 24 (36) | 65 (61) | 0.001 |
| DLT | 43 (64) | 42 (39) | |
| Ischemia first lung (min) | 187 ± 64 | 208 ± 68 | 0.04 |
| Ischemia second lung (min) | 297 ± 61 | 296 ± 69 | 0.90 |
| Estimated blood loss (ml) | 1500 (200, 8000) | 800 (300, 10000) | 0.005 |
| Crystalloids (ml) | 3059 ± 1871 | 3171 ± 1667 | 0.68 |
| Colloids (ml) | 500 (0, 3500) | 500 (0, 4700) | 0.97 |
| Blood products (ml) | 700 (0, 6500) | 0 (0, 7650) | 0.002 |
| Aprotinin use | 44 (66) | 44 (41) | 0.001 |
| Post-operative | |||
| Primary graft dysfunction | 13 (12) | 2 (3) | 0.04 |
| Pneumonia | 6 (6) | 2 (3) | 0.42 |
| Sepsis | 8 (7) | 4 (6) | 0.71 |
| Length of ICU (days) | 10 ± 116 ± 1 | 6 ± 12 | 0.02 |
| Length of hospitalization | 40 ± 34 | 26 ± 14 | <0.001 |
| (days) | |||
| Cyclosporine/tacrolimus | 38 (57)/29 (43) | 74 (69)/33 (31) | 0.10 |
Data are presented as number (%) or mean ± standard deviation.
CPB: cardiopulmonary bypass; DL.T: double lung transplantation; ICU: Intensive care unit; and SLT: single lung transplantation.
RESULTS
Incidence of post-lung transplantation ARF
The cohort was subdivided into two groups based on renal function status. Group I consisted of 67 patients who developed post-operative ARF, and group II consisted of 107 patients with preserved renal function. Overall, 67 out of the 174 patients developed ARF in the early post-operative period (39%). ARF occurred at 4.5 ± 4.4 post-operative days. Patients suffering from ARF presented similar pre-operative characteristics to those without ARF (Table 1) and presented a mean age of 46 years with a body mass index of 23 kg m−2. Female patients were more represented in that group, but in a non-significant manner. Diabetes and systemic hypertension were similar in both groups. Emphysema and cystic fibrosis were the two main underlying diagnoses accounting for one-third of patients each. During the operative period, group I patients were more frequently DLT, had more blood loss, and received more transfusions and aprotinin. The mean pre-operative CrCl in group I was 107 ± 31 ml min−1 and the mean post-operative CrCl was 39 ± 16 ml min−1 (64% decrease), whereas group II patients showed a decrease of 26% in CrCl (mean pre-operative CrCl: 89 ± 27 μmol l−1; mean post-op CrCl: 66 ± 21 ml min−1).
Predictive factors of post-lung transplantation ARF
The baseline demographic and medical conditions, as well as the indications for lung transplantation, were similar in both groups (Table 1). Male donors were more prevalent in group II. Follow-up was complete in all patients with a mean length of follow-up of 39 ± 28 months (group I: 36 ± 26 months; group II: 41 ± 29 months). DLT was more common in group I (Table 2). The mean graft ischemic time was longer in group II for SLT (group I: 187 ± 64 min; group II: 208 ± 68 min; p = 0.04). Significantly more blood transfusions were required in group I (group I: 1143 ± 1457 vs group II: 648 ± 1289; p = 0.002). The use of aprotinin was more prevalent in group I (group I: 66% vs group II: 41%; p = 0.001). Duration of stay in the ICU (group I: 10 ± 11 days vs group II: 6 ± 12; p = 0.02) and length of hospital stay (group I: 40 ± 34 vs group II: 26 ± 14; p < 0.001) were both longer in group I. On multivariate analysis, predictive factors for developing ARF after lung transplantation were the use of aprotinin (OR 2.20 (1.11; 4.36), p = 0.03) and DLT (OR 2.61 (1.32; 5.15), p = 0.04) (Table 3).
Table 3:
Predictive factors
| Univariate | Multivariate | Multivariate | |
|---|---|---|---|
| p-value | p-value | Adjusted OR | |
| Acute renal failure | |||
| Double lung transplantation | 0.0008 | 0.0405 | 2.606 [1.320; 5.145] |
| Aprotinin use | 0.0011 | 0.0292 | 2.203 [1.114; 4.357] |
| Female gender | 0.2176 | 0.8708 | 0.928 [0.380; 2.271] |
| Inotropic support | 0.0064 | 0.1289 | 0.187 [0.021; 1.629] |
| Total graft ischemia | 0.039 | 0.3177 | 0.997 [0.990; 1.003] |
| Blood products | 0.0278 | 0.5076 | 1.000 [1.000; 1.001] |
| CPB | 0.1033 | 0.5671 | 0.644 [0.143; 2.902] |
| Primary graft dysfunction | 0.8893 | 0.7733 | 1.173 [0.397; 3.468] |
| Univariate | Multivariate | Multivariate | |
| p-value | p-value | Adjusted HR | |
| Long-term death | |||
| Age at transplantation | 0.0449 | 0.0221 | 1.030 [1.004; 1.057] |
| Female gender | 0.1569 | 0.0384 | 1.960 [1.036; 3.707] |
| Indication other than emphysema | 0.0008 | 0.0038 | 6.529 [1.835; 23.230] |
| Length of ICU stay | 0.0012 | 0.0072 | 1.029 [1.008; 1.051] |
| Coronary artery disease | 0.2683 | 0.3269 | 1.467 [0.682; 3.158] |
| ARF | 0.2592 | 0.2036 | 1.539 [0.792; 2.994] |
| Aprotinin use | 0.3242 | 0.1583 | 1.718 [0.810; 3.641] |
| Double lung transplantation | 0.0010 | 0.2569 | 1.659 [0.691; 3.979] |
ARF: acute renal failure; ICU: intensive care unit; HR: hazard ratio; and OR: odds ratio.
Long-term renal function and survival post-lung transplantation
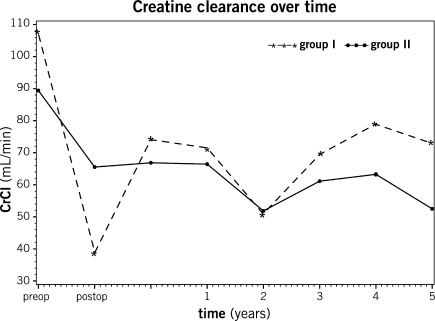
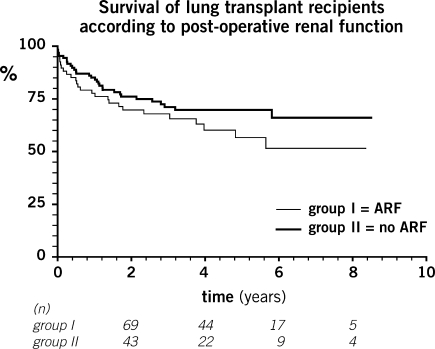
Patients in both groups showed a similar evolution of creatinine clearance levels over the 5-year follow-up. Mean CrCl at 5 years was 73 ± 41 ml min−1 in group I vs 53 ± 36 ml min−1 in group II (p = 0.69) (Fig. 1). At 5-year follow-up, patients receiving cyclosporine presented a non-significant decrease in CrCl (−19 ± 29 ml min−1) when compared to those receiving tacrolimus (−18 ± 32 ml min−1) (p = 0.81). The overall survival following lung transplantation was 82% at 1 year and 65% at 5 years. There was no statistically significant difference in survival between the two groups (p = 0.18) (Fig. 2). The 30-day mortality was 6.3%. Five patients died in the first 30 days of their transplant in group I, and six in group II (p = 0.62). Causes of early death were stroke in one patient, cardiac arrest in one, incoercible hemothorax in one, septic shock in one, right ventricular heart failure in two, primary graft failure in two, and multiorgan failure in three. Multivariate analysis showed that age at the time of transplantation (HR 1.030 (1.004; 1.057), p = 0.02), female recipients (HR 1.960 (1.036; 3.707), p = 0.04), length of stay in the ICU (HR 1.029 (1.008; 1.051), p = 0.007), and indication for lung transplantation other than emphysema (HR 6.529 (1.835; 23.230), p = 0.004) were predictive of decreased survival (Table 3).
Figure 1:
Creatinine clearance overtime. ARF: acute renal failure and CrCl: creatinine clearance.
Figure 2:
Survival of lung transplant recipients according to post-operative renal function. Patients at risk at each time point listed under the abscissa. ARF: acute renal failure.
DISCUSSION
The definition of ARF varies in the scientific literature. In 2004, the Adult Dialysis Quality Initiative (ADQI) workgroup recommended the use of a consensus definition for ARF in the critically ill patient [11, 12]. These criteria, known under the acronym RIFLE, were chosen in this study to allow better comparison of results with the current literature. The RIFLE-Injury criterion (defined as a 50% decrease in Glomerular filtration rate (GFR)) was chosen to provide a sensitive marker for significant renal impairment. Based on this threshold, ARF was observed in 39% of the patients in this series in the early post-operative period. Paradoxically, patients presenting ARF in the post-operative period (group I) had significantly higher pre-operative creatinine clearance levels when compared to group II. This underlines the importance of the proportional decrease in creatinine clearance in the definition of ARF. As noticeable in Table 1, both groups were similar at baseline in terms of demographic and associated medical conditions, except for pre-operative renal function (Fig. 1). No difference was noted in terms of predictive factors such as systemic hypertension, diabetes, and age. In comparison, Rocha et al. reporting on renal complications of lung transplantation using the above mentioned criteria observed a 56% prevalence of post-operative ARF [4].
The development of post-operative ARF has been attributed to a number of factors. Volume depletion is generally favored to prevent reperfusion pulmonary edema and primary graft dysfunction. Hemodynamic instability often requires the use of inotropic agents with vasoconstrictive effects on the kidney [4,7]. In the present study, volume loss and vasopressors have not been identified as risk factors for ARF (Table 3). Multivariate analysis identified DLT as a predictive factor for developing post-operative ARF. Interestingly, ischemia times were similar in both groups when DLT was performed (Table 2). Moreover, mean total ischemia time was similar in both groups regardless of the performance of a SLT or a DLT (Table 3). These results suggest that the longer time required to perform a DLT, which is an indicator of the surgical stress, is not sufficient to explain all of the relationship between the ARF and the performance of a DLT. It is probable that those patients in poor general condition undergoing DLT under CPB, with aprotinin administration, were those at risk for ARF post-operatively. Despite the correctness of the multivariate analysis, some interactions and confounding variables may have influenced the results not accounted for. The other factor identified as predictive for development of ARF after lung transplantation was the exposure to aprotinin. This finding is interesting as it adds to the controversy that exists with respect to this particular drug. In lung transplantation, Kesten et al. reported that the protease inhibitor aprotinin was beneficial in patients at high risk for bleeding [16]. Bittner et al. reported in 2006 that aprotinin decreased post-transplant reperfusion injury in lung transplant recipients [17]. The same year, Mangano et al. alerted the scientific community reporting a higher risk on renal failure and death among cardiac surgery patients [18]. In 2007, the BART trial comparing the use of aprotinin to lysine analog in high-risk cardiac surgery patients was stopped prematurely due to excessive mortality related to the drug [19]. Consequently, Bayer HealthCare voluntarily stopped marketing the drug on a worldwide basis since October 2007. The Food and Drug Administration and Health Canada, among other health authorities in collaboration with Bayer have created a restricted access programs for the drug. Healthcare authorities still exhort physicians to carefully monitor patients receiving aprotinin for cardiovascular and renal complications [20]. The present study was not designed to measure the specific impact of aprotinin use in lung transplant patients, but its identification as an independent predictor of ARF serves to heighten the concerns about its safety in cohorts other than cardiac revascularization surgery. In accordance with other risk factors for ARF published in the literature, the present study also considered patient gender, the diagnosis of pre-operative arterial hypertension, the diagnosis of idiopathic pulmonary hypertension, the prolonged length of stay in the intensive care unit, the use of antibiotics, the use of radiographic contrast dye, the use of the cardiopulmonary bypass, and the use of calcineurin inhibitors in the immediate post-operative period, among others, as potential risk factors [21, 22]. Of these factors, some were included in the multivariate model, while others were denied. There was no difference in the positive factors identified in the final model when variables were forced into the model. The final model presented comprises only variables that reached a p-value of 0.20 at the univariate analysis.
Defining the development of ARF according to a relative clearance decline is elegant as it standardizes the definition. However, from a clinical point of view, there is a clear distinction between these patients with those in whom dialysis becomes a life-threatening requirement. In a comparable report, Rocha et al. identified a lower baseline CrCl as a predictor for ARF requiring dialysis [4]. Ojo et al. reported a fivefold increase in late death (5 years) with ESRD after non-renal solid organ transplantation and a twofold increase for chronic, but not end-stage, renal dysfunction compared to recipients without chronic renal failure [8]. In the present series, only one patient required long-term dialysis and no predictive factors for this specific complication were identified.
In this study, the groups were classified according to post-operative renal status, as group I had renal failure, while group II did not. The fact that the ANOVA did not show a difference over a 5-year follow-up period suggests that ARF in itself does not have a significant impact on long-term renal function in our study population. It is noteworthy that the two groups presented a decrease in renal function in the immediate post-operative period and that both groups recovered their renal function in the months following transplantation. Group I, with a better baseline CrCl, showed a greater recuperation within 6 months, and overall the CrCl in group I recovered to levels superior to those of group II, despite the occurrence of ARF immediately following operation. This is consistent with other reports, such as Barraclough et al., identifying baseline creatinine clearance as a predictive factor for long-term renal function [23]. In our study, the calcineurin inhibitor chosen for maintenance therapy did not impact on the evolution of creatinine clearance over time. At 5 years, both groups had a significantly lower creatinine clearance compared to baseline values (Fig. 1). This may reflect the influence of the immunosuppressant therapy, regardless of the agent chosen. The two groups presented a decline in CrCl at 2 years, but this did not correlate with changes in immunosuppressive management.
The overall survival rate of 82% at 1 year and 65% at 5 years compares favorably with the data from the ISHLT Registry on lung transplantation (78% at 1 year; 50% at 5 years), and is similar to the data from Rocha et al., who reported survivals of 85% at 1 year and 60% at 5 years, both for no ARF and ARF non-dialyzed patients [10]. In the later series, survival was markedly decreased with requirement for dialysis, perhaps due to dialysis-related complications [4]. In the present study, as already mentioned, only one patient required dialysis and no survival difference was found between the ARF and non-ARF groups. This underlines the fact that post-operative ARF may not be a major threat to survival but the requirement for dialysis is. In the current study, multivariate analysis identified age at the time of transplantation, female recipients, indications other than emphysema, and length of stay in the ICU as significant factors for decreased survival. Similarly, ISHLT data show the following factors to be risk factors for long-term mortality: diagnosis of PPH, IV inotropes in the recipient pre-operatively, ventilator dependant or ICU patient, prior sternotomy, history of diabetes [10]. Advanced age, by definition, imposes a burden on survival, while a longer stay in the ICU is a marker of a suboptimal peri-operative course, worse outcomes, and lower survival. Occurrence of ARF and the baseline creatinine did not correlate with decreased survival in our analysis. Use of aprotinin did not decrease survival contrary to other reports [16,17].
Study limitations
The present study is a single-center retrospective review, with a limited number of patients thus subject to a number of inherent biases, which also limit power. Because this was an observational study, no formal power calculation was undertaken before starting the study. However, with our sample size, we would have been able to detect a difference in creatinine clearance of around 35 ml min−1 with an 80% power. The results may not be applicable to lung transplant patient populations from other centers. Survival and renal dysfunction prevalence correspond to data reported in the literature; the results of these analyses are likely to be representative of the mainstream results obtained in current clinical practice. Limited long-term follow-up may also be a significant factor. The use of the Cockroft–Gault formula to predict renal function systematically may overestimate the real glomerular filtration rate because of the secreted portion of serum creatinine. However, pre-operative, post-operative, and long-term CrCl were obtained using the same calculation.
CONCLUSION
ARF is a frequent complication following lung transplantation. DLT and the use of aprotinin carry a greater risk for renal impairment in this series. The occurrence of post-operative ARF does not seem to be predictive of long-term renal function and does not appear to affect long-term survival.
Funding
This work was supported by the Alfonso Minicozzi and Family Chair in Thoracic Surgery and Lung Transplantation and the Thoracic Surgery Research Foundation of Montreal.
Conflict of interest: none declared.
REFERENCES
- 1.Navis G, Broekroelofs J, Mannes GP, van der Bij W, de Boer WJ, Tegzees AM, de Jong PE. Renal hemodynamics after lung transplantation. A prospective study. Transplantation. 1996;61:1600–5. doi: 10.1097/00007890-199606150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Pattison JM, Peterson J, Kuo P, Valantine V, Robbins RC, Theodore J. The incidence of renal failure in one hundred consecutive heart-lung transplant recipients. Am J Kidney Dis. 1995;26:643–8. doi: 10.1016/0272-6386(95)90602-9. [DOI] [PubMed] [Google Scholar]
- 3.Broekroelofs J, Stegemen CA, Navis G, de Jong PE. Prevention of renal function loss after non-renal solid organ transplantation—how can nephrologists help to keep the kidneys out of the line of fire? Nephrol Dial Transplant. 1999;14:1841–3. doi: 10.1093/ndt/14.8.1841. [DOI] [PubMed] [Google Scholar]
- 4.Rocha PN, Rocha AT, Palmer SM, Davis RD, Smith SR. Acute renal failure after lung transplantation: incidence, predictors and impact on perioperative morbidity and mortality. Am J Transplant. 2005;5:469–76. doi: 10.1111/j.1600-6143.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 5.Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl. 1998;66:S16–24. [PubMed] [Google Scholar]
- 6.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–21. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 7.Bloom RD, Doyle AM. Kidney disease after heart and lung transplantation. Am J Transplant. 2006;6:671–9. doi: 10.1111/j.1600-6143.2006.01248.x. [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of nonrenal organ. N Eng J Med. 2003;349:931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 9.Ishani A, Ertuk S, Hertz MI, Matas AJ, Savik K, Rosenberg ME. Predictors of renal function following lung or heart-lung transplantation. Kidney Int. 2002;61:2228–34. doi: 10.1046/j.1523-1755.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- 10.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-fourth Official Adult Lung and Heart–Lung Transplantation Report—2007. J Heart Lung Transplant. 2007;26:782–95. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Kellum J, Ronco C. Acute renal failure: time for consensus. Intensive Care Med. 2001;27:1685–8. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure: definition, outcomes measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, McNeil KD, Reed EF, Reinsmoen NL, Scott JP, Studer SM, Tazelaar HD, Wallwork JL, Westall G, Zamora MR, Zeevi A, Yousem SA. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Clelland CA, Higenbottam TW, Otulana BA, Stewart S, Scott JP, McGoldrick J, Wallwork J. The histological changes in transbronchial biopsy after treatment of acute lung rejection in heart-lung transplants. J Pathol. 1990;161:105. doi: 10.1002/path.1711610204. [DOI] [PubMed] [Google Scholar]
- 15.Sibley RK, Berry GJ, Tazelaar HD, Kraemer MR, Theodore J, Marshall SE, Billingham ME, Starnes VA. The role of transbronchial biopsies in the management of lung transplant recipients. J Heart Lung Transplant. 1993;12:308. [PubMed] [Google Scholar]
- 16.Kesten S, Hoyas A, Chaparro C, Mauer J. Aprotinin reduces blood loss in lung transplant recipients. Ann Thorac Surg. 1995;59:877–9. doi: 10.1016/0003-4975(95)00051-l. [DOI] [PubMed] [Google Scholar]
- 17.Bittner HB, Richter M, Kuntze T, Rahmel A, Dahlberg P, Hertz M, Mohr FW. Aprotinin decreases reperfusion injury and allograft dysfunction in clinical lung transplantation. Eur J Thorac Cardiovasc Surg. 2006;29:210–5. doi: 10.1016/j.ejcts.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 19.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 20.Hiatt WR. Observational studies of drug safety: aprotinin and the absence of transparency. N Eng J Med. 2006;355:2171–3. doi: 10.1056/NEJMp068252. [DOI] [PubMed] [Google Scholar]
- 21.Myers BD, Ross J, Newton J, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Eng J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 22.Esposito C, De Mauri A, Vitulo P, Oggionni T, Cornacchia F, Valentino R, Grosjean F, Torreggiani M, Dal Canton A. Risk factors for chronic renal dysfunction in lung transplant recipients. Transplantation. 2007;84:1701–3. doi: 10.1097/01.tp.0000295989.63674.53. [DOI] [PubMed] [Google Scholar]
- 23.Barraclough K, Menahem SA, Bailey M, Thomson NM. Predictors of decline in renal function after lung transplantation. J Heart Lung Transplant. 2006;25:1431–5. doi: 10.1016/j.healun.2006.09.023. [DOI] [PubMed] [Google Scholar]