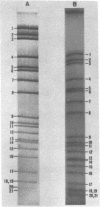
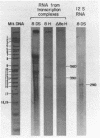
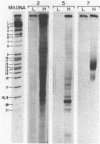
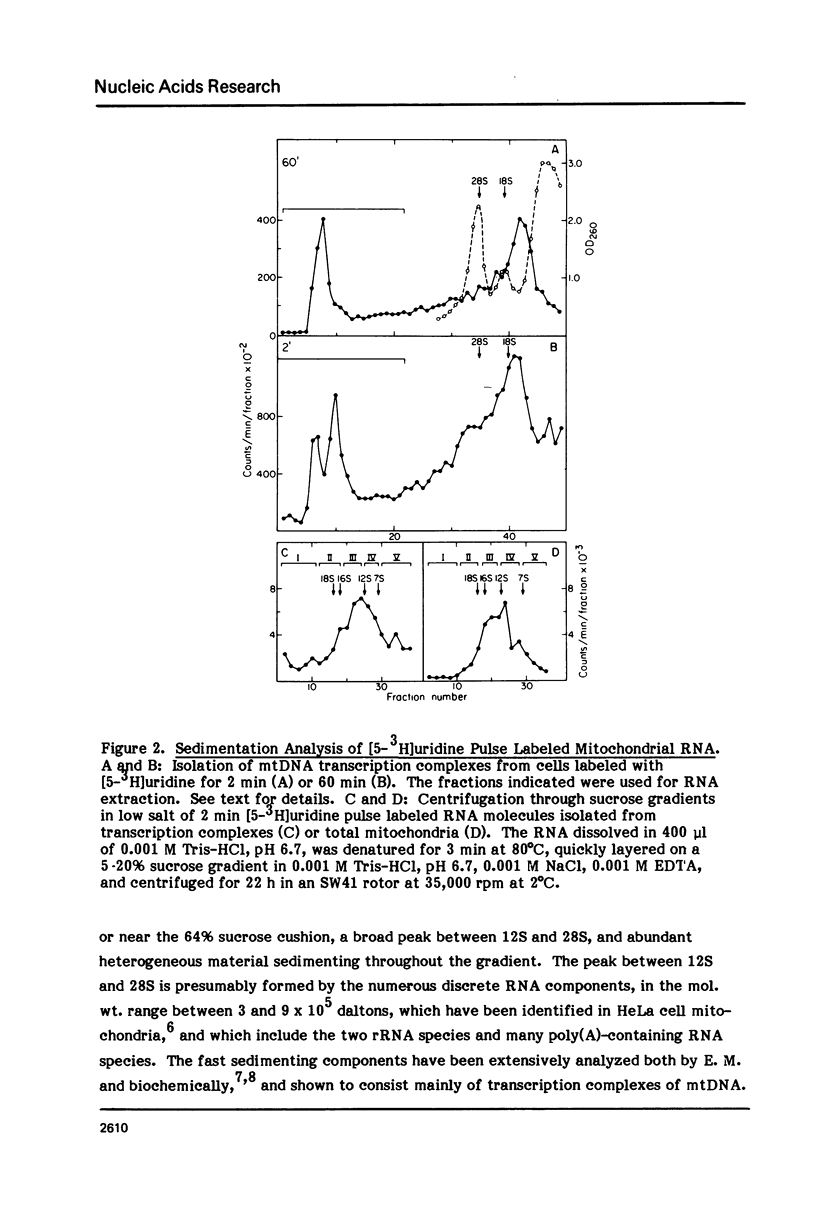
Abstract
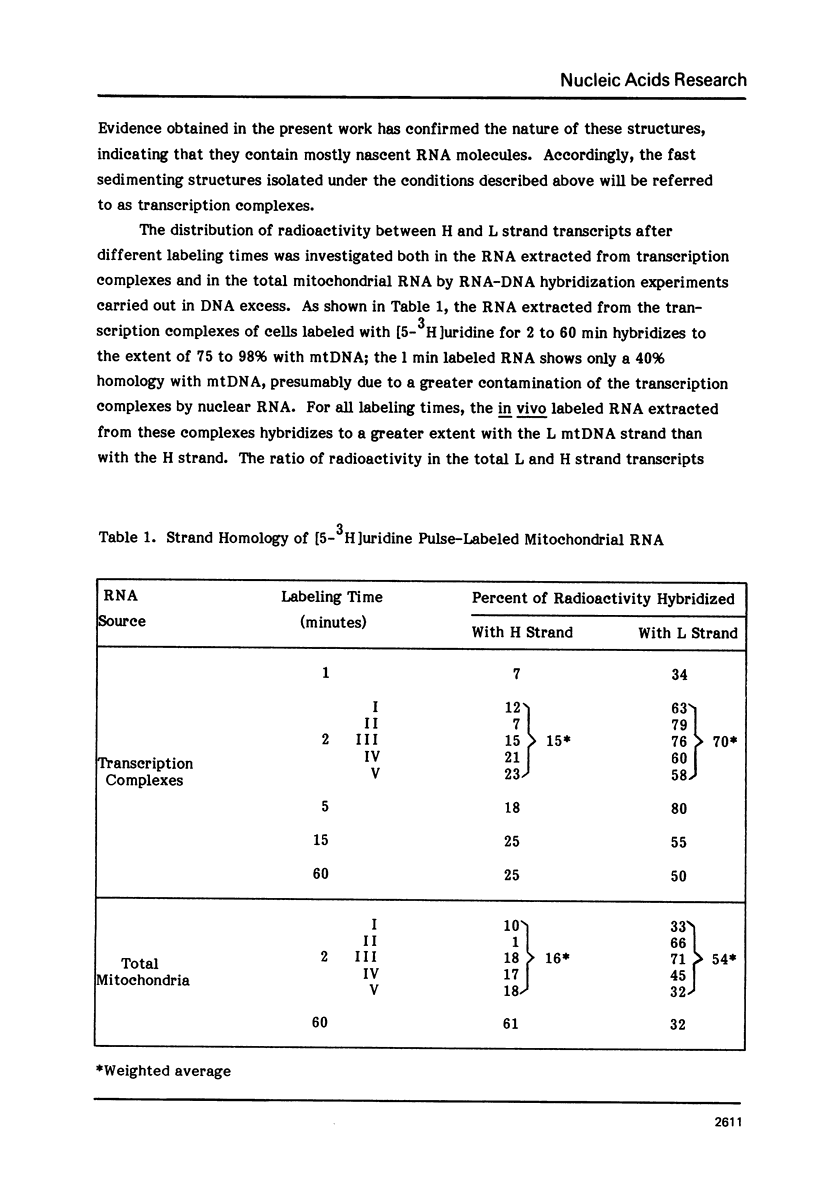
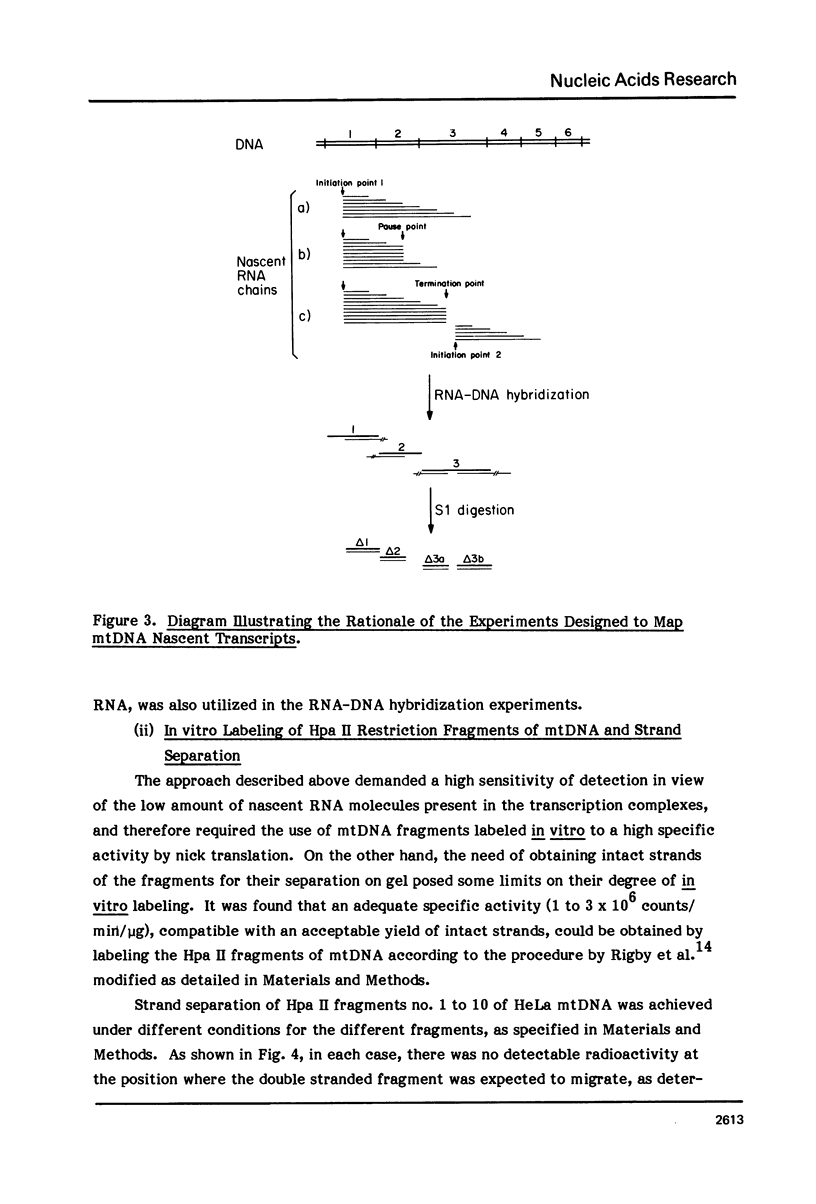
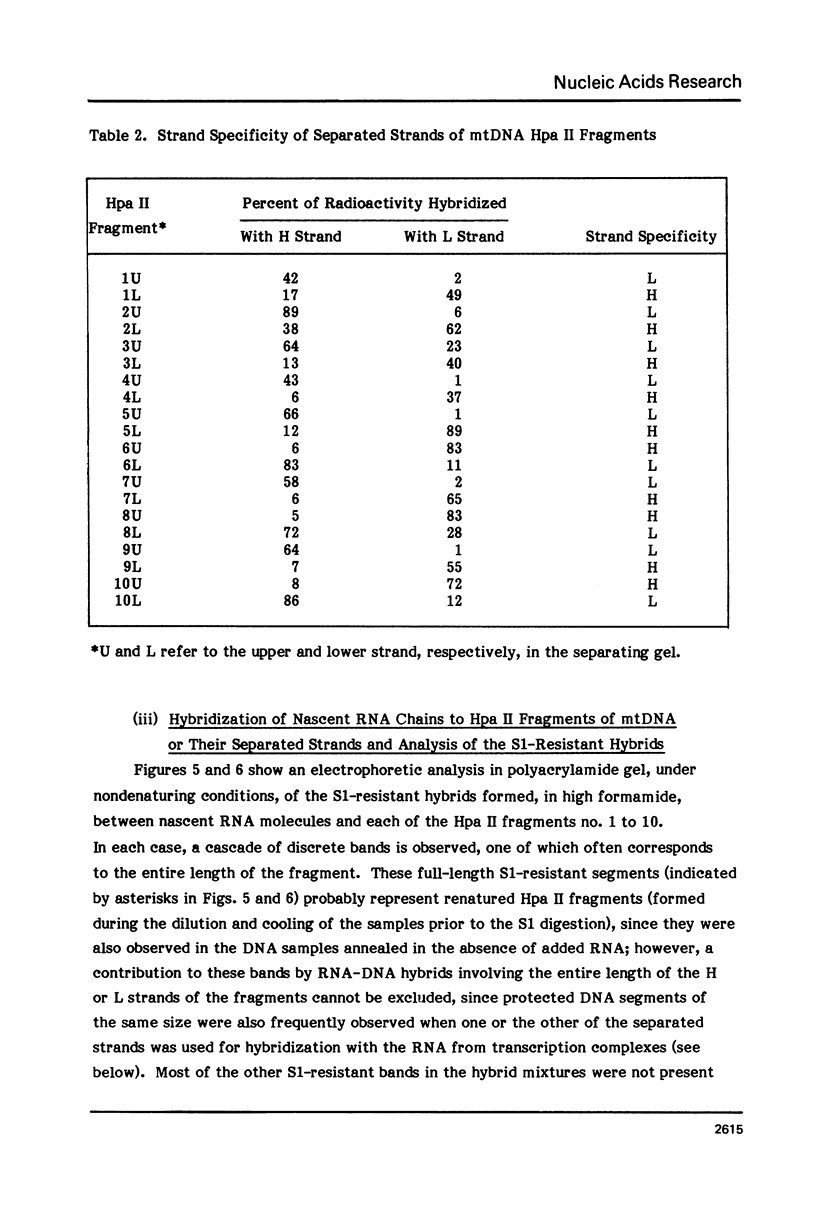
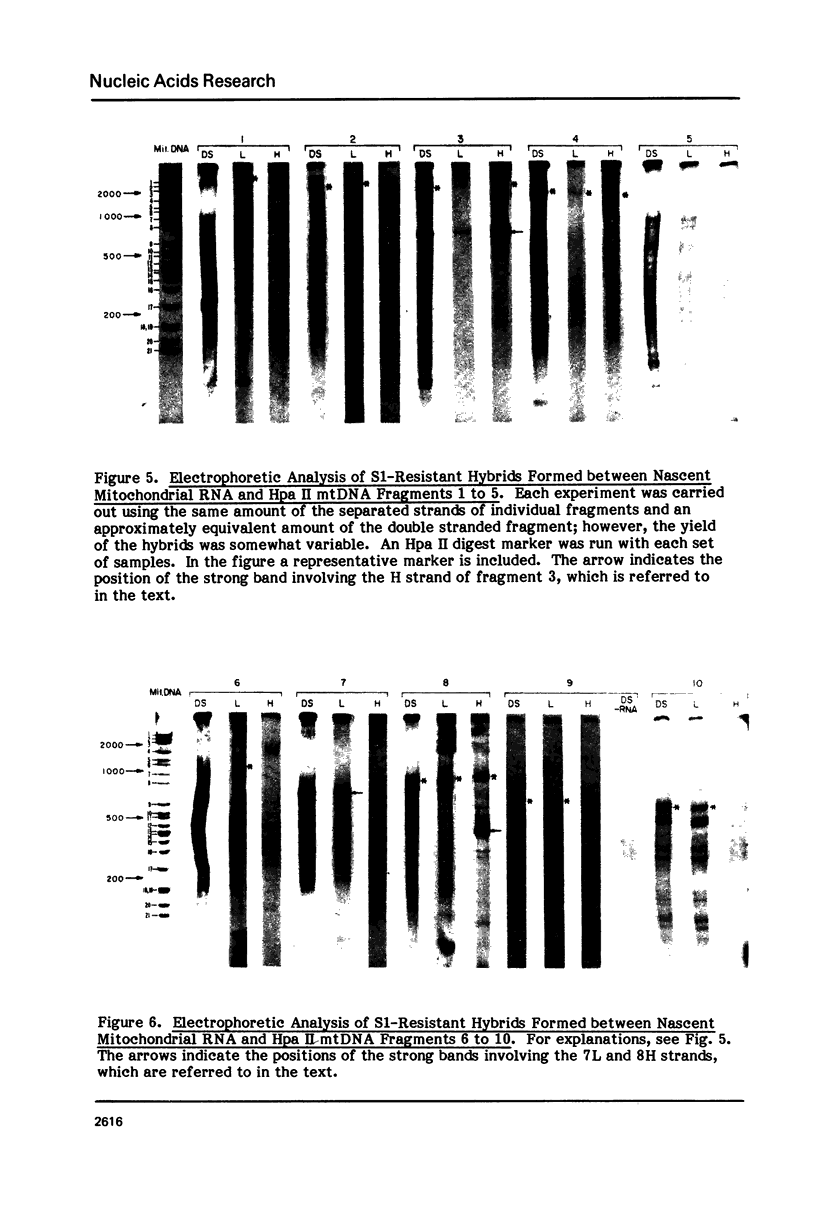
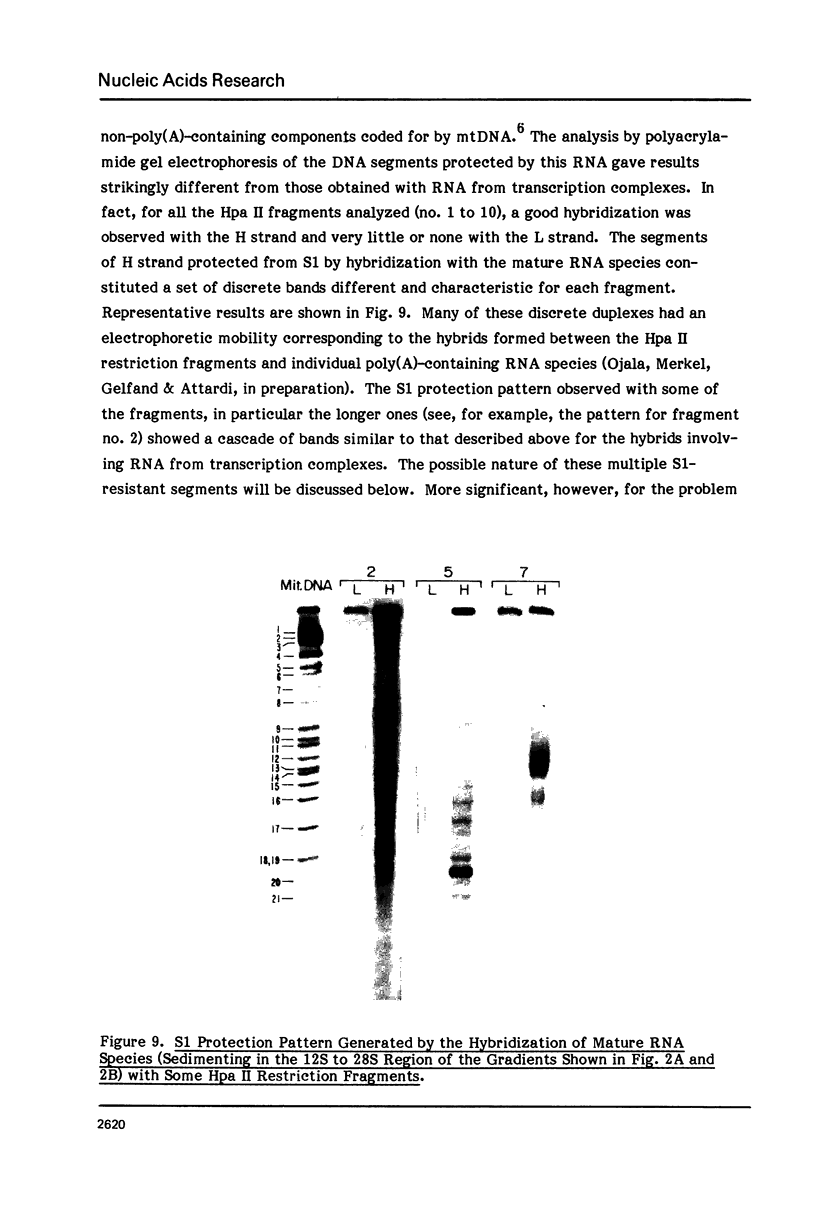
The sequences complementary to the nascent RNA molecules isolated from transcription complexes of HeLa cell mtDNA have been mapped on the H and L strands of mtDNA by the S1 protection technique. The distribution of these sequences among different Hpa II restriction fragments was found to reflect the position of these fragments in the Hpa II map of mtDNA. Thus, the S1-resistant hybrids formed with the L strand corresponded almost exclusively to the right half of the genome past the origin of replication in the direction of L strand transcription, and were especially concentrated in the region immediately adjacent to the origin. By contrast, the hybrid duplexes involving the H strand appeared to be localized in the left half of the genome, and in particular in the quadrant of the map adjacent to the origin in the direction of H strand transcription. These results strongly suggest that the region of mtDNA around the origin of replication contains an initiation site for L strand transcription and an initiation site for H strand transcription.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Expression of the mitochondria genome in HeLa cells. IV. Titration of mitochondrial genes for 16 s, 12 s and 4 s RNA. J Mol Biol. 1971 Jan 28;55(2):271–276. doi: 10.1016/0022-2836(71)90197-5. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. XI. Isolation and characterization of transcription complexes of mitochondrial DNA. J Mol Biol. 1972 Sep 28;70(2):363–373. doi: 10.1016/0022-2836(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Amalric F., Merkel C., Gelfand R., Attardi G. Fractionation of mitochondrial RNA from HeLa cells by high-resolution electrophoresis under strongly denaturing conditions. J Mol Biol. 1978 Jan 5;118(1):1–25. doi: 10.1016/0022-2836(78)90241-3. [DOI] [PubMed] [Google Scholar]
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré D., Attardi G. Biochemical and electron microscopic characterization of DNA-RNA complexes from HeLa cell mitochondria. Biochemistry. 1978 Aug 8;17(16):3263–3273. doi: 10.1021/bi00609a014. [DOI] [PubMed] [Google Scholar]
- Crews S., Attardi G. The sequences of the small ribosomal RNA gene and the phenylalanine tRNA gene are joined end to end in human mitochondrial DNA. Cell. 1980 Mar;19(3):775–784. doi: 10.1016/s0092-8674(80)80053-5. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E., Jr Multiple discrete sites for premature RNA chain termination late in adenovirus-2 infection: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2571–2575. doi: 10.1073/pnas.76.6.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. C., Attardi G. Amino acid specificity of the transfer RNA species coded for by HeLa cell mitochondrial DNA. J Mol Biol. 1976 Mar 25;102(1):125–141. doi: 10.1016/0022-2836(76)90077-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Murphy W. I., Attardi B., Tu C., Attardi G. Evidence for complete symmetrical transcription in vivo of mitochondrial DNA in HeLa cells. J Mol Biol. 1975 Dec 25;99(4):809–814. doi: 10.1016/s0022-2836(75)80187-2. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. A detailed physical map of HeLa cell mitochondria DNA and its alignment with the positions of known genetic markers. Plasmid. 1977 Nov;1(1):78–105. doi: 10.1016/0147-619x(77)90010-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Precise localization of the origin of replication in a physical map of HeLa cell mitochondrial DNA and isolation of a small fragment that contains it. J Mol Biol. 1978 Jul 5;122(3):301–319. doi: 10.1016/0022-2836(78)90192-4. [DOI] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Preparation of large quantities of separated strands from simian virus 40 DNA restriction fragments by low-temperature low-salt agarose gel electrophoresis. Anal Biochem. 1977 Dec;83(2):666–677. doi: 10.1016/0003-2697(77)90071-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]