Abstract
OBJECTIVE
Diabetes mellitus has been recognized as a risk factor for mortality and morbidity after coronary bypass grafting, but a significant association between diabetes mellitus and postoperative atrial fibrillation (AF) has not been found. Although a recent study demonstrated a potential link between preoperative hemoglobin A1c level and risk of postoperative AF, there has not been sufficient examination of this relationship. We aimed to investigate the association between preoperative hemoglobin A1c and AF after isolated off-pump coronary bypass grafting.
METHODS
Of 912 consecutive patients undergoing isolated coronary bypass surgery, 805 were retrospectively analyzed for AF after excluding the following 107 cases: emergency (n = 81), chronic AF (n = 18), and pacemaker rhythm (n = 8). We performed a group analysis with hemoglobin A1c levels categorized into tertiles of the baseline distribution and a continuous analysis based on 1% increments in hemoglobin A1c levels. The cutoff points for the tertiles were as follows: lower, 3.8–5.6% (n = 283); middle, 5.7–6.7% (n = 282); upper, 6.8–11.4% (n = 240).
RESULTS
AF occurred in 159 patients (19.8%) after surgery. The median value (25th–75th percentile) of preoperative hemoglobin A1c was significantly lower in patients who developed AF than in those who did not (5.8 (5.4–6.3) vs 6.1 (5.5–7.2), p = 0.01). The incidence of postoperative AF was 28.3% (80/283) in the lower tertile, 17.4% (49/282) in the middle tertile, and 12.5% (30/240) in the upper tertile (p for trend = 0.01). The unadjusted odds ratio (95% confidence interval) for the association between hemoglobin A1c and postoperative AF was 0.70 (0.61–0.83) per 1% increase and 0.42 (0.29–0.70) for the upper versus the lower tertile. This association persisted after adjustment for the univariate predictors (0.74 (0.60–0.92) per 1% increase; 0.54 (0.31–0.90) for upper vs lower tertile) and the known risk factors (0.78 (0.63–0.95) per 1% increase; 0.55 (0.35–0.88) for upper vs lower tertile). The area under the receiver operator characteristic curve (95% confidence interval) for preoperative hemoglobin A1c as a predictor of postoperative AF was 0.70 (0.65–0.75) (p = 0.01).
CONCLUSIONS
Preoperative hemoglobin A1c independently predicts the occurrence of AF after isolated off-pump coronary bypass grafting.
Keywords: Atrial fibrillation, Coronary artery bypass graft surgery, Diabetes mellitus, Off-pump surgery
INTRODUCTION
Diabetes mellitus has been recognized as a risk factor for mortality and morbidity after coronary artery bypass grafting (CABG), but a significant association between diabetes mellitus and postoperative atrial fibrillation (AF) has not been found [1–13]. A recent study demonstrated a potential link between preoperative HbA1c level and risk of AF after CABG [14], but there has not been sufficient examination of this relationship. In the present study, we examined data on patients undergoing isolated off-pump CABG by a single surgeon and investigated the association between preoperative HbA1c and the occurrence of postoperative AF.
MATERIALS AND METHODS
Between January 2002 and November 2010, 912 consecutive patients underwent isolated CABG by a single surgeon at our institution. Except for six emergent cases in which percutaneous cardiopulmonary support was applied preoperatively because of hemodynamic collapse, all patients underwent CABG using the off-pump technique without conversion to cardiopulmonary bypass during surgery. A total of 805 patients was retrospectively analyzed after excluding the following 107 cases: emergent (n = 81), chronic AF (n = 18), and pacemaker rhythm (n = 8). Emergent cases were excluded because HbA1c levels could not be measured. All patients had previously granted permission for use of their medical records for research purposes. The study was approved by the Institutional Review Board. The endpoint was new-onset AF after surgery, which was diagnosed when there was an irregular cardiac rhythm without P-waves lasting more than 60 min that required further administration of antiarrhythmics, cardioversion, or anticoagulation therapy. After surgery, all patients were subjected to continuous electrocardiographic monitoring, using a bedside monitor in the intensive care unit and telemetry on the hospital ward, until postoperative day 7. When episodes of arrhythmia were captured by an automatic alarm function, a 12-lead electrocardiogram was performed and assessed by the physicians. HbA1c levels were measured in the morning under fasting before surgery by high-performance liquid chromatography (HLC-723G8, Tosoh, Tokyo). All patients included in the study were admitted to our hospital at least 5 days before surgery. In principle, we did not alter the medication prescribed by the referring cardiologist.
Definition
Body mass index of ≥25 kg m−2 was considered as obesity in the present study (in contrast to ≥30 kg m−2 in Western populations) according to the criteria of the Japan Society for the Study of Obesity [15]. Diabetes was defined as a history of diabetes regardless of duration of disease or need for antidiabetic agents. Patients, who required chronic (>3 months) bronchodilator therapy to avoid disability from obstructive airway disease and had a forced expiratory volume in 1 s less than 75% of the predicted value or less than 1.25 l, were considered having chronic pulmonary disease.
Anesthetic and surgical technique
The induction of anesthesia was achieved with intravenous administration of fentanyl citrate, midazolam, and vecuronium bromide. Anesthesia was maintained with intravenous administration of fentanyl and propofol and inhalation administration of low concentrations of sevoflurane as necessary. Anticoagulation was achieved with heparin after the conduits were harvested. The activated clotting time was maintained at more than 250 s. The off-pump technique was used for all patients. The details of the surgical technique were described previously [16].
Statistical analysis
We performed group analysis with HbA1c levels categorized into tertiles of the baseline distribution and continuous analysis based on 1% increments in HbA1c levels. The cutoff points for the tertiles were as follows: lower, 3.8–5.6% (n = 283); middle, 5.7–6.7% (n = 282); and upper, 6.8–11.4% (n = 240). The association of HbA1c with risk factors for postoperative AF was assessed on the basis of trend tests across the HbA1c tertiles using linear regression analysis for continuous variables and logistic regression analysis for binary variables. The correlation of two continuous variables was checked using Spearman’s rank correlation test. The association of preoperative HbA1c with postoperative AF was assessed by logistic regression analysis, and results were presented as odds ratio (OR) and 95% confidence interval (95% CI). To determine whether HbA1c remained predictive after adjustment for possible confounders of the relationship, we constructed three models. The first model included HbA1c alone, the second HbA1c and the covariates with p value of <0.25 in univariate logistic regression analyses, and the third HbA1c and the known risk factors for postoperative AF: age, sex, body mass index, chronic kidney disease, chronic pulmonary disease, hypertension, triple vessel disease, ejection fraction of <40%, left atrial dimension, preoperative beta blockers, preoperative statins, inotropic support of >24 h, and transfusion. To identify significant interactions, interaction terms were created between HbA1c and other covariates and introduced into the models in addition to the other covariates. To examine multicollinearity of the models, we calculated the variance inflation factor for each variable in the models and confirmed that none of them was exceeded 2 (highest, 1.29), which indicates that multicollinearity was not a significant issue. To evaluate the impact of HbA1c in explaining postoperative AF, a receiver operating characteristic curve was constructed and the area under the curves determined. All statistical testing was two sided. Results were considered statistically significant at a level of p < 0.05. All analyses were performed with the SPSS statistical package version 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
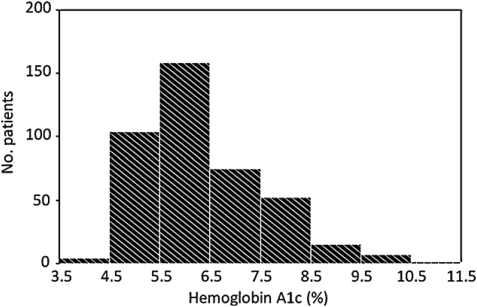
The distribution of HbA1c was right skewed (Fig. 1). The median value (25th–75th percentile) was 6.0 (5.4–7.1). AF occurred in 159 of 805 patients (19.8%) after surgery, most often on postoperative day 2 (29.7%), with 78.0% of occurrences on postoperative days 1, 2, 3, or 4 (Fig. 2). The incidence of postoperative AF was 28.3% (80/283) in the lower tertile, 17.4% (49/282) in the middle tertile, and 12.5% (30/240) in the upper tertile (p for trend = 0.01). The mean time to AF occurrence was similar between the groups (2.1 ± 0.5 days in the lower tertile, 2.2 ± 0.6 in the middle tertile, and 2.1 ± 0.6 in the upper tertile, p for trend = 0.74). The median value (interquartile range) of baseline HbA1c was significantly lower in patients who developed postoperative AF than in those who did not (5.8 (5.4–6.3) vs 6.1 (5.5–7.2), p = 0.01).
Figure 1:
Distribution of preoperative hemoglobin A1c.
Figure 2:
Onset of atrial fibrillation after surgery.
The distribution of risk factors for postoperative AF by preoperative HbA1c level is shown in Table 1. Patients with higher HbA1c were younger and more likely to have chronic kidney disease, peripheral arterial disease, triple vessel disease, and low left ventricular ejection fraction and to use preoperative oral antidiabetics and insulin. They also required longer operation time, more insulin during intensive care, and more transfusion than patients with lower HbA1c. The total dose of insulin infused during intensive care was positively correlated with HbA1c level (r = 0.55, p = 0.01). No correlation was found between age and HbA1c (r = −0.19, p = 0.54).
Table 1:
Distribution of risk factors by baseline hemoglobin A1c levels
| Hemoglobin A1c |
p for trend | |||
|---|---|---|---|---|
| Tertile 1 (n = 283) | Tertile 2 (n = 282) | Tertile 3 (n = 240) | ||
| Age, year | 69.3 ± 10.8 | 69.2 ± 9.1 | 66.1 ± 9.6 | 0.01 |
| Male gender | 233 (82.3) | 217 (77.0) | 192 (80.0) | 0.47 |
| Body mass index, kg m2 | 23.0 ± 3.7 | 23.5 ± 2.8 | 23.6 ± 3.1 | 0.07 |
| Obesity (body mass index ≥ 25) | 86 (30.4) | 85 (30.1) | 74 (30.4) | 0.92 |
| Diabetes | 17 (6.0) | 153 (54.3) | 236 (98.3) | 0.01 |
| Hematocrit, % | 37 ± 5 | 37 ± 6 | 37 ± 6 | 0.83 |
| Logistic EuroSCORE | 3.5 [1.8–6.2] | 3.6 [2.0–6.1] | 3.3 [1.8–6.4] | 0.56 |
| Smoking | 150 (53.0) | 164 (58.2) | 127 (52.9) | 0.98 |
| Chronic kidney disease | 133 (47.0) | 173 (61.4) | 153 (63.8) | 0.01 |
| Peripheral arterial disease | 26 (9.2) | 32 (11.4) | 46 (19.2) | 0.01 |
| Chronic pulmonary disease | 54 (19.1) | 57 (20.2) | 47 (19.6) | 0.74 |
| Hypertension | 208 (73.5) | 210 (74.5) | 166 (69.2) | 0.30 |
| Prior myocardial infarction | 107 (37.8) | 117 (41.5) | 113 (47.1) | 0.07 |
| Triple vessel disease | 154 (54.4) | 193 (68.4) | 170 (70.8) | 0.01 |
| Ejection fraction < 40% | 16 (5.7) | 28 (9.9) | 29 (12.1) | 0.01 |
| Left atrial dimension, mm | 39 ± 7 | 40 ± 6 | 40 ± 6 | 0.10 |
| Preoperative drugs | ||||
| Oral antidiabetics | 4 (1.4) | 39 (13.8) | 84 (35.0) | 0.01 |
| Insulin | 4 (1.4) | 40 (14.2) | 93 (38.8) | 0.01 |
| Renin angiotensin system inhibitors | 113 (39.9) | 120 (42.6) | 100 (41.7) | 0.45 |
| Beta blockers | 199 (70.3) | 200 (70.9) | 169 (70.4) | 0.68 |
| Statins | 185 (65.4) | 188 (66.7) | 156 (65.9) | 0.31 |
| Operative data | ||||
| Operative duration, h | 4.4 ± 1.1 | 4.6 ± 1.1 | 4.6 ± 1.1 | 0.01 |
| Complete revascularization | 275 (97.2) | 274 (97.2) | 233 (97.1) | 0.92 |
| Postoperative data | ||||
| Inotropic support for >24 h | 22 (7.8) | 22 (7.8) | 21 (8.8) | 0.69 |
| Insulin used during intensive care | 0.01 | |||
| None | 211 (74.6) | 127 (45.0) | 38 (15.8) | |
| 1–20, units | 60 (21.2) | 115 (40.8) | 78 (32.5) | |
| ≥ 21, units | 12 (4.2) | 40 (14.2) | 124 (51.7) | |
| Transfusion | 70 (24.7) | 83 (29.4) | 84 (35.0) | 0.01 |
| Drugs initiated on postoperative day 1 | ||||
| Renin angiotensin system inhibitors | 188 (66.4) | 186 (66.0) | 158 (65.8) | 0.66 |
| Beta blockers | 240 (84.8) | 245 (86.9) | 204 (85.0) | 0.54 |
| Statins | 253 (89.4) | 253 (89.7) | 214 (89.2) | 0.74 |
Data are number (percent), mean ± standard deviation, or median [25th—75th percentile]. Cutoff points for tertiles: lower, 3.8-5.6%; middle, 5.7-6.7%; upper, 6.8–11.4%. EuroSCORE: European System for Cardiac Operative Risk Evaluation.
Patients with postoperative AF stayed in hospital significantly longer than those without AF (median value (25th–75th percentile) was 14 [11–17] vs 12 [10–15], p = 0.01) and were more likely to suffer from stroke (2.2% vs 0.6%, p = 0.03), pneumonia (4.4% vs 1.8%, p = 0.02), and perioperative myocardial infarction (5.0% vs 0.6%, p = 0.01). The incidence of 30-day mortality was 1.3% in patients with AF and 0.9% in patients without AF (p = 0.71).
The results of univariate comparisons between patients with and without postoperative AF are summarized in Table 2. The following variables with p value of <0.25 were included in the model 2: age, smoking, chronic kidney disease, chronic pulmonary disease, hypertension, preoperative oral antidiabetics, preoperative statins, operative duration, inotropic support for >24 h, and insulin used during intensive care.
Table 2:
Univariate comparison between patients with and without postoperative atrial fibrillation
| No AF (n = 646) | AF (n = 159) | Odds ratio (95% CI) | P | |
|---|---|---|---|---|
| Age, year | 67.7 ± 9.8 | 70.6 ± 10.2 | 1.38 (1.13–1.64) | 0.01 |
| Male gender | 516 (79.9) | 126 (79.2) | 0.92 (0.60–1.41) | 0.84 |
| Body mass index, kg m2 | 23.3 ± 3.1 | 23.5 ± 3.7 | 1.01 (0.95–1.06) | 0.45 |
| Obesity (body mass index ≥ 25) | 195 (30.2) | 50 (31.4) | 1.06 (0.73–1.54) | 0.76 |
| Diabetes mellitus | 352 (54.5) | 54 (34.0) | 0.43 (0.30–0.61) | 0.01 |
| Hematocrit, % | 37 ± 6 | 38 ± 5 | 1.01 (0.98–1.05) | 0.59 |
| Logistic EuroSCORE | 3.5 [1.9–6.1] | 3.9 [2.0–6.9] | 1.02 (0.96–1.05) | 0.54 |
| Smoking | 366 (56.7) | 75 (47.2) | 0.73 (0.51–0.95) | 0.02 |
| Chronic kidney disease | 375 (58.0) | 84 (52.8) | 0.85 (0.68–1.16) | 0.14 |
| Peripheral arterial disease | 86 (13.3) | 18 (11.3) | 0.77 (0.43–1.29) | 0.44 |
| Chronic pulmonary disease | 130 (20.1) | 28 (17.6) | 0.90 (0.55–1.21) | 0.22 |
| Hypertension | 472 (73.1) | 112 (70.4) | 0.85 (0.58–1.18) | 0.19 |
| Prior myocardial infarction | 269 (41.6) | 68 (42.8) | 1.08 (0.78–1.52) | 0.68 |
| Triple vessel disease | 421 (65.2) | 96 (60.4) | 0.98 (0.70–1.41) | 0.33 |
| Ejection fraction <40% | 61 (9.4) | 12(7.5) | 0.84 (0.45–1.54) | 0.41 |
| Left atrial dimension, mm | 40 ± 7 | 40 ± 6 | 0.97 (0.94–1.04) | 0.91 |
| Preoperative drugs | ||||
| Oral antidiabetics | 111 (17.2) | 16 (10.1) | 0.56 (0.34–0.94) | 0.03 |
| Insulin | 117 (18.1) | 20 (12.6) | 0.60 (0.35–1.07) | 0.10 |
| Renin angiotensin system inhibitors | 268 (41.5) | 65 (40.9) | 0.93 (0.60–1.44) | 0.80 |
| Beta blockers | 458 (70.9) | 110 (69.2) | 0.91 (0.61–1.41) | 0.65 |
| Statins | 439 (68.0) | 90 (56.6) | 0.72 (0.50–0.88) | 0.01 |
| Operative data | ||||
| Operative duration, h | 4.3 ± 1.1 | 4.6 ± 1.1 | 1.24 (1.11–1.58) | 0.03 |
| Complete revascularization | 628 (97.2) | 154 (96.9) | 0.99 (0.67–1.49) | 0.56 |
| Postoperative data | ||||
| Inotropic support for >24 h | 45 (7.0) | 20 (12.6) | 1.70 (1.12–2.53) | 0.03 |
| Insulin used during intensive care | 0.03 | |||
| None | 292 (45.2) | 84 (52.8) | 1 (Ref) | |
| 1–20, units | 202 (31.3) | 51 (32.1) | 1.05 (0.77–1.40) | |
| ≥ 21, units | 152 (23.5) | 24 (15.1) | 0.56 (0.35–0.92) | |
| Transfusion | 282 (43.7) | 77 (48.4) | 1.22 (0.89–1.82) | 0.28 |
| Drugs initiated on postoperative day 1 | ||||
| Renin angiotensin system inhibitors | 410 (63.5) | 101 (63.5) | 0.99 (0.66–1.32) | 0.38 |
| Beta blockers | 548 (84.8) | 136 (85.5) | 0.98 (0.77–1.28) | 0.81 |
| Statins | 552 (85.4) | 135 (84.9) | 0.94 (0.65–1.30) | 0.59 |
Data are number (percent), mean ± standard deviation, or median [25th–75th percentile]. EuroSCORE: European System for Cardiac Operative Risk Evaluation
The unadjusted OR (95% CI) for the association between HbA1c and postoperative AF calculated using a logistic regression model was 0.70 (0.61–0.83) per 1% increase in HbA1c; patients with HbA1c levels in the upper tertile had a 58% lower risk of postoperative AF (OR 0.42; 95% CI 0.29–0.70) than those with HbA1c levels in the lower tertile (Table 3). Adding the univariate predictors (model 2) and the known risk factors into the model (model 3) attenuated this association, but HbA1c remained a predictive indicator of postoperative AF. Other variables statistically significant in the model 2 were age (OR 1.34 per 10-year increase; 95% CI 1.08–1.65; p = 0.01), preoperative statin (OR 0.70; 95% CI 0.56–0.91; p = 0.02), operative duration (OR 1.23 per 1-h increase; 95% CI 1.07–1.48; p = 0.02), and inotropic support for >24 h (OR 1.54; 95% CI 1.08–2.33; p = 0.03). No interaction term was significantly associated with postoperative AF in the models. The area under the receiver operator characteristic curve (95% CI) for the association between HbA1c and postoperative AF was 0.70 (0.65–0.75) (p = 0.01).
Table 3:
Odds ratios and 95% confidence intervals for association between hemoglobin A1c (per 1% increase and by hemoglobin A1c tertile) and postoperative atrial fibrillation
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Per 1 % increase | 0.70 (0.61–0.83) | 0.74 (0.60–0.92) | 0.78 (0.63–0.95) |
| Lower tertile | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Upper tertile | 0.42 (0.29–0.70) | 0.54 (0.31–0.90) | 0.55 (0.35–0.88) |
Values are odds ratio (95% confidence interval). Model 1 includes HbA1c alone. Model 2 HbA1c, age, smoking, chronic kidney disease, chronic pulmonary disease, hypertension, preoperative oral antidiabetics, preoperative statins, operative duration, inotropic support for >24 h, and insulin used during intensive care, and model 3 HbA1c, age, sex, body mass index, chronic kidney disease, chronic pulmonary disease, hypertension, triple vessel disease, ejection fraction of <40%, left atrial dimension, preoperative beta blockers, preoperative statins, inotropic support for >24 h, and transfusion. Cutoff points for tertiles: lower, 3.8–5.6%; upper, 6.8–11.4%.
To identify independent effect of diabetes, obesity, and use of statins on the incidence of postoperative AF, we created a logistic regression model, including diabetes, obesity, and use of statins as covariates, and interaction terms (diabetes × obesity, diabetes × use of statins, and obesity × use of statins) were introduced into the model to identify significant interactions. Diabetes (OR 0.55, 95% CI 0.32–0.95, p = 0.03) and use of statins (OR 0.79, 95% CI 0.55–0.98, p = 0.04) were significantly associated with postoperative AF. No interaction terms were significantly associated with postoperative AF.
DISCUSSION
The major finding of the present study, which enrolled 805 patients undergoing elective off-pump coronary bypass surgery by a single surgeon, was that higher preoperative HbA1c was independently associated with a lower risk of postoperative AF.
A number of investigators have demonstrated the association between preoperative HbA1c and AF after coronary bypass surgery. Halkos and colleagues examined 3089 patients undergoing elective primary coronary surgery and demonstrated that patients with HbA1c of ≥7.0% were younger, more often female, more likely to have renal failure and advanced coronary disease, received more grafts, and had higher intra- and postoperative glucose levels; the incidence of AF was 20.9% in patients with HbA1c of <7.0% and 15.1% in those with HbA1c of ≥7.0% (p = 0.007), the adjusted OR (95% CI) for association with postoperative AF at HbA1c of ≥6.8% was 0.73 (0.55–0.96), and the area under the receiver operator characteristic curve was 0.687 [14]. These findings concur with our results. However, unlike in our study, in which all patients underwent off-pump surgery, in Halkos’s study, cardiopulmonary bypass was used in 29.1% of patients with HbA1c of <7.0% and 34.7% of patients with HbA1c of ≥7.0% (p = 0.01). Matsuura and colleagues investigated outcomes in 101 diabetic patients undergoing off-pump CABG and reported that the incidence of postoperative AF was 29.7% in patients with HbA1c of <6.5% and 22.2% in those with HbA1c of >6.5% (p = 0.49) [17].
The exact mechanisms that explain the protective effect of elevated HbA1c on postoperative AF are not clear. One possible explanation is that patients with elevated HbA1c require more insulin, which has been reported to reduce the risk of postoperative AF [18,19], to control the postoperative blood glucose level. In the present study, patients with higher HbA1c required more insulin than those with lower HbA1c. Postoperative insulin infusion was significantly associated with postoperative AF in a univariate model but was not significant in a multivariate model. Another possible explanation is that patients with higher HbA1c were significantly younger than those with lower HbA1c in the present study. Both age and HbA1c were independent predictors of postoperative AF, but the two factors were not correlated.
Postoperative complications, especially pneumonia and myocardial infarction, might have contributed to the occurrence of postoperative AF. In the present study, the incidence of pneumonia and myocardial infarction was significantly higher in patients with postoperative AF than in those without AF. These adverse events are associated with increased vulnerability for AF because of hypoxia, systemic inflammation, disturbance in electrolyte balance, and ventricular impairment. However, it is not clear whether postoperative AF was the etiology or the result of the adverse events. Several investigators also reported the association between postoperative AF and these adverse events. Aranki and colleagues examined the outcome in 570 consecutive patients undergoing coronary bypass surgery and reported that the incidence of pneumonia (11% vs 1%, p < 0.001), myocardial infarction (5% vs 3%, p = 0.40), and stroke (3.7% vs 1% p = 0.025) after surgery was significantly higher in patients with postoperative AF than in those without AF [12]. In a large observational study of 4657 patients undergoing coronary bypass surgery, the incidence of pneumonia was significantly higher in patients with postoperative AF (8.75% vs 3.4%, p < 0.001) [13].
In clinical practice, HbA1c can be easily determined. Although the present study was not designed to identify patients who would benefit from prophylactic use of antiarrhythmic drugs such as beta blocker, amiodarone, sotalol, and statin, preoperative measurement of HbA1c might be useful for noninvasive risk stratification of patients at high risk of postoperative AF.
The present study has a number of potential limitations. First, all enrolled subjects were Japanese patients who underwent revascularization using the off-pump technique at a single center, which limits the generality of the findings; second, we had no information about the dose of catecholamine used in the operating theater, which may have influenced the occurrence of AF; third, HbA1c levels were based on a single measurement; finally, episodes of postoperative AF may have been missed because electrocardiographic telemetry is susceptible to the effect of motion artifact and electrocardiographic monitoring was not performed after postoperative day 8.
In conclusion, preoperative HbA1c independently predicts the occurrence of postoperative AF after isolated off-pump CABG. Further studies are necessary to investigate the mechanism underlying the association between preoperative HbA1c and postoperative AF and to work out a strategy to improve outcomes for high-risk patients identified by HbA1c analysis.
Conflict of interest: none declared.
REFERENCES
- 1.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–23. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 3.Woods SE, Smith JM, Sohail S, Sarah A, Eagle A. The influence of type 2 diabetes mellitus in patients undergoing coronary artery bypass graft surgery. Chest. 2004;126:1789–95. doi: 10.1378/chest.126.6.1789. [DOI] [PubMed] [Google Scholar]
- 4.Rajakaruna C, Rogers CA, Suranimala C, Angelini GD, Ascione R. The effect of diabetes mellitus on patients undergoing coronary surgery: a risk-adjusted analysis. J Thorac Cardiovasc Surg. 2006;132:802–10. doi: 10.1016/j.jtcvs.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Calafiore AM, Di Mauro M, Di Giammarco G, Contini M, Vitolla G, Iaco AL, Canosa C, D’Alessandro S. Effect of diabetes on early and late survival after isolated first coronary bypass surgery in multivessel disease. J Thorac Cardiovasc Surg. 2003;125:144–54. doi: 10.1067/mtc.2003.73. [DOI] [PubMed] [Google Scholar]
- 6.Kubal C, Srinivasan AK, Grayson AD, Fabri BM, Chalmers JAC. Effect of risk-adjusted diabetes on mortality and morbidity after coronary artery bypass surgery. Ann Thorac Surg. 2005;79:1570–6. doi: 10.1016/j.athoracsur.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Whang W, Bigger JT. Diabetes and outcomes of coronary artery bypass graft surgery in patients with severe left ventricular dysfunction: results from the CABG Patch Trial Database. J Am Coll Cardiol. 2000;36:1166–72. doi: 10.1016/s0735-1097(00)00823-8. [DOI] [PubMed] [Google Scholar]
- 8.Szabo Z, Hakanson E, Svedjeholm R. Early postoperative outcome and medium-term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2002;74:712–9. doi: 10.1016/s0003-4975(02)03778-5. [DOI] [PubMed] [Google Scholar]
- 9.Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR, Griffith BP. Predictors and impact of atrial fibrillation after isolated coronary artery bypass grafting. Crit Care Med. 2002;30:330–7. doi: 10.1097/00003246-200202000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosokawa K, Nakajima Y, Umenai T, Ueno H, Taniguchi S, Matsukawa T, Mizobe T. Predictors of atrial fibrillation after off-pump coronary artery bypass graft surgery. Br J Anaesth. 2007;98:575–80. doi: 10.1093/bja/aem067. [DOI] [PubMed] [Google Scholar]
- 11.Choi YS, Shim JK, Hong SW, Kimc DH, Kima JC, Kwak YL. Risk factors of atrial fibrillation following off-pump coronary artery bypass graft surgery: predictive value of C-reactive protein and transfusion requirement. Eur J Cardiothorac Surg. 2009;36:838–43. doi: 10.1016/j.ejcts.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr., Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 13.Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, Browner WS. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–6. [PubMed] [Google Scholar]
- 14.Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, Guyton RA, Thourani VH. Elevated preoperative HbA1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136:631–40. doi: 10.1016/j.jtcvs.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 15. Examination Committee of Criteria for “Obesity Disease” in Japan, Japan Society for the Study of Obesity. New criteria for “obesity disease” in Japan. Circ J 2002;66:987–92. [DOI] [PubMed]
- 16.Kinoshita T, Asai T, Nishimura O, Suzuki T, Kambara A, Matsubayashi K. Off-pump bilateral versus single skeletonized internal thoracic artery grafting in patients with diabetes. Ann Thorac Surg. 2010;90:1173–9. doi: 10.1016/j.athoracsur.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Matsuura K, Imamaki M, Ishida A, Shimura H, Niitsuma Y, Miyazaki M. Off-pump coronary artery bypass grafting for poorly controlled diabetic patients. Ann Thorac Cardiovasc Surg. 2009;15:18–22. [PubMed] [Google Scholar]
- 18.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–21. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 19.Lazar HL, Chipkin S, Philippides G, Bao Y, Apstein C. Glucose–insulin–potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg. 2000;70:145–50. doi: 10.1016/s0003-4975(00)01317-5. [DOI] [PubMed] [Google Scholar]