Abstract
OBJECTIVE
Oxygen is routinely administered to patients undergoing acute myocardial infarction as well as during revascularization procedures and cardiac surgery. Because reactive oxygen species are mediators of ischemia/reperfusion injury, increased oxygen availability might theoretically aggravate myocardial injury during reperfusion. We hypothesized that ventilation with a hyperoxic gas at start of reperfusion might increase ischemia/reperfusion injury.
METHODS
Rats were anesthetized with isoflurane and ventilated with 40% oxygen. The animals were subjected to 40 min of regional myocardial ischemia and 120 min of reperfusion. In the test group, rats (n = 11) were ventilated with a normobaric hyperoxic gas (95% O2) during the last 10 min of ischemia and the first 10 min of reperfusion. Control rats (n = 14) were ventilated with 40% O2 throughout the experiments. Due to irreversible reperfusion arrhythmias, one animal in the hyperoxia group and six animals in the control group were excluded. Hearts (n = 8 in the control group and n = 10 in the test group) were harvested for measurement of infarct size.
RESULTS
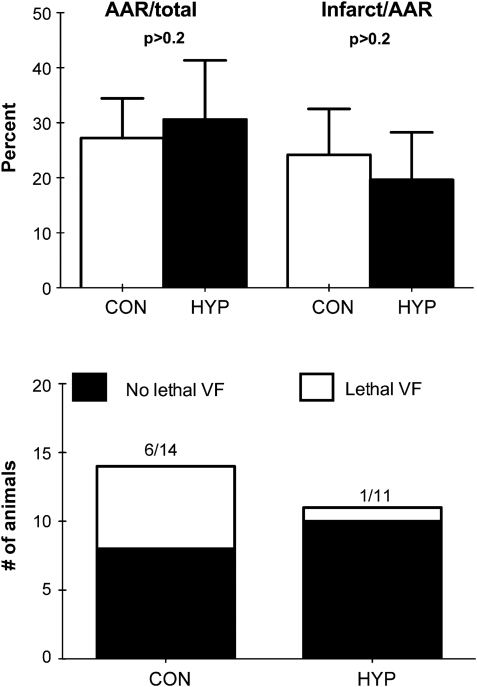
The incidence of lethal arrhythmias was 1/11 in the test group and 6/14 in the control group (p = 0.06). Reperfusion with normobaric hyperoxia did not influence infarct size (20 ± 8% of area at risk) compared with the normoxia group (24 ± 8% and of area at risk), respectively (mean ± SD, p > 0.2).
CONCLUSION
Normobaric hyperoxia during early reperfusion did not increase ischemia/reperfusion injury.
Keywords: Heart, Ischemia, Reperfusion, Hyperoxia
INTRODUCTION
Revascularization and early reperfusion is the preferred mode of treatment for patients with acute myocardial infarction [1]. However, reperfusion may be a ‘double-edged sword’ [2]. The restoration of blood flow causes irreversible cellular damage in post-ischemic tissue. The mechanisms of reperfusion injury are multifaceted and complex, but there is abundant evidence that production of reactive oxygen species (ROS) is an important pathogenic mechanism [3,4]. ROSs at low concentrations are byproducts of normal metabolism [5]. ROSs such as superoxide (O2−), hydrogen peroxide (H2O2), the hydroxyl radical (HO•), and singlet oxygen (O2•) are involved in cellular signaling and microbicidal defenses [6]. On the other hand, excess production of ROS causes lipid peroxidation and oxidative damage to membranes, proteins, and DNA [4]. Zweier and co-workers showed that there is a burst of superoxide anions in the first 20–30 s after reperfusion and ROS scavenger administration led to improvement of postischemic function [7].
In theory, increased availability of oxygen might increase ROS production and thus aggravate the reperfusion injury. Furthermore, it has been shown that hyperoxia induces coronary vasoconstriction and decreases coronary flow in both healthy volunteers and patients with coronary artery disease [8]. Hypoxic or controlled reperfusion alleviates post-ischemic injury in skeletal muscle [9] and stomach [10]. In principle, oxygen supplementation during reperfusion by thrombolysis or percutaneous coronary interventions might be harmful. The optimal oxygenation strategy of patients with acute myocardial infarction undergoing reperfusion has not been studied in large randomized investigations. In a systematic review, Wijesinghe and co-workers concluded that ‘the limited evidence that does exist suggests that the routine use of high-flow oxygen in uncomplicated acute myocardial infarction may result in a greater infarct size and possibly increase the risk of mortality [11].’ The Cochrane collaboration recently concluded: ‘nonetheless, since the evidence suggests that oxygen may in fact be harmful, we think it is important to evaluate this widely used treatment in a large trial, as soon as possible, to make sure that current practice is not causing harm to people who have had a heart attack [12].’ A recent editorial in the British Medical Journal stated, ‘The potential dangers of hyperoxia needs to be recognized,’ and that ‘Oxygen therapy remains a cornerstone of modern medical practice. To further quantify the risks associated with hyperoxia more trials are needed [13].’
In cardiac surgery using cardiopulmonary bypass and cardioplegia, there is a postcardioplegic reperfusion of the ischemic myocardium. Studies in different models have suggested that normoxia during reoxygenation of the hypoxic or cardioplegic heart reduces reperfusion injury [14–16].
We hypothesized that normobaric hyperoxia at the time of reperfusion might increase ischemia/reperfusion injury. In the present study, the effect of exposing rats to hyperoxia during early reperfusion was investigated.
MATERIALS AND METHODS
Animal care
The protocols were approved by the Norwegian Animal Health Authority, and the animals received humane care in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. Male Wistar rats (Scanbur AS, Norway) of 250–320 g weight were used. All animals had conventional microbiological status. Environmental conditions regarding food (RM3 from Scanbur BK AS, Norway), water (ad libitum), humidity (55–60%), light (12 h light and 12 h dark), and environmental enrichment were the same for all animals. Animals were acclimatized for at least 4 days before the experiments.
Myocardial ischemia/reperfusion injury
Anesthesia was induced with isoflurane (Abbott Scandinavia, Solna, Sweden) in a clear plastic induction chamber (VetEquip, CA, USA) before the animals underwent tracheostomy and cannulation of the left common carotid artery for measurement of blood pressure and heart rate. The animals were fixed on a water-heated operating table and ventilated at a tidal volume of 3.5 ml at a rate of 30 breaths per min with a small animal ventilator (Harvard Apparatus, Massachusetts, USA). Anesthesia was maintained during the entire procedure with isoflurane. The major and minor pectoral muscles were cut and the fourth left rib excised to gain access to the thoracic cavity. The pericardium was dissected and opened, and a 5/0 silk suture was placed under the left anterior descending artery approximately 2 mm distal to its eminence behind the pulmonary trunk. The animals were allowed 10 min of stabilization before tightening the ligature.
All animals were subjected to 40 min of regional myocardial ischemia and 120 min of reperfusion. In the hyperoxia group, animals were ventilated with >95% oxygen for the last 10 min of ischemia and the first 10 min of reperfusion. The coronary artery was occluded with a plastic occluder over a Teflon pledget. Tissue ischemia was confirmed by visible cyanosis before closing the chest provisionally with surgical tongs. Reperfusion was also confirmed visibly. Functional data were recorded with Powerlab 4/30 and Graph 5.2 acquisition software (ADInstruments, UK). Animals with lethal ventricular fibrillation (VF) during reperfusion were excluded from infarct evaluation, but the incidence of irreversible VF was calculated for each group.
Measurement of infarct size
After reperfusion, the animals were euthanized by excision of the heart. The ligature was then retightened and the aorta was perfused retrogradely with 2% Evans Blue dye (Sigma–Aldrich, USA) to delineate the area at risk. The heart was cut into eight transverse slices from apex to base, where the four central slices were 1 mm thick, and the apical and basal slices were 2 mm. The four central ones were used for determination of infarct size.
These slices were incubated in 1% triphenyl tetrazolium chloride (TTC) (Sigma–Aldrich, USA) in a 37 °C water bath for 15 min and then placed between two glass plates and photographed in a light chamber using a Nikon Coolpix 5400 digital camera (Nikon, Japan). The area at risk and infarct size were calculated using Adobe Photoshop CS3 Extended (Adobe, USA) by a researcher blinded to the groups.
Arterial blood gas measurements
Partial pressure of oxygen in the blood (PaO2) was measured using an i-Stat1 (Abbott Laboratories, IL, USA) and CG4+ cartridges at baseline and 1, 5, and 10 min after the inspired oxygen concentration was changed from 21% to >95%. The inspired oxygen concentration was measured using a PMA 10 Oxygen Analyzer (M&C TechGroup, Rattingen, Germany), calibrated with 100% N2, room air, and 95% O2. Partial pressure of carbon dioxide in the blood (PaCO2) was normally between 4.0 and 5.4.
To demonstrate that hyperoxemia was present before start of reperfusion, serial arterial blood gases were obtained from the common carotid artery of four rats with the same model of ischemia/reperfusion. These rats were not included in infarct size measurement.
Statistical analyses
Graphpad Prism 5 (Graphpad Software, CA, USA) was used for all statistical analyses. Student’s t-test was used for comparison of infarct size. The chi-square test was used for comparison of lethal VF. Analysis of variance (ANOVA) repeated measurements were used to evaluate blood pressure and heart rate. Exact p-values are presented except when p > 0.2.
RESULTS
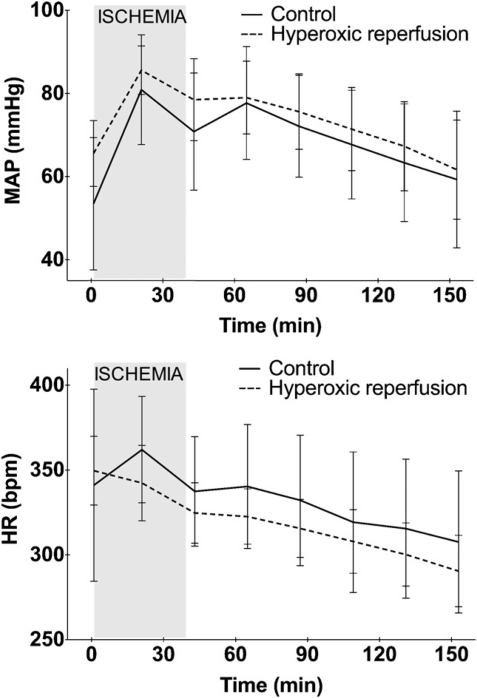
Increasing inspired O2 concentration to >95% had no effect on PaCO2, bicarbonate, pH, or lactate (data not shown). However, the saturation level of oxygen in hemoglobin (SaO2) and PaO2 increased after 1 min of hyperoxia from 98.8% to 100% and from 100 to >520 mmHg, respectively. SaO2 and PaO2 did not increase further during the next 5 or 10 min. The time from induction of anesthesia to stabilization after placement of the ligature did not differ between the groups. Hyperoxic ventilation during reperfusion did not influence heart rate or mean arterial blood pressure (Fig. 1). It did not affect either area at risk or infarct size (Fig. 2). The incidence of irreversible lethal arrhythmias was lower in the hyperoxic group (1/10) versus the control group (6/14) (Fig. 2).
Figure 1:
Hemodynamic parameters in rats undergoing 40 min of in vivo regional myocardial ischemia and 120 min of reperfusion. MAP: mean arterial blood pressure, HR: heart rate.
Figure 2:
(Upper panel) Area-at-risk (AAR) and infarct size (mean ± SD) in rat hearts undergoing 40 min of regional ischemia and 120 min of reperfusion in vivo. CON: control group. HYP: animals ventilated with >95% O2 during the last 10 min of ischemia and the first 10 min of reperfusion (n = 8 and 10 in CON and HYP, respectively). (Lower panel) Incidence of lethal reperfusion arrhythmias (VF) in rats (groups as in upper panel), p = 0.06.
DISCUSSION
The main finding of the present study is that ventilation with normobaric hyperoxia at the onset of reperfusion neither augmented infarct size nor impaired hemodynamic function in rats. These results are contrary to our hypothesis, but they are in agreement with findings from a study in rabbits undergoing 45 min of coronary ligation and 3 h of reperfusion [17]. Shnier and co-workers [17] found no difference in infarct size between animals receiving normoxic or hyperoxic ventilation during reperfusion. Their study, however, is different from ours in design. Although reporting flushing the ventilation circuit with oxygen, the investigators began oxygen supplementation ‘just before coronary artery ligature release,’ possibly delaying the increase in substrate for ROS past the critical first seconds of reperfusion [3]. There was no measurement of arterial blood gases at the start of reperfusion.
To our knowledge, the present study is the first investigation of the effects of hyperoxic ventilation during early reperfusion following coronary artery occlusion in an in vivo rat model. In our study, oxygen was administered 10 min prior to reperfusion to ensure high availability of oxygen at the onset of reperfusion. To allow time for oxygen to equilibrate with the extravascular space and cells is important. Although PaO2 exceeded 500 mmHg after 1 min of hyperoxic ventilation, it may take longer before hyperoxia has reached an equilibrium in the cardiomyocytes. Two hours of reperfusion is normally a sufficient length of reperfusion for evaluation of infarct size. In the present study, we had an intervention that we hypothesized would increase the infarct size by increasing the amount of ROS during reperfusion. As generation of oxygen species is a phenomenon primarily during the first seconds and minutes of reperfusion, we feel that 2 h of reperfusion is safe for detecting any detrimental effect of hyperoxia. It may also be argued that TTC staining is not a complete endpoint of ischemia/reperfusion injury. It is possible to include other markers of ischemia/reperfusion injury, such as apoptosis, autophagy, and release of biochemical markers. However, necrosis is the ‘hardest’ end point and the other markers are usually in parallel with necrosis detected by TTC.
At moderate concentrations, ROSs are important second messengers, but, at high concentrations, they offset the oxidation–reduction balance of the cell and may be deleterious [18]. Antioxidants have been shown to reduce infarct size and improve function after ischemia/reperfusion in several experimental models [4,19]. Thus, hyperoxygenation during very early reperfusion might be damaging. Our study showed that oxygen at the time of reperfusion is not detrimental. If any effect was found, it was that hyperoxia was preventive against lethal VF. We have no good explanation for this observation. The positive effects on arrhythmias seem to be believable, as the threshold of p = 0.05 is an artificial one, and we do have a statistical error type I because the study was underpowered to detect this difference.
A group size of about 10 animals is usually sufficient for these type of studies to find important differences. Small differences in patient studies may still have a clinical relevance; however, in animal studies we look for a more ‘hard’ effect. The present difference in infarct size is very small. A retrospective power calculation suggests that 25 animals in each group would find a difference. If we look for ‘hard’ effects in experimental studies, a group size of 25 to achieve statistical significance may be less important. In fact, we have never seen an experimental study in small rodents, where group size is 25 to compare infarct size.
Conceivably, hyperoxemia during late ischemia might supply oxygenated blood to the risk zone via collateral coronary arteries, masking possibly the detrimental effects of hyperoxic reperfusion by limiting the severity of the ischemic insult. However, rats have negligible collateral coronary blood flow [20], making this possible pitfall less likely. Collateral blood flow might also explain the infarct-sparing effect of ventilation with 100% oxygen following coronary artery occlusion in dogs [21], as canines have extensive collaterals in the coronary circulation [20]. The border zone or the area at risk is critical for generation of reperfusion arrhythmias, and improved oxygenation of this area might be preventive toward lethal arrhythmias.
We have previously found that pre-treatment with hyperoxic gas has a precondition-like, cardioprotective effect, possibly by a nuclear factor-kappa B (NFκB)-dependent mechanism [22,23]. However, oxygen is obviously a double-edged sword because it may be detrimental in the cardiac surgery setting [15], in particular in combination with hypoxic, immature hearts [14,16].
It is worthwhile to carefully consider the following: ‘The results of well conducted trials may lead to refinements in the use of oxygen. Unfortunately, due to its accepted role in therapeutic practice and virtually non-existent potential for commercial development, oxygen therapy has attracted little research funding in recent years. At present doctors should strive to ensure that oxygen is prescribed, administered, and monitored with care. This will enable us to achieve optimal tissue oxygenation for more of our patients [13].’
To date, only one randomized trial has investigated the effect of oxygen supplementation to patients with myocardial infarction [24]. The care offered to patients enrolled in this study, which was published in 1976, differs largely from current treatment with rapid revascularization. Though reporting only in-hospital outcomes and being seriously underpowered to evaluate clinical outcomes, the study suggested that oxygen supplementation might even be harmful to patients with acute myocardial infarction. Oxygen is commonly administered to patients presenting with chest pain and with verified myocardial infarction. Evidence for the optimal mode, timing, and dosage, or even benefit, is absent. Although oxygen supplementation to hypoxemic patients is intuitively more palatable, optimal oxygenation has not been determined for these patients either. The few experimental studies that exist show conflicting findings [17,22,23,25], which may be due to species differences and model heterogeneity. The important message from our study is that, in the present model with regional ischemia, hyperoxia during reperfusion is not detrimental. This is in contrast with studies which have been conducted in animal models on cardiopulmonary bypass [14–16]. It might well be that the situation is different between a regional ischemia with a localized infarct and a global, but weaker ischemic insult. The limitation of the present study is that findings from rats cannot be transferred to patients; hence, large clinical studies are necessary.
In conclusion, in a rat model of in vivo myocardial ischemia and reperfusion, ventilation with normobaric hyperoxia at onset of reperfusion tended to reduce lethal arrhythmias and did not influence infarct size in rats. Consequently, increased availability of oxygen during early reperfusion is not harmful in this model in contrast to other studies in other models.
Funding
The investigation was supported by grants from Ulleval University Hospital, University of Oslo, The Norwegian Health Association, The Southeastern Regional Health Trust, and The Gjensidige Foundation. LHM is the recipient of a medical student’s scholarship from the University of Oslo and the Norwegian Research Council.
Conflict of interest: none declared.
REFERENCES
- 1.Van De Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg P, Tubaro M, Verheugt F, Weidinger F, Weis M, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Hellemans I, Kristensen S, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano J, Aguirre F, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Mario C, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip G, Rutten F. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. New Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–7. [PubMed] [Google Scholar]
- 4.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986;74:1424–33. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 5.Bartosz G. Reactive oxygen species: destroyers or messengers? Biochem Pharmacol. 2009;77:1303–15. doi: 10.1016/j.bcp.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46:531–42. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA. 1987;84:1404–7. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz W, Donoso R, Marcus H, Swan HJ. Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation. 1972;45:763–8. doi: 10.1161/01.cir.45.4.763. [DOI] [PubMed] [Google Scholar]
- 9.Korthuis RJ, Smith JK, Carden DL. Hypoxic reperfusion attenuates postischemic microvascular injury. Am J Physiol. 1989;256:H315–9. doi: 10.1152/ajpheart.1989.256.1.H315. [DOI] [PubMed] [Google Scholar]
- 10.Perry MA, Wadhwa SS. Gradual reintroduction of oxygen reduces reperfusion injury in cat stomach. Am J Physiol. 1988;254:G366–72. doi: 10.1152/ajpgi.1988.254.3.G366. [DOI] [PubMed] [Google Scholar]
- 11.Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Weatherall M, Beasley R. Routine use of oxygen in the treatment of myocardial infarction: systematic review. Heart. 2008;95:198–202. doi: 10.1136/hrt.2008.148742. [DOI] [PubMed] [Google Scholar]
- 12.Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD007160.pub2. [Art. No. CD007160] [DOI] [PubMed] [Google Scholar]
- 13.Thomson AJ, Webb DJ, Maxwell SRJ. Oxygen therapy in acute medical care. Br Med J. 2002;324:1406–7. doi: 10.1136/bmj.324.7351.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihnken K, Morita K, Buckberg G, Winkelmann B, Beyersdorf F, Sherman M. Reduced oxygen tension during cardiopulmonary bypass limits myocardial damage in acute hypoxic immature piglet hearts. Eur J Cardiothorac Surg. 1996;10:1127–34. doi: 10.1016/s1010-7940(96)80361-7. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Ku K, Kaneda T, Zang Z, Otaki M, Oku H. Cardioprotective effects of lowering oxygen tension after aortic unclamping on cardiopulmonary bypass during coronary artery bypass grafting. Circ J. 2002;66:718–22. doi: 10.1253/circj.66.718. [DOI] [PubMed] [Google Scholar]
- 16.Morita K, Ihnken K. Studies of hypoxemic/reoxygenation injury: with aortic clamping: XII. Delay of cardiac reoxygenation damage in the presence of cyanosis: a new concept of controlled cardiac reoxygenation. J Thorac Cardiovasc Surg. 1995;110:1265–73. doi: 10.1016/s0022-5223(95)70013-7. [DOI] [PubMed] [Google Scholar]
- 17.Shnier CB, Cason BA, Horton AF, Hickey RF. Hyperoxemic reperfusion does not increase myocardial infarct size. Am J Physiol. 1991;260:H1307–12. doi: 10.1152/ajpheart.1991.260.4.H1307. [DOI] [PubMed] [Google Scholar]
- 18.Kutala VK, Khan M, Angelos MG, Kuppusamy P. Role of oxygen in postischemic myocardial injury. Antioxid Redox Signal. 2007;9:1193–206. doi: 10.1089/ars.2007.1636. [DOI] [PubMed] [Google Scholar]
- 19.Zweier JL, Rayburn BK, Flaherty JT, Weisfeldt ML. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest. 1987;80:1728–34. doi: 10.1172/JCI113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21:737–46. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 21.Malm A, Arborelius MJ, Bornmyr S, Lilja B, Gill RL. Effects of oxygen on acute myocardial infarction: a thermographic study in the dog. Cardiovasc Res. 1977;11:512–8. doi: 10.1093/cvr/11.6.512. [DOI] [PubMed] [Google Scholar]
- 22.Tähepold P, Ruusalepp A, Li G, Vaage J, Starkopf J, Valen G. Cardioprotection by breathing hyperoxic gas-relation to oxygen concentration and exposure time in rats and mice. Eur J Cardiothorac Surg. 2002;21:987–94. doi: 10.1016/s1010-7940(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 23.Tähepold P, Vaage J, Starkopf J, Valen G. Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg. 2003;125:650–60. doi: 10.1067/mtc.2003.36. [DOI] [PubMed] [Google Scholar]
- 24.Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1:1121–3. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly RF, Hursey TL, Parrillo JE, Schaer GL. Effect of 100% oxygen administration on infarct size and left ventricular function in a canine model of myocardial infarction and reperfusion. Am Heart J. 1995;130:957–65. doi: 10.1016/0002-8703(95)90194-9. [DOI] [PubMed] [Google Scholar]