Abstract
OBJECTIVES
We have previously demonstrated the functionality and growth of autologous, living, tissue-engineered vascular grafts (TEVGs) in long-term animal studies. These grafts showed substantial in vivo tissue remodeling and approximation to native arterial wall characteristics. Based on this, in vitro and in vivo matrix metalloproteinase (MMP) activity of TEVGs is investigated as a key marker of matrix remodeling.
METHODS
TEVGs fabricated from biodegradable scaffolds (polyglycolic-acid/poly-4-hydroxybutyrate, PGA/P4HB) seeded with autologous vascular cells were cultured in static and dynamic in vitro conditions. Thereafter, TEVGs were implanted as pulmonary artery replacements in lambs and followed up for 2 years. Gelatin gel zymography to detect MMP-2 and -9 was performed and collagen content quantified (n = 5). Latent (pro) and active MMP-2 and -9 were detected.
RESULTS
Comparable levels of active MMP-9 and pro-MMP-2 were detected in static and dynamic culture. Higher levels of active MMP-2 were detected in dynamic cultures. Expression of MMP-2 and -9 was minimal in native grafts but was increased in implanted TEVG. Pro-MMP-9 was expressed 20 weeks post implantation and persisted up to 80 weeks post implantation. Collagen content in vitro was increased in dynamically conditioned TEVG as compared with static constructs and was increased in vivo compared with the corresponding native pulmonary artery.
CONCLUSIONS
MMPs are up-regulated in vitro by dynamic culture conditions and could contribute to increased matrix remodeling, native analogous tissue formation and functional growth of TEVGs in vivo. Monitoring of MMP activity, for example, by molecular imaging techniques, may enable the non-invasive assessment of functional tissue quality in future clinical tissue-engineering applications.
Keywords: Matrix metalloproteinases, Tissue engineering, Vascular graft, Autologous, Growth, Cells
INTRODUCTION
Tissue-engineering technologies continue to address the critical need for living autologous tissue substitutes with growth potential in congenital cardiac surgery. Applications of biological and engineering principles strive to construct tissues with characteristics of healthy native tissues from their cellular components. For reconstruction of the right-ventricular outflow tract, the use of homografts, xenografts, and polytetrafluoroethylene (PTFE) grafts remains the mainstay of surgical treatment options, despite variable results and degrees of success. The need, however, for surgical re-interventions is inevitable in many situations due to conduit failure as well as the inability of these conduits to grow with the child.
The feasibility of creating implantable large- and small-caliber tissue-engineered vessels for systemic and low-pressure applications has been demonstrated experimentally and in initial clinical trials. Functional arteries have been grown in vitro both using animal and human cells [1–3]. Recently, we have demonstrated that tissue-engineered vascular grafts (TEVGs) implanted as autologous, living, pulmonary artery replacements showed excellent functionality and growth in long-term large animal follow-up studies covering the full biological growth cycle of the animal model [4]. Interestingly, these growing and functional tissue-engineered arteries demonstrated substantial remodeling over time, and, most importantly, structural approximation to native arterial wall tissue composition. The normal arterial wall consists of an intimal layer, a media layer, and an adventitial layer. Vascular remodeling and structural adaptation of tissue-engineered grafts in response to hemodynamic stresses based on constant cellular activity appear to be a prerequisite for growth and sustained functionality [4].
Vascular smooth muscle cells (VSMCs) are the main cellular components of the arterial wall and provide vascular tone and the arterial wall extracellular matrix. Facilitating SMC growth, migration, basement-membrane breakdown, angiogenesis, and other aspects of vascular remodeling necessitates an ongoing degradation and reorganization of extracellular matrix. First described in 1962 by Gross and Lapiere [5], 26 described matrix metalloproteinases (MMPs) contribute to extracellular matrix remodeling. MMPs are zinc-dependent peptidases broadly classified according to their substrate-degradation properties. They are synthesized in the latent form as zymogens and secreted as pro-enzymes (pro-MMPs). Activation occurs via a process known as the cysteine-Zn+ switch [6], which enables proteolytic activity. In this study, we identified MMPs as important enzymes in TEVG remodeling. The functions of MMPs are not fully understood; however, they are of interest in physiological processes, such as pregnancy [7], and have also been reported to be associated with pathologic phenomena [8–10]. In vascular tissue engineering, MMP-related processes are expected to be of critical importance for tissue homeostasis and functional growth. With the aim of a more meticulous understanding of long-term remodeling mechanisms, tissue-engineered autologous living pulmonary arteries were analyzed for MMP-2 and -9 expression as well as collagen content and compared with native arterial tissue. SMCs produce pro-MMP-2 in normal arterial wall and in culture, but active MMP-2 and pro- and active MMP-9 are absent [11–13]. By contrast, injured and diseased vessels undergoing remodeling produce active MMP-2 and MMP-9 [11,12,14]. Consequently, in this study, we focused on the expression of the gelatinases MMP-2 and-9. MMP-2 has been shown to degrade native type IV, V, VI, and X collagen as well as denatured collagen, proteoglycans, elastin, and growth factors [15]. MMP-9 degrades gelatin, an irreversibly hydrolyzed collagen, and is expressed following injury or inflammatory stimulation [16]. In the light of these observations and the so-far limited understanding with regard to tissue engineering [17], the long-term MMP expression in autologous, living TEVGs implanted as pulmonary artery replacements in a growing large animal model is investigated.
MATERIAL AND METHODS
The general approach to cell isolation, culture, and seeding has been described in detail [4,18]. All investigations performed in this work are based on tissue samples generated in a previous study focusing on functional performance of tissue-engineered arteries in a growing long-term animal model [4]. All procedures were conducted according to Swiss regulations on animal welfare and permission granted by the ethical committee (Permission Nr. 71/2002, 91/2004).
In vitro production of TEVGs
Bioabsorbable pulmonary artery scaffolds were fabricated from non-woven polyglycolic-acid mesh (PGA, thickness 1.0 mm, specific gravity 70–90 mg cm−3, Cellon SA, Luxembourg) coated with poly-4-hydroxybutyrate (P4HB, molecular weight 1 × 106, PHA 4400, Tepha Inc., Lexington, MA, USA). Based on biopsies of peripheral blood vessel (Swiss White Alpine lambs), endothelial cells were obtained using a collagenase-instillation technique. Following isolation, they were cultured in tissue culture flasks (Nunc™, Roskilde, Denmark) with endothelial basal medium (EBM-2) medium supplemented with 10% lamb serum (GIBCO, NY, USA). To obtain myofibroblasts, the remaining de-endothelialized vessel segments were minced and cultured in Dulbecco’s modified Eagle Medium (DMEM) or advanced DMEM (GIBCO, NY, USA) supplemented with 10% lamb serum, 2 mM glutaMAX (GIBCO, NY, USA) and 50 μg ml−1 gentamycin (GIBCO, NY, USA). After myofibroblast migration onto the dishes (after 5–7 days), the cells were serially passaged and expanded in a humidified incubator at 37 °C and 5% CO2. Sufficient cell numbers for cell seeding were obtained in pure culture after 10–14 days.
Myofibroblasts (4.5–5.5 × 106 cm−2) were sequentially seeded onto the vascular scaffolds, followed by endothelial cell seeding (1 × 106 cm−2) phenotypically confirmed by von Willebrand Factor (vWF) presence. Thereafter, the constructs were transferred into a pulse duplicator system [18] and grown under gradually increasing nutrient medium flow conditions (50–550 ml min−1, 1 Hz) for 14 days. Control TEVGs were grown accordingly, but in static nutrient. Media were changed every 3–4 days.
In vivo implantation of TEVGs
The TEVGs were surgically implanted as replacements of the main pulmonary artery into lambs, as described in detail as previously described in Circulation 2006 [4].
Computed tomography (CT)–angiography analysis
Computed tomography (CT)–angiography scans were performed under general anesthesia on a 64-slice scanner with a 0.37-s rotation time (Somatom Sensation 64™, Siemens, Forchheim, Germany). A bolus of Iodixanol (270 mg ml−1, Amersham Health, UK) was injected into a forearm vein at a flow rate of 4 ml s−1, using 2 ml of contrast agent per kg. Slices with a thickness of 0.75 mm (increment 0.5 mm) and a medium soft-tissue reconstruction kernel (B30f) were used for evaluation.
Analyses of TEVG explants
Out of a total of 14 animals [4], a subgroup of five TEVGs samples was investigated as to MMP profiles, microstructure, and tissue composition. A representative longitudinal sample of each TEVG was fixed in 10% phosphate-buffered formalin (pH 7.0) and embedded in paraffin. Each cross section included native tissue proximal as well as distal to the entire length of the TE construct. Routine histological staining was by hematoxylin and eosin (H&E) and Masson’s trichrome. Immunohistochemical stainings were performed using the Ventana Benchmark automated staining system (Ventana Medical Systems, Tucson, AZ, USA) with Ventana reagents for the entire process. Primary antibodies against the following antigens were applied: vWF(polyclonal rabbit anti-vWF antibodies; all from DakoCytomation, Glostrup, Denmark), alpha smooth muscle actin (monoclonal mouse anti-αSMA, clone 1A4; Sigma), and desmin (monoclonal mouse anti-desmin, clone D33). Primary antibodies were detected with the Ventana iVIEW DAB detection kit, resulting in a brown reaction product. Counterstaining was performed with hematoxylin. As an indicator for collagen formation, the hydroxyproline content of lyophilized samples was determined, as described by Huszar et al. [19].
MMP-2 and -9 were detected by gelatin zymography, as previously described [14]. Briefly, sodium dodecylsulfate (SDS) cell lysates were subjected to electrophoresis on 7.5% (w/v) polyacryalamide gel electrophoresis (PAGE) gel containing 0.2% (w/v) SDS supplemented with 2 mg ml−1 gelatin. All gels were calibrated with a high-molecular-mass protein standard mixture. In addition, for standardization, on each gel, an aliquot of a standard conditioned medium collected from HT1080 cells (which secrete both MMP-2 and -9) was run.
Statistical analysis
Analysis of variance was performed to estimate the means and the significance of the categorical factors using Statistical Package for Social Sciences (SPSS) version 16 (SPSS, Inc., Chicago, IL, USA). A paired t-test was performed comparing native tissue to tissue-engineered samples. A p-value <0.05 was considered as significant.
RESULTS
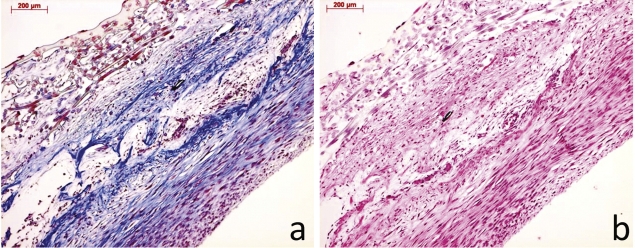
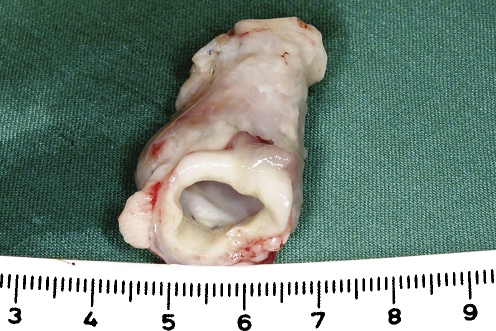
Static and dynamically cultured TEVGs were analyzed and compared with tissue engineered and native in vivo samples at 20 (TE20, n = 1), 50 (TE50, n = 2), and 80 (TE80, n = 2) weeks post implantation. A macroscopic image of the tissue-engineered graft at time of implantation is shown in Fig. 1. Masson’s trichrome staining of a pre-implant conduit showed collagen within the extracellular matrix, as blue color. H&E staining showed layered tissue formation of the pre-implant conduit (Fig. 2(a) and (b)). As early as 20 weeks post implantation, the macroscopic external appearance of the pulmonary conduit was indistinguishable from native tissue (Fig. 3).
Figure 1:
Tissue engineered vascular graft (TEVG) after of surgical implantation as main pulmonary artery replacement.
Figure 2:
(a) Trichrome Masson (TM) staining of pre-implant TEVG showing collagen staining in blue. (b) Hematoxylin and Eosin (H&E) staining of pre-implant TEVG showing layered tissue formation within the pre-implant TEVG (the bar represents 200 μm).
Figure 3:
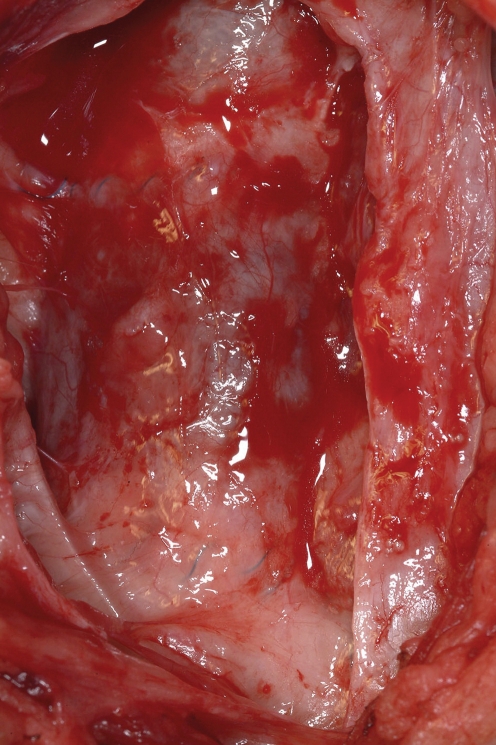
Macroscopic image of an explanted TEVG at 20 weeks post implantation. There is no evidence of thrombus or calcification and the TEVG shows a smooth luminal surface.
Morphology
At the time of sacrifice, the tissue-engineered pulmonary artery graft was dissected out in toto. The TEVGs were carefully observed for signs of thrombus formation, calcification and obvious stenosis, or aneurysmal formation. There were no signs of morphological abnormalities in any of the animals included. The luminal layer appeared smooth and continuous with the native pulmonary artery and free of calcification or thrombus formation.
Tissue microstructure and composition
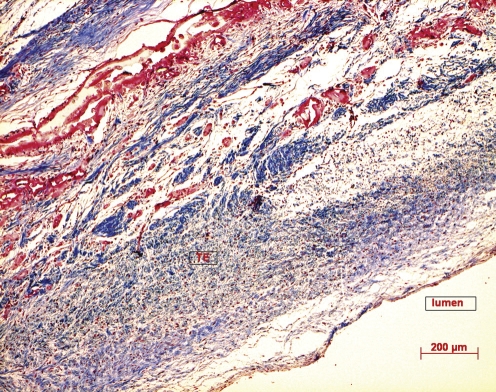
The triple-layered tissue-engineered pulmonary artery consisted of an intima, a media, and an adventitial layer reminiscent of native artery. At TE20, TE50, and TE80, there is an increasing vascular SMC distribution within the TEVGs, with a corresponding increased collagen deposition in the extracellular matrix with time (Fig. 4).
Figure 4:
Trichrome Masson (TM) staining of explanted TEVG at 20 weeks post implantation showing collagen staining in blue (The bar represents 200 μm).
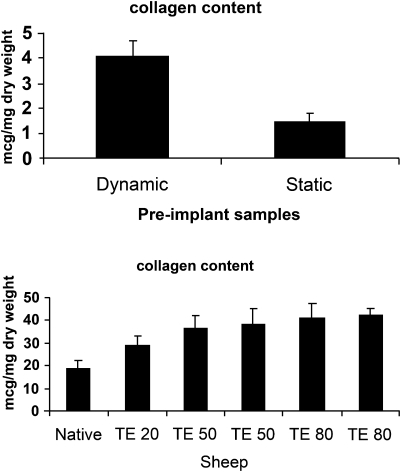
Collagen content in vitro and in vivo, as measured by hydroxyproline content, increased significantly in dynamically conditioned constructs as compared with static constructs (Fig. 5(a)) with the dynamically generated TEVGs having a hydroxyproline content of 4.08 ± 1.9 μg mg−1 dry weight. The hydroxyproline content was significantly increased in tissue-engineered constructs compared with native pulmonary artery (Fig. 5(b)). Native pulmonary artery collagen content was 18.8 ± 3.6 μg mg−1 dry weight. At 20 weeks post implantation (TE20), the tissue-engineered conduit had a collagen content of 29.03 ± 4.14 μg mg−1 dry weight. As seen in Fig. 5(b), the collagen content increases up to 42.3 ± 2.98 μg mg−1 dry weight at TE80.
Figure 5:
(a) Collagen content of static versus dynamic pre implant grafts. There is a significant difference between dynamic versus static samples (p = 0.05) (b). Collagen content of 5 TEVG at 20 (TE20), 50 (TE50) and 80 (TE80) weeks post implantation versus native (N) pulmonary artery. There is a significant difference between native and TE 50 (p < 0.05) and TE 80 (p < 0.05) samples.
MMP activity
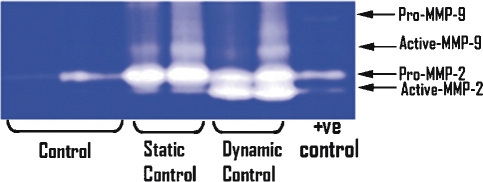
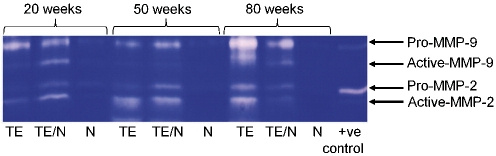
MMP expression was detected as clear bands on the blue background of the stained gel, due to lysis of the gelatin. These bands correspond to the predicted molecular weights of MMP-2 and -9 and correspond with the positive control. MMP-2 and MMP-9 exist in both latent and active forms, with molecular weight of latent MMP-2 being 72 kDa and that of latent MMP-9 being 92 kDa. Comparable levels of active MMP-9 and pro-MMP-2 were detected in VSMCs in static and dynamic culture. However, higher levels of active MMP-2 were detected in TEVGs in dynamic cultures as compared with static culture (Fig. 6). Expression of MMP-2 and MMP-9 was minimal in native grafts but was increased in implanted tissue-engineered grafts (Fig. 7). Both latent and pro-MMP-2 and -9 expression was observed as early as TE20 and persists up to TE80 (Fig. 7).
Figure 6:
MMP-2 and -9 expression in vascular smooth muscle controls, dynamic (n = 2) and static (n = 2) culture conditions. Gelatinolytic zymography was performed to detect MMP-2 and MMP-9. Active and pro(latent) forms are indicated.
Figure 7:
MMP-2 and -9 expression in TEVG (TE) versus native (N) at 20, 50 and 80 weeks after implantation. The TE pulmonary artery (TE), site of anastomosis (TE/N) between TE and native as well as native (N) PA were subjected to gelatin zymography. Both active and pro(latent) MMPs are indicated.
DISCUSSION
More than 20 years ago, Weinberg and Bell produced a collagen-based vascular conduit by isolating aortic bovine endothelial, smooth muscle, and adventitial fibroblast cells [20]. Although not implanted in vivo, these constructs showed sufficient burst strength and structural features of arterial tissue. Since these early days, tissue engineering has combined principles of cell biology with biomechanical engineering, leading to advances in the field [21].
Various cell sources have been investigated for cardiovascular applications both in animal and in human models [1]. Shin’oka et al. used bone-marrow-derived cells intra-operatively seeded onto vascular scaffolds as pulmonary artery replacements in pediatric patients with adequate functionality [3]. The histological results elucidating the growth potential of these grafts are awaited. Others have used human fibroblast cells in scaffold-free tissue-engineering applications for small-diameter blood vessels [22].
However, little is known regarding the post-implantation tissue-remodeling processes observed to a substantial degree in most experimental studies. It appears that, apart from the initially seeded cells, endogenous cell re-population phenomena contribute importantly to these remodeling processes comprising somatic growth and long-term functionality. With regard to future routine clinical use of the tissue-engineering technology, a meticulous understanding of the underlying remodeling mechanisms will be of crucial importance. Furthermore, safety parameters assessable in a non-invasive fashion after implantation may be a prerequisite for the safe translation of clinical tissue engineering into clinical practice. It is anticipated that such assessments may be performed by molecular-imaging methodologies, such as magnetic resonance imaging, which can monitor enzymatic activities of MMPs [23].
Ideal tissue-engineered conduits should show durability, absence of thrombus formation, resistance to infections, lack of immunological complications, and, most importantly, potential for growth. We have shown functional growth and approximation of tissue-engineered pulmonary arteries to native tissue in animal studies. Long-term follow-up of animals with autologous ovine cells seeded onto a biodegradable scaffold and implanted in the pulmonary position has confirmed both feasibility and functional growth of the tissue-engineered conduits based on CT and echocardiography data [4].
Improvements in bioreactor technology and physiological preconditioning in vitro have been shown to stimulate extracellular matrix formation in vitro. Collagen formation was significantly increased in the dynamic pre-implantation TEVG as compared with static culture. In our lamb model, as early as 20 weeks postoperatively, collagen formation in the tissue-engineered graft had already exceeded native pulmonary artery, with continued deposition leveling in at TE80.
The long-term role of MMPs in pulmonary artery tissue engineering in a growing animal model has been unknown so far. MMPs play a significant role in tissue remodeling and have been extensively investigated in pregnancy [7], as well as in pathological processes, such as hypertension [8], atherosclerosis [10], and aneurysmal formation [9]. All of these processes show degradation of extracellular matrix and remodeling by MMP activity.
To date, there is only limited knowledge as to the role of MMPs in the remodeling of tissue-engineered cardiovascular structures [17], particularly with regard to MMP activity during somatic growth. Our results have confirmed MMP-2 and MMP-9 as key enzymes in tissue-engineered vascular remodeling with ongoing MMP-2 and MMP-9 activity up to TE80 weeks. In vitro, there was an up-regulation of active MMP-2 as a result of biomimetic, dynamic culture conditions. Once implanted, there appears to be constant recruitment of cells into the implanted tissue-engineered graft to maintain tissue homeostasis and sustained functionality [4]. The origin of these cells is under investigation and may include blood-born stem cells as well as cells from the peri-vascular environment [24]. Histologically, the media layer of the tissue-engineered graft showed fibroblasts and collagen deposition with a rich capillary network. This, together with the hemodynamic stresses of the pulmonary circulation, would account in part for the increased activated MMP-2 and MMP-9 activity in the tissue-engineered graft. The enzymatic activity is ongoing throughout the TE80 period, even though there is complete degradation of the biodegradable polymer and therefore absence of foreign body phenomena in the graft. The MMP-2 activity in native tissue does not appear to vary significantly at the various time points, despite being subjected to the same hemodynamic stresses as the TEVGs. This implies that hemodynamic stresses alone do not account for the increased MMP activity in vivo, which rather reflects the adaptive tissue remodeling activities resulting in the observed functionality and growth of the TEVGs. Therefore, MMP activity could be regarded and used as an indicator of ‘guided healing processes’. MMP-9 activity is markedly up-regulated in the tissue-engineered grafts in conjunction with a significantly increased collagen production as compared with native tissue. Histologically, a corresponding increase in VSMCs in tissue-engineered implants is observed with time.
Indeed, MMPs have been demonstrated to be linked with aneurysms and atherosclerotic processes [8–10]. However, both echocardiography and CT data did not display any aneurysmal formation or atherosclerotic signs as also confirmed by detailed histology [4]. To the contrary, functional growth with arterial wall structures approximating native arterial layers over time were observed. Thus, the increased MMP activity in TEVGs appears not to be attributed to these pathological processes, but the result of increased cellular recruitment, which use MMP-2 and -9 to migrate or invade the tissue-engineered graft similarly seen in classical healing processes. In addition, there was no evidence of calcification, thrombus formation, or stenosis within the tissue-engineered graft, further suggesting the absence of pathological processes.
Our observations raise questions in terms of the differences which exist between tissue-engineered and native vascular grafts. The presence of increased MMP activity even in the long-term grafts suggests a sustained remodeling activity to maintain tissue homeostasis and functionality. Further studies will be necessary to evaluate the role of other classes of MMPs and the effects of inhibiting MMP activity with regard to functionality, long-term growth potential, and performance of tissue-engineered vascular grafts. Based on a more detailed understanding of matrix remodeling, modern magnetic resonance and molecular imaging techniques may allow for non-invasive tracking of MMP activity in clinical settings [25]. Such methodologies enabling the definition of clinically relevant quality criteria may contribute to the safe translation of tissue-engineering technologies to routine clinical practice. In addition, this growing insight into the remodeling mechanisms involving MMPs could help to specifically design intelligent scaffold materials. These may support remodeling phenomena within tissue-engineered constructs in the future by attaching transcription factors and/or cytokines to the starter matrices.
Conflict of interest: none declared.
REFERENCES
- 1.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 2.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, McKee JA, Klinger RY, Counter CM, Niklason LE. Blood vessels engineered from human cells. Lancet. 2005;365:2122–4. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 3.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Hoerstrup SP, Cummings Mrcs I, Lachat M, Schoen FJ, Jenni R, Leschka S, Neuenschwander S, Schmidt D, Mol A, Gunter C, Gossi M, Genoni M, Zund G. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–66. doi: 10.1161/CIRCULATIONAHA.105.001172. [DOI] [PubMed] [Google Scholar]
- 5.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014–22. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG. 2004;111:931–9. doi: 10.1111/j.1471-0528.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 8.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–8. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 9.Keeling WB, Armstrong PA, Stone PA, Bandyk DF, Shames ML. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39:457–64. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- 10.Zaltsman AB, Newby AC. Increased secretion of gelatinases A and B from the aortas of cholesterol fed rabbits: relationship to lesion severity. Atherosclerosis. 1997;130:61–70. doi: 10.1016/s0021-9150(96)06046-7. [DOI] [PubMed] [Google Scholar]
- 11.Zempo N, Kenagy RD, Au YP, Bendeck M, Clowes MM, Reidy MA, Clowes AW. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209–17. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 12.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–45. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 13.Southgate KM, Davies M, Booth RF, Newby AC. Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem J. 1992;288:93–9. doi: 10.1042/bj2880093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George SJ, Zaltsman AB, Newby AC. Surgical preparative injury and neointima formation increase MMP-9 expression and MMP-2 activation in human saphenous vein. Cardiovasc Res. 1997;33:447–59. doi: 10.1016/s0008-6363(96)00211-8. [DOI] [PubMed] [Google Scholar]
- 15.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whatling C, McPheat W, Hurt-Camejo E. Matrix management: assigning different roles for MMP-2 and MMP-9 in vascular remodeling. Arterioscler Thromb Vasc Biol. 2004;24:10–11. doi: 10.1161/01.ATV.0000100562.63144.C1. [DOI] [PubMed] [Google Scholar]
- 17.Stock UA, Wiederschain D, Kilroy SM, Shum-Tim D, Khalil PN, Vacanti JP, Mayer JE, Jr., Moses MA. Dynamics of extracellular matrix production and turnover in tissue engineered cardiovascular structures. J Cell Biochem. 2001;81:220–8. doi: 10.1002/1097-4644(20010501)81:2<220::aid-jcb1037>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, Martin DP, Moran AM, Guleserian KJ, Sperling JS, Kaushal S, Vacanti JP, Schoen FJ, Mayer JE., Jr Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:III44–9. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- 19.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105:424–9. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 21.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 22.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancelot E, Amirbekian V, Brigger I, Raynaud JS, Ballet S, David C, Rousseaux O, Le Greneur S, Port M, Lijnen HR, Bruneval P, Michel JB, Ouimet T, Roques B, Amirbekian S, Hyafil F, Vucic E, Aguinaldo JG, Corot C, Fayad ZA. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler Thromb Vasc Biol. 2008;28:425–32. doi: 10.1161/ATVBAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 24.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–27. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Rubbens MP, Driessen-Mol A, Boerboom RA, Koppert MM, van Assen HC, TerHaar Romeny BM, Baaijens FP, Bouten CV. Quantification of the temporal evolution of collagen orientation in mechanically conditioned engineered cardiovascular tissues. Ann Biomed Eng. 2009;37:1263–72. doi: 10.1007/s10439-009-9698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]