Abstract
Sternal cleft is a chest wall malformation that can expose mediastinal viscera and vessels to injuries. It can be classified into two forms, complete and partial. Its etiology and incidence are unknown and it is often associated with other defects. The aim of this article is to review the literature and report our experience with this rare anomaly, focusing on clinical presentation and management. We reviewed the English written literature about sternal cleft and collected the clinical data of all the published series. We present seven new cases that we have observed and treated since 1999. Literature reports 51 series including 86 patients, more frequently female (62%) and affected with partial superior form (67%). Sternal cleft is often asymptomatic (74%) and associated with other defects (72%). Surgical treatments include primary closure (73%), bone graft interposition (10%), prosthetic closure (7%), and muscle flap interposition (3%). In our series, primary closure was possible in four cases, while in three cases we placed a prosthesis. Five patients had associated defects and two were affected with PHACES (posterior fossa abnormalities, hemangiomas, arterial lesions, cardiac abnormalities/aortic coarctation, abnormalities of the eye, and sternum defects) syndrome. We report for the first time the association of sternal cleft with connectival nevi in three of our patients. At follow-up, we observed no major complication or recurrences. Although primary closure is the preferred option and should be performed in the neonatal period, the use of prostheses warrants good results as well. Prior to treatment, associated defects and syndromes should be excluded.
Keywords: Congenital sternal cleft, Sternum abnormalities, Chest wall malformations, Musculoskeletal abnormalities
INTRODUCTION
The sternum develops from mesenchymal cells that start to gather around the 6th week of gestation [1]. A defect in the fusion process causes the sternal cleft (SC) (Fig. 1), a rare idiopathic congenital chest wall malformation (CWM). Acastello et al. found that SC accounted for 0.15% of all CWMs [2]. The Hoxb gene might be involved in the development of SC [3].
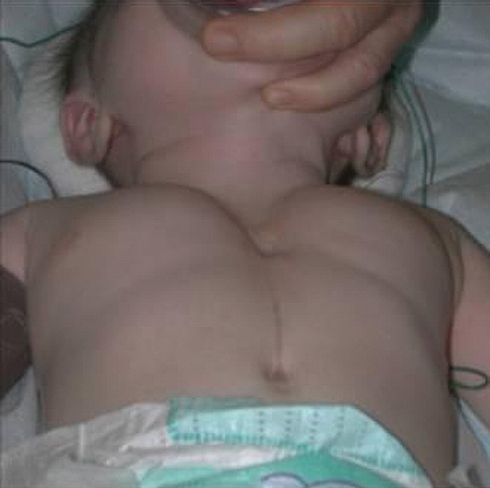
Figure 1:
Partial sternal cleft.
SC can be classified into two major forms, complete and partial, which can be superior or inferior [2]. The partial inferior form is often associated with ectopia cordis. A particular condition is the sternal foramen in which there is not a clear SC but a fusion defect of the sternum in its middle portion.
SC causes paradoxical respiratory movements and increases the risk of harmful events on mediastinal viscera. Surgical correction is therefore recommended, if possible in the neonatal age [4,5], to achieve primary closure. However, the reduction in thoracic volume can cause cardiovascular impairment. Different techniques have been developed to avoid this problem, such as with the use of prostheses [6–8], partial or total thymectomy [9], sliding chondrotomies, and clavicle dislocation [10–12]. These techniques can be an alternative to primary closure if the latter is challenging or impossible due to a stiff thorax.
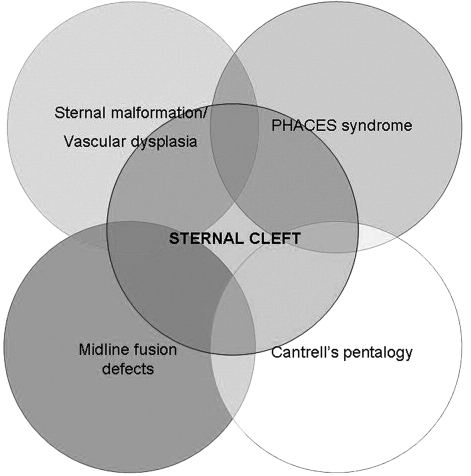
Other defects, such as vascular dysplasia, PHACES syndrome, midline fusion defects, and Cantrell’s pentalogy [1,12,13] (Fig. 2) are possibly associated with SC.
Figure 2:
The relationship between sternal cleft and other rare syndromes/associations.
The aim of this article is to review the series of SC patients reported in the literature and report our experience, focusing on clinical presentation and management of the patients with this rare malformation.
MATERIALS AND METHODS
Review of the literature
We reviewed the literature searching for articles, written in English, concerning patients affected by SC, regardless of age. We searched Pubmed for words like ‘sternal cleft’ and ‘bifid sternum’. We excluded, from the study, articles written in languages other than English, articles concerning nonhumans, and articles reporting no clinical data on patients but just genetic or embryologic aspects of SC. Collected data included: age at diagnosis, age at treatment, sex, type of SC, symptoms, physical examination, associated defects, surgical intervention, perioperative complications, and surgical outcome.
Institutional series
From 1999 to 2010, at the Giannina Gaslini Institute, we observed and treated seven patients affected by SC. Patients were studied retrospectively, retrieving the following data from the clinical records: age at diagnosis, age at treatment, sex distribution, type of SC, symptoms, physical examination, associated defects, surgical approach, perioperative complications, and surgical outcome.
All the patients were studied preoperatively with chest X-ray, computerized tomography (CT) (Fig. 3), and cardiologic evaluation (electrocardiogram and echocardiography).
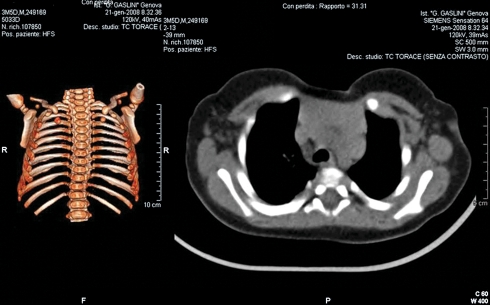
Figure 3:
Radiologic imaging in a patient with sternal cleft. CT scan with 3D-reconstruction of the thoracic wall (on the left side) shows an increased interclavicular space, a typical sign of sternal cleft. CT scan (on the right side) shows bulging of the mediastinal viscera.
For surgery, all the patients were placed in a supine position, with their arms abducted at 90°. All the patients received antibiotic prophylaxis with a cephalosporin or penicillin. The skin incision was vertical on the midline, downward from the jugular notch to the end of the defect. Pectoralis major muscles were dissected to expose the sternal bars. Vertical strap muscles of the neck were divided at their insertion on the sternal upper margins. The medial edges of the two sternal halves were freed from the underlying pleura and pericardium. The inferior aspect of partial SC was incised, when U shaped, to make closure easier. The sternal bars were approximated on the midline. If closure was deemed possible, it was completed by juxtaposing nonabsorbable sutures (primary closure). Otherwise, a partial or complete thymectomy [9] was attempted prior to primary closure. If the SC was too wide or there was a mediastinal compression after sternal bar juxtaposition, we closed the defect by placing different prostheses in multiple layers. Mainly, we have used nonabsorbable prostheses, made of Gore-tex® or Gore® DualMesh® biomaterials (W.L. Gore and Associates, Inc., Flagstaff, AZ, USA), calcium phosphate cement (EuroBone®, FH Orthopedics, F-68990 Heimsbrubb, France), or polyester. In two cases, we also placed absorbable prostheses in LactoSorb® (Biomet microfixation, Jacksonville, FL, USA).
Pain control was obtained with a 24-h intravenous infusion of morphine (dose 20γ kg−1 h−1) followed by oral or endorectal administration of paracetamol/codeine. Pain was assessed according to the face, legs, activity, cry, and consolability (FLACC) scale in infants, and with the faces pain scale or the numeric scale in older children.
RESULTS
Literature review
In the literature, 51 series including 86 SC patients have been reported between 1879 and 2011. The largest single-center population has been described by de Campos et al. [14], who observed 15 patients in a 27-year period, followed by the series reported by Acastello et al. [2] and Daum and Zachariou [4], including eight patients each. All the other reported series included less than three cases [3,5,6,15–59] (Table 1).
Table 1:
SC series published in the literature. Institutional series is highlighted in bold
| Authors | No. of patients | Period | Age at diagnosis | Mean age at surgery | M | F | Type of cleft |
|---|---|---|---|---|---|---|---|
| De Campos et al. [14] | 15 | 1979–2007 | 1.5–19 years (mean 8.96) | 1.5–19 years (mean 8.96) | 4 | 11 | 1 comp – 1 PI – 13 PS |
| Daum and Zachariou [4] | 8 | 1965–▪1999 | 15 days | 15 days | – | – | 5 PS – 3 comp |
| Acastello et al. [2] | 8 | 1987–2001 | 15 days to 15 years | 15 days to 15 years | 2 | 6 | PS |
| Present series | 7 | 1999–▪2010 | Birth — 6 years | 1.16 years | 2 | 5 | 5 PS – 2 comp |
| Zidere et al. [15] | 3 | – | 11-16 weeks (mean 13.6) | – | – | – | – |
| Vivero et al. [16] | 2 | – | Birth | Neonatal | 2 | – | 1 comp – 1 PI |
| Padalino et al. [17] | 2 | 2006 | Neonatal | 21 days to 9 months | – | 2 | PS |
| Domini et al. [5] | 2 | – | Birth – 6 days | 2—4 weeks | 2 | – | PS |
| Singh et al. [18] | 2 | – | 15 days to 6 months | 6 months to 18 days | 2 | – | PS |
| Forzano et al. [3] | 2 | – | 15–17 (16 years) | – | – | 1 | PI |
| Case reports [6–18–59] | 43 | 1967–▪2009 | 33 < 1 month; 10 > 1 month | 18 < 1 year; 12 > 1 year | 16 | 24 | 23 PS – 12 comp – 5 PI – 2 SF |
PS: partial superior; PI: partial inferior; comp: complete; and SF: sternal foramen.
SC was mostly diagnosed in the neonatal period (55/86, 64%) and also prenatally (5/86, 6%), and after 1 year of age (26/86, 30%). Females were affected by SC more frequently than males (61% vs 39%, respectively).
The most represented form was the partial superior type, accounting for 67% of all patients, followed by the complete form (19.5%), the partial inferior form (11%), and the sternal foramen (2.5%).
Twenty-three patients were symptomatic (23/86, 27%) and presented mostly respiratory symptoms (dyspnea, respiratory distress, and recurrent respiratory tract infections).
Sixty-three patients (63/86, 73%) had associated defects (Table 2), mainly cardiac defects (22%) and vascular anomalies (9%). Supraumbilical raphe, which per se represents only a cosmetic anomaly, was also common (7%) and was associated with syndromes such as PHACES (6%) or midline fusion defects (3.5%).
Table 2:
The associated anomalies in SC series reported in the literature and at our institute
| Associated defects | n/88 | % | Gaslini (n/7) | % |
|---|---|---|---|---|
| Cardiac defects | 19 | 22.1 | 1 | 14.2 |
| Aortic malformations | 8 | 9.3 | 1 | 14.2 |
| Supraumbilical raphe | 6 | 7 | 2 | 28.5 |
| Cantrell's pentalogy | 6 | 7 | – | – |
| PHACES syndrome | 5 | 5.8 | 2a | 28.5 |
| Pectus excavatum | 5 | 5.8 | 1 | 14.2 |
| Hemangiomas | 4 | 4.7 | 3 | 42.8 |
| Midline fusion defects | 3 | 3.5 | – | |
| Maxillo–facial defects | 3 | 3.5 | – | – |
| Extrathoracic hamartoma | 2 | 2.3 | – | – |
| CDH | 2 | 2.3 | – | – |
| Connectival nevi | – | – | 5 | 71.4 |
a PHACES syndrome involves more defects reported in the table.
Seventy patients (81%) were treated surgically, mostly under 1 year of age (45/70, 64%). The oldest patient, aged 52 years, underwent primary closure and, after 3 years of follow-up, was doing well [36]. The surgical repair was successfully completed after mobilization of the pectoralis major muscles that was re-approximated on the midline.
Primary closure resulted the preferred treatment (55/70, 78.5%), with or without chondrotomies, periosteal flaps, or cartilage resections (Table 3). Alternative procedures included bone graft interposition (7/70, 10%), prosthetic closure (5/70, 7%), and muscle flap interposition (2/70, 3%).
Table 3:
The types of surgical procedures used to repair SC as reported in the literature and in our series
| Surgical procedure | n/70 | % | Gaslini (n/7) | % |
|---|---|---|---|---|
| Primary closure alone | 27 | 38.6% | 4 | 57.14% |
| Primary closure with periosteal flap | 14 | 20.0% | – | – |
| Primary closure with sliding chondrotomies | 10 | 14.3% | – | – |
| Primary closure with cartilage resection | 4 | 5.7% | – | – |
| Bone graft placement | 7 | 10.0% | – | – |
| Prosthetic closure | 5 | 7.1% | 3 | 42.86% |
| Muscle flap | 2 | 2.9% | – | – |
| 2 stage primary closure | 1 | 1.4% | – | – |
Three intraoperative complications (3/70, 4%) were reported, due to pericardial or pleural tears during sternal dissection. Postoperative complications were 12 (17%), mostly represented by retrosternal seromas and pneumothorax. Death occurred in three patients affected with cardiac defects (one ventricular septal defect, one stenotic slit-like coronary ostium, and one double-outlet right ventricle). One patient died before surgery because of heart failure due to ventricular septal defect. The other two patients died during the first 24 h after surgery. These unfavorable events seemed to be more related to the underlying diseases than to the type of repair. No recurrences of SC after repair have been described in the literature.
Eight patients (10%) were also affected with pectus excavatum (diagnosed preoperatively or at follow-up); in two of them, pectus excavatum and SC were treated simultaneously, while in the other six, pectus excavatum was not repaired.
Institutional series
Between 1999 and 2010, we have observed seven consecutive SCs (five females and two males). All but one were diagnosed in the neonatal period. Mean age at surgery was 1.16 years (±2.19 standard deviation, SD; range 1 month to 6.5 years).
Five patients had a partial SC (71%) and two a complete form (29%). Only one patient was symptomatic, complaining of respiratory symptoms associated with frequent respiratory infections.
On physical examination, only two patients had an isolated SC. The other five patients had associated defects, such as maxillo-facial hemangiomas, precordial skin tags, or a supraumbilical median raphe (Fig. 4). Two of these patients (40%) were affected with PHACES syndrome [13]. One patient (14%) had a ventricular septal defect and vertebral anomalies; another patient (14%) developed pectus excavatum at follow-up. Five patients (71%) had precordial skin tags on physical examination. In the last three cases with skin tags, these were analyzed after removal and the histopathology revealed they were hamartomatous congenital skin lesions, known as ‘connectival nevi’.
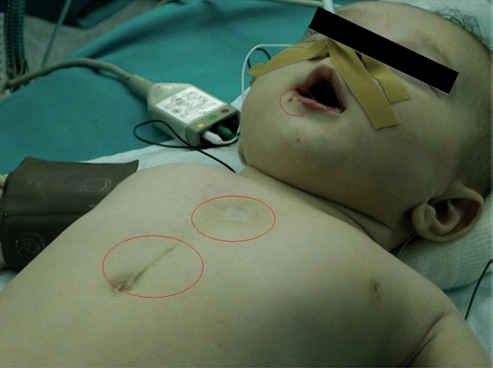
Figure 4:
Maxillo-facial hemangiomas, precordial skin tags, and supraumbilical raphe.
Four patients (57%) underwent primary closure, all between 3 and 4 months of age. In the other three patients (43%), prostheses were used. In one case aged 6.5 years, SC was repaired with a deep layer of Gore-tex®, a middle layer of EuroBone,® and a superficial layer of LactoSorb®. In another patient, aged 6 months, double-layer reconstruction, with a deep layer of polyester and a superficial one in LactoSorb®, was performed. In the last patient, aged 1 month, we used a double-layer repair (polyester deep and Gore® DualMesh® superficial). These two cases, despite being of a favorable age, were repaired with prostheses because the defect was wide and a primary closure could cause cardiovascular impairment.
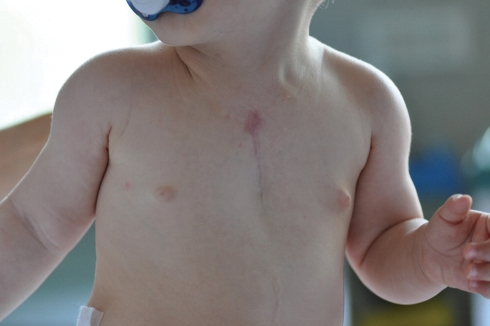
The surgical outcome was excellent (Fig. 5), and SC repair was completed successfully in all cases, without any cardiovascular impairment.
Figure 5:
Follow-up at 5 months after primary closure of sternal cleft. No paradoxical respiratory movements were observed.
Mean hospital stay was 12 days (±8.82 SD) with a range of 6–33 days after surgery. The longest hospital stay was observed in a patient who developed mild respiratory distress after surgery. Fiberoptic bronchoscopy revealed a laryngeal hemangioma that was treated successfully with oral beta-blockers.
A patient who had a sternal reconstruction with EuroBone® and LactoSorb® prostheses developed a postoperative presternal seroma. The seroma was drained and, at follow-up, we did not observe any structural or cosmetic changes.
No recurrences were observed. Pain control was satisfactory in all patients.
One patient developed pectus excavatum 4 years after primary closure of the SC. We decided to repair the pectus excavatum through an open approach, using the Ravitch technique modified by DeLuca–Wesselhoeft [60], placing a steel bar through the sternum. Pectus excavatum repair was completed successfully, without complications or recurrences.
Two out of five female patients reached pubertal age and did not complain of any anomaly or asymmetry in breast development.
DISCUSSION
SC is a rare anomaly with a reported incidence <1% of all CWMs [2]. As reported in the literature [58], we observed a higher incidence of partial superior type SC and a female predominance.
In the neonatal period, patients are mostly asymptomatic; on physical examination, a paradoxical midline thoracic bulging (protrusion of the mediastinal viscera during expiration) is noticeable in all patients. If not treated surgically, patients with SC can have impaired gas exchange, present respiratory symptoms such as dyspnea and cough, or develop chest infections [8]. These data are confirmed also in our small series, in which the only symptomatic patient was 6.5 years old and complained of frequent respiratory infections.
According to the literature, SC was frequently associated with other defects (63/88, 72%). These must be carefully looked for before any surgical procedure, since they can lead to major complications. Some of them are evident on physical examination such as maxillo-facial hemangiomas [2], cleft lip or palate [15], pectus excavatum [13,26], precordial skin tags, supraumbilical raphe [48], or gastroschisis [45]. Other defects must be ruled out, such as cardiac defects, aortic coarctation, eye abnormalities, posterior fossa anomalies, and hidden hemangiomas [58]. In some cases, SC is also part of clinical conditions such as PHACES syndrome [11,16,61,62], sternal malformation/vascular dysplasia, midline fusion defects, or Cantrell’s pentalogy [1,9,52] (Table 2, Fig. 2).
We found PHACES syndrome and maxillo-facial hemangiomas with a frequency, respectively, 5 and 9 times higher than reported in the literature. This could be explained by a very careful clinical evaluation, being careful not to miss hemangiomas and skin tags on the thorax or abdomen. A cardiological evaluation was performed in all cases. In our opinion, this is mandatory before surgery, as well as radiologic imaging (X-ray and CT scan). More specific evaluations, like neuroradiologic imaging or ophthalmologic evaluation, must be reserved for patients with a high suspicion of associated syndromes. Genetic evaluation can be useful. Laryngo-tracheo-bronchoscopy is recommended to rule out subglottic hemangiomas in patients with other evidence of hemangiomas.
We pointed out that skin tags were frequently identifiable on physical examination. These resulted to be in all three cases in which they were sent to histopathology evaluation as hamartomatous congenital skin lesions that are often associated with bone defects like osteopoikilosis [63,64]. To our knowledge, this is the first report in the literature of such an association of SC with connectival nevi. However, a study on a larger series is mandatory to confirm that the connectival nevus of the anterior thoracic wall can be part of the clinical spectrum.
If left untreated, SC potentially increases the risk of trauma-related injury to the heart, the lungs, and the major vessels. To our knowledge, there are no reports in the literature regarding traumas in patients with SC [41]. However, we agree with the literature that a surgical procedure is highly recommendable and should be performed as soon as possible [2,3,7,13], possibly during the neonatal period. The thoracic wall has a higher compliance at this age, making a primary closure easier without any other additional procedures such as chondrotomies, osteotomies, or clavicular dislocation. Even the risk of cardiovascular impairment is lower. In older patients, the rib cage is stiff and repair is difficult. For this reason, sliding chondrotomies, osteotomies, or clavicular dislocation [8,10,13] are helpful because they can increase thoracic wall compliance and allow primary closure. In certain circumstances, a partial or complete thymectomy [9] can be necessary to reduce the risk of mediastinal compression and to perform primary closure. If the SC is too wide or the risk of cardiovascular compression is too high, the SC can be closed with the use of prostheses or bone grafts. The result of the literature review and our experience showed that the use of prostheses is a valid alternative to the primary closure in older or difficult cases. There was no difference in the surgical outcome between the group treated with primary closure and the group treated with prostheses, as both warranted satisfactory results. In our patients, we have used both absorbable and nonabsorbable prostheses without any major complication. Cosmetic results were not affected by the kind of prostheses that was implanted, and at palpation no difference was observed in sternal resistance between patients with different kinds of prostheses.
Even though SC is easily identifiable at birth, it is often misdiagnosed and a third of cases have no diagnosis before 1 year of age. A late referral to a thoracic surgeon with expertise in CWMs treatment can make primary closure more difficult or even impossible. We had a similar experience in our population, whose mean age at surgery was relatively high (1.16 years).
After SC repair, patients should be followed up carefully since they can develop other CWMs such as pectus excavatum. If this complication occurs, a surgical correction should be considered.
Breast development in female patients with SC is normal; however, special care must be taken during SC repair not to damage mammary glands.
In conclusion, SC is a rare CWM that should be diagnosed at birth and treated surgically, if possible in the neonatal period. Prior to surgery, accurate physical examination is mandatory to rule out possible associated syndromes. If there is a suspicion of other anomalies, specific examinations should be performed. The connectival nevus of the anterior thoracic wall can be part of the clinical spectrum of SC. Surgery in the first months of life is recommended achieve primary closure. However, surgery is also feasible and successful in older patients, sometimes with the use of prostheses.
ACKNOWLEDGEMENT
We thank Anna Capurro for her help in revising the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Engum SA. Embryology sternal clefts, ectopia cordis, and Cantrell’s pentalogy. Semin Pediatr Surg. 2008:154–60. doi: 10.1053/j.sempedsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Acastello E, Majluf R, Garrido P, Barbosa LM, Peredo A. Sternal cleft: a surgical opportunity. J Pediatr Surg. 2003:178–83. doi: 10.1053/jpsu.2003.50038. [DOI] [PubMed] [Google Scholar]
- 3.Forzano F, Daubeney PE, White SM. Midline raphe, sternal cleft, and other midline abnormalities: a new dominant syndrome? Am J Med Genet A. 2005;135:9–12. doi: 10.1002/ajmg.a.30682. [DOI] [PubMed] [Google Scholar]
- 4.Daum R, Zachariou Z. Total and superior sternal clefts in newborns: a simple technique for surgical correction. J Pediatr Surg. 1999:408–11. doi: 10.1016/s0022-3468(99)90487-6. [DOI] [PubMed] [Google Scholar]
- 5.Domini M, Cupaioli M, Rossi F, Fakhro A, Aquino A, Chiesa PL. Bifid sternum: neonatal surgical treatment. Ann Thorac Surg. 2000:267–9. doi: 10.1016/s0003-4975(99)01206-0. [DOI] [PubMed] [Google Scholar]
- 6.Hazari A, Mercer NS, Pawade A, Hayes AM. Superior sternal cleft: construction with a titanium plate. Plast Reconstr Surg. 1998;101:167–70. doi: 10.1097/00006534-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman E. Surgical correction of bifid sternum using Marlex mesh. Arch Surg. 1965;88:76–80. doi: 10.1001/archsurg.1965.01320070078017. [DOI] [PubMed] [Google Scholar]
- 8.Ravitch MM. Disorders of the sternum and thoracic wall. In: Sabiston Jr DC, Spencer FC., editors. Gibbon’s surgery of the chest. 4th ed. Philadelphia: W.B. Saunders; 1983. pp. 318–60. [Google Scholar]
- 9.Torre M, Rapuzzi G, Guida E, Costanzo S, Jasonni V. Thymectomy to achieve primary closure of total sternal cleft. J Pediatr Surg. 2008:e17–20. doi: 10.1016/j.jpedsurg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Fokin AA. Thoracic defects: cleft sternum and Poland syndrome. Thorac Surg Clin. 2010:575–82. doi: 10.1016/j.thorsurg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 11.de Campos JR, Filomeno LT, Fernandez A, Ruiz RL, Minamoto H, Werebe Ede C, Jatene FB. Repair of congenital sternal cleft in infants and adolescents. Ann Thorac Surg. 1998:1151–4. doi: 10.1016/s0003-4975(98)00596-7. [DOI] [PubMed] [Google Scholar]
- 12.Fokin AA, Robicsek F. Management of chest wall deformities. In: Franco KL, Putnam Jr JB, editors. Advanced therapy in thoracic surgery. 2nd ed. Hamilton: BC Decker; 2005. p. 145e62. [Google Scholar]
- 13.Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J, Frieden I, Drolet B. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics. 2009:1447–56. doi: 10.1542/peds.2009-0082. [DOI] [PubMed] [Google Scholar]
- 14.de Campos JR, Das-Neves-Pereira JC, Velhote MC, Jatene FB. Twenty seven-year experience with sternal cleft repair. Eur J Cardiothorac Surg. 2009:539–41. doi: 10.1016/j.ejcts.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Zidere V, Allan LD. Changing findings in pentalogy of Cantrell in fetal life. Ultrasound Obstet Gynecol. 2008;32:835–7. doi: 10.1002/uog.6223. [DOI] [PubMed] [Google Scholar]
- 16.Vivero RJ, Fort A, Ruiz JW, Roy S. Airway implications of congenital sternal agenesis. Am J Otolaryngol. 2010:364–7. doi: 10.1016/j.amjoto.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Padalino MA, Zanon GF, Migneco F, Rubino MG, Fusaro F, Stellin G. Surgical repair of incomplete cleft sternum and cardiac anomalies in early infancy. Ann Thorac Surg. 2006:2291–4. doi: 10.1016/j.athoracsur.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Lahoti BK, Garge S, Negi A, Jain V. Sternal cleft repair: a report of two cases and review of literature. Afr J Paediatr Surg. 2010:211–3. doi: 10.4103/0189-6725.70432. [DOI] [PubMed] [Google Scholar]
- 19.Jabbad H, Shehata R, Al-Ebrahim K. Successful surgical repair of complete sternal cleft in an adult. Asian Cardiovasc Thorac Ann. 2010:376–8. doi: 10.1177/0218492310376015. [DOI] [PubMed] [Google Scholar]
- 20.Twomey EL, Moore AM, Ein S, McAuliffe F, Seaward G, Yoo SJ. Prenatal ultrasonography and neonatal imaging of complete cleft sternum: a case report. Ultrasound Obstet Gynecol. 2005;25:599–601. doi: 10.1002/uog.1835. [DOI] [PubMed] [Google Scholar]
- 21.Aytac A, Saylam A. Successful surgical repair of congenital total cleft sternum with partial ectopia cordis. Thorax. 1976;31:466–9. doi: 10.1136/thx.31.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luthra S, Dhaliwal RS, Singh H. Sternal cleft—a natural absurdity or a surgical opportunity. J Pediatr Surg. 2007:582–4. doi: 10.1016/j.jpedsurg.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Mathai J, Cherian VK, Chacko J, Sen S, Karl S, Pandyan MS, Mathew AK. Bridging the cleft over the throbbing heart. Ann Thorac Surg. 2006:2310–1. doi: 10.1016/j.athoracsur.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Eijgelaar A, Bijtel JH. Congenital cleft sternum. Thorax. 1970;25:490–8. doi: 10.1136/thx.25.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadhav V, Rao S, D’Cruz A. Autologous repair of isolated complete sternal cleft in an adolescent. J Pediatr Surg. 2009:2414–6. doi: 10.1016/j.jpedsurg.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Yavuzer S, Kara M. Primary repair of a sternal cleft in an infant with autogenous tissues. Interact Cardiovasc Thorac Surg. 2003:541–3. doi: 10.1016/S1569-9293(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 27.Sarper A, Oz N, Arslan G, Demircan A. Complete congenital sternal cleft associated with pectus excavatum. Tex Heart Inst J. 2002;29:206–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Cozzi DA, Ceccanti S, Mele E, Frediani S, Totonelli G, Passariello M. Low cervical skin crease approach for superior sternal cleft repair. J Pediatr Surg. 2009:1856–8. doi: 10.1016/j.jpedsurg.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Hirata Y, Arkovitz MS, Marboe CC, Mosca RS. A successful neonatal repair of congenital aortic aneurysm with cleft sternum. J Thorac Cardiovasc Surg. 2009:769–71. doi: 10.1016/j.jtcvs.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Padalino MA, Stellin G, Thiene G, Rubino M, Rizzo S, Milanesi O, Basso C. Giant congenital aortic aneurysm with cleft sternum in a neonate: pathological and surgical considerations for optimal management. Cardiovasc Pathol. 2010:183–6. doi: 10.1016/j.carpath.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Baqain EB, Lataifeh IM, Khriesat WM, Fraiwan NM, Armooti MA. Primary repair of a large incomplete sternal cleft in an asymptomatic infant with Prolene mesh. J Pediatr Surg. 2008:e39–41. doi: 10.1016/j.jpedsurg.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Slone T, Emil S, Meissner N, Behjatnia B, Fairbanks T, Romansky S. Sternal cleft, Morgagni hernia, and ectopic liver: a unique chest wall anomaly. J Pediatr Surg. 2007:2132–5. doi: 10.1016/j.jpedsurg.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 33.Levin JH, Kaler SG. Non-random maternal X-chromosome inactivation associated with PHACES. Clin Genet. 2007:345–50. doi: 10.1111/j.1399-0004.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 34.Hebra A, Davidoff A, O’Neill JA., Jr Neonatal sternal cleft associated with an extrathoracic cystic mass. J Pediatr Surg. 1997:627–30. doi: 10.1016/s0022-3468(97)90724-7. [DOI] [PubMed] [Google Scholar]
- 35.Rose NC, Coleman BG, Wallace D, Gaupman K, Ruchelli E. Prenatal diagnosis of a chest wall hamartoma and sternal cleft. Ultrasound Obstet Gynecol. 1996;7:453–5. doi: 10.1046/j.1469-0705.1996.07060453.x. [DOI] [PubMed] [Google Scholar]
- 36.Firmin RK, Fragomeni LS, Lennox SC. Complete cleft sternum. Thorax. 1980;35:303–6. doi: 10.1136/thx.35.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santini F, Faggian G, Pessotto R, Mazzucco A. Successful repair of complete sternal cleft associated with congenital heart disease. Report of one case. J Cardiovasc Surg. 1995;36:75–7. [PubMed] [Google Scholar]
- 38.Sen S, Posacioglu H, Cikirikcioglu M, Guner H. Upper sternal cleft associated with unusual symptoms. Thorac Cardiovasc Surg. 2000;48:375–7. doi: 10.1055/s-2000-8340. [DOI] [PubMed] [Google Scholar]
- 39.Snyder BJ, Robbins RC, Ramos D. Primary repair of complete sternal cleft with pectoralis major muscle flaps. Ann Thorac Surg. 1996;61:983–4. doi: 10.1016/0003-4975(95)00786-5. [DOI] [PubMed] [Google Scholar]
- 40.Abel RM, Robinson M, Gibbons P, Parikh DH. Cleft sternum: case report and literature review. Pediatr Pulmonol. 2004;37:375–7. doi: 10.1002/ppul.10441. [DOI] [PubMed] [Google Scholar]
- 41.Chang T, Qian Y, Tang S, Dai K, Sun B, Jin R. Sternal cleft and ectopia cordis: a case report. Chin Med J. 1999;112:188–90. [PubMed] [Google Scholar]
- 42.Biswas G, Khandelwal NK, Venkatramu NK, Chari PS. Congenital sternal cleft. Br J Plast Surg. 2001:259–61. doi: 10.1054/bjps.2000.3527. [DOI] [PubMed] [Google Scholar]
- 43.Suri RK, Sharma RK, Jha NK, Sharma BK. Complete congenital sternal cleft in an adult: repair by autogenous tissues. Ann Thorac Surg. 1996:573–5. [PubMed] [Google Scholar]
- 44.Hill CA, Argenta LC, Hines M. Superior sternal cleft repair using autologous rib grafts in an infant with complex congenital heart disease. Ann Thorac Surg. 2007:673–4. doi: 10.1016/j.athoracsur.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 45.Shalak L, Kaddoura I, Obeid M, Hashem H, Haidar R, Bitar FF. Complete cleft sternum and congenital heart disease: review of the literature. Pediatr Int. 2002:314–6. doi: 10.1046/j.1442-200x.2002.t01-1-01545.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt AI, Jesch NK, Gluer S, Ure BM. Surgical repair of combined gastroschisis and sternal cleft. J Pediatr Surg. 2005:e21–23. doi: 10.1016/j.jpedsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Cudi Tuncer M, Ufuk Aluclu M, Karabulut O, Ulku R, Savas Hatipoglu E, Nazaroglu H. The demonstration of the inferior sternal cleft using three-dimensional reconstruction: a case report. Rom J Morphol Embryol. 2009:513–6. [PubMed] [Google Scholar]
- 48.Slavotinek AM, Dubovsky E, Dietz HC, Lacbawan F. Report of a child with aortic aneurysm, orofacial clefting, hemangioma, upper sternal defect, and marfanoid features: possible PHACE syndrome. Am J Med Genet. 2002;110:283–8. doi: 10.1002/ajmg.10455. [DOI] [PubMed] [Google Scholar]
- 49.Raas-Rothschild A, Nir A, Gillis R, Rein AJ. Giant congenital aortic aneurysm with cleft sternum, supraumbilical raphe, and hemangiomatosis: report and review. Am J Med Genet. 2000:243–5. doi: 10.1002/(sici)1096-8628(20000131)90:3<243::aid-ajmg11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 50.Hoeffel CC, Do MB, Le LH, Phan HT, Nguyen KQ, Chen YB. Partial failure of sternal fusion in an adolescent. South Med J. 1999;92:1204–6. doi: 10.1097/00007611-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Pivnick EK, Kaufman RA, Velagaleti GV, Gunther WM, Abramovici D. Infant with midline thoracoabdominal schisis and limb defects. Teratology. 1998:205–8. doi: 10.1002/(SICI)1096-9926(199811)58:5<205::AID-TERA7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 52.Correa-Rivas MS, Matos-Llovet I, Garcia-Fragoso L. Pentalogy of Cantrell: a case report with pathologic findings. Pediatr Dev Pathol. 2004;7:649–52. doi: 10.1007/s10024-004-9104-5. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, Nagesh NK, Mahajan J, Chellani HK, Mohan M, Anand NK. Superior sternal cleft. Indian Pediatr. 1988;25:203–6. [PubMed] [Google Scholar]
- 54.Liu SW, How CK, Chen JD. Isolated superior sternal cleft. Otolaryngol Head Neck Surg. 2009:130–1. doi: 10.1016/j.otohns.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Gunay E, Simsek Z, Guneren G, Celikyay F. E-page original images. A rare case of isolated complete congenital sternal cleft. Anadolu Kardiyol Derg. 2010;10:E30. doi: 10.5152/akd.2010.182. [DOI] [PubMed] [Google Scholar]
- 56.Pousios D, Panagiotopoulos N, Piyis A. Congenital sternal cleft in an adult male not associated with cardiac defects or ectopia cordis. Ann Thorac Surg. 2007:1900. doi: 10.1016/j.athoracsur.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 57.Battal B, Karademir I, Bozlar U, Saglam M, Bulakbasi N, Tasar M. Case report. Isolated complete congenital sternal cleft in an adult: MDCT imaging findings. Br J Radiol. 2009:e202–3. doi: 10.1259/bjr/76694119. [DOI] [PubMed] [Google Scholar]
- 58.Durusoy C, Mihci E, Tacoy S, Ozaydin E, Alpsoy E. PHACES syndrome presenting as hemangiomas, sternal clefting and congenital ulcerations on the helices. J Dermatol. 2006:219–22. doi: 10.1111/j.1346-8138.2006.00050.x. [DOI] [PubMed] [Google Scholar]
- 59.Chakkarapani E, Barnard I, Couriel J. Superior sternal cleft, cutaneous, and airway haemangiomas. Arch Dis Child Fetal Neonatal Ed. 2007:F3. doi: 10.1136/adc.2005.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesselhoeft CW, Jr, DeLuca FG. A simplified approach to the repair of pediatric pectus deformities. Ann Thorac Surg. 1982;34:640–6. doi: 10.1016/s0003-4975(10)60902-2. [DOI] [PubMed] [Google Scholar]
- 61.Metry DW, Garzon MC, Drolet BA, Frommelt P, Haggstrom A, Hall J, Hess CP, Heyer GL, Siegel D, Baselga E, Katowitz W, Levy ML, Mancini A, Maronn ML, Phung T, Pope E, Sun G, Frieden IJ. PHACE syndrome: current knowledge, future directions. Pediatr Dermatol. 2009:381–98. doi: 10.1111/j.1525-1470.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 62.James PA, McGaughran J. Complete overlap of PHACE syndrome and sternal malformation—vascular dysplasia association. Am J Med Genet. 2002;110:78–84. doi: 10.1002/ajmg.10398. [DOI] [PubMed] [Google Scholar]
- 63.Yadegari M, Whyte MP, Mumm S, Phelps RG, Shanske A, Totty WG, Cohen SR. Buschke-Ollendorff syndrome: absence of LEMD3 mutation in an affected family. Arch Dermatol. 2010:63–8. doi: 10.1001/archdermatol.2009.320. [DOI] [PubMed] [Google Scholar]
- 64.Assmann A, Mandt N, Geilen CC, Blume-Peytavi U. Buschke-Ollendorff syndrome—differential diagnosis of disseminated connective tissue lesions. Eur J Dermatol. 2001;11:576–9. [PubMed] [Google Scholar]