Abstract
OBJECTIVE
The study aimed to evaluate the effectiveness of two new nodal classifications based on the number of metastatic lymph nodes (LNs) or ratio of metastatic to examined LNs (LNR) in making a prognosis, compared with the current nodal classification based on the location of metastatic LNs.
METHODS
We analyzed 651 non-small-cell lung cancer patients who had undergone complete resection with the removal of more than five LNs between 1986 and 2003, excluding preoperative treatment cases, and a Tis, T4, N3, and M1 status, along with limited resection and operative death cases. The cutoff numbers for each category in the two new nodal classifications (number of metastatic LNs (nN0–2): 0, 1–2, and >3, and LNR (rN0–2): 0, 1–12, and >12%) were defined so that the numbers corresponded with paired categories within the current nodal classification.
RESULTS
The 5-year survival rate was 75.4% for patients with the N0 categories in all three classifications. The 5-year survival rates for patients with N1 and N2 categories were 52.2% and 42.6% according to the current nodal classification, 54.3% and 39.8% according to the number of metastatic LNs, and 58.8% and 35.0% according to the LNR, respectively. Although all three nodal classifications were independent prognostic factors along with the age and pathological T status, when the three nodal classifications were entered into multivariate analysis individually, the hazard ratio of rN2 was the highest, at 3.15, followed by that of nN2 at 2.96.
CONCLUSIONS
The LNR followed by the number of metastatic LNs may be more effective prognostic indicators than the current nodal classification based on the location of metastatic LNs. For the future revision, the number of metastatic LNs and LNR should be evaluated as indicators for the nodal classification of lung cancer.
Keywords: Non-small-cell lung cancer, Staging, Lymph node staging, Lymph node metastasis, Number of metastatic lymph node, Prognostic factor
INTRODUCTION
The nodal classification in the current 7th edition of the TNM classification (TNM, tumor, node, metastasis) [1], which has recently been revised, is defined based on the location of metastatic lymph nodes (LNs), as in the previous 6th edition. Concerns regarding the current nodal classification for lung cancer have been pointed out that N1 and N2 patient groups consist of heterogeneous subgroups with regard to the prognosis. Cases with multiple-station metastases have been reported to be a poorer prognostic subgroup compared with those with single-station metastasis in both N1 [2] and N2 patients [3,4]. In addition, patients with skip metastasis, which is defined as having N2 disease without N1 disease, have been reported to comprise a more favorable prognostic subgroup [5].
In the TNM staging system for other major malignancies such as colorectal carcinoma, gastric carcinoma, and breast cancer, the number of metastatic LNs is included in the nodal classification [6], because it has been shown to be a more effective prognostic indicator than the location of metastatic LNs. If the number of metastatic LNs is included in the nodal classification of the lung cancer staging system as a more effective prognostic indicator, some of the concerns regarding the current nodal classification could also be resolved.
Conversely, the ratio of metastatic to examined LNs (LNR) has been suggested to be a more favorable prognostic indicator than the number of metastatic LNs in some malignancies [7,8]. Furthermore, stage migration was less frequently observed in the nodal classification based on the LNR, when compared with the nodal classification based on the number of metastatic LNs [8].
The aim of this study was to elucidate which nodal classification is the best of the three: the current nodal classification based on the anatomical location of metastatic LNs (Current NC), a nodal classification based on the number of metastatic LNs (Number NC), and a nodal classification based on the LNR (LNR NC).
MATERIALS AND METHODS
Patients
Between October 1986 and December 2003, 972 patients with lung cancer underwent surgical resection of the lung at our hospital. We excluded patients who had small-cell carcinoma, residual disease at surgery, concomitant double cancer, pathological Tis disease, stage IIIB disease, and stage IV disease. Furthermore, patients with induction treatment, limited resection, or operative death were also eliminated. A total of 674 patients met the criteria. Of these, the number of LNs removed ranged from 1 to 66, with a median of 19. Twenty-three patients had less than six LNs removed, and we excluded these patients because six or more LNs/stations are recommended to be examined according to the 7th edition of the TNM classification. Finally, we analyzed 651 patients, and the patient characteristics are shown in Table 1. The Tochigi Cancer Center institutional review board approved this retrospective study, and waived the requirement of patient consent.
Table 1:
Univariate analysis of overall survival according to clinicopathologic factors and three nodal classifications in 651 patients
| Variable | No. of patients | 5-year OS (%) | 95% CI | P-value |
|---|---|---|---|---|
| Age | ||||
| < 66 | 331 | 72.8 | 68.0–77.6 | <0.001 |
| > 67 | 320 | 60.3 | 54.6–65.7 | |
| Gender | ||||
| Female | 212 | 75.9 | 70.1–81.7 | <0.001 |
| Male | 439 | 62.2 | 57.7–66.7 | |
| Histology | ||||
| Adenocarcinoma | 392 | 73.2 | 68.8–77.6 | <0.001 |
| Non-adenocarcinoma | 259 | 56.8 | 50.8–62.8 | |
| Pathological T status | ||||
| T1a | 149 | 91.3 | 86.8–95.8 | <0.001 |
| T1b | 126 | 73.8 | 66.1–81.5 | |
| T2a | 242 | 58.7 | 52.5–64.9 | |
| T2b | 55 | 60.0 | 47.0–73.0 | |
| T3 | 79 | 38.0 | 27.3–48.7 | |
| Surgical type | ||||
| Lobectomy | 602 | 68.6 | 64.9–72.3 | <0.001 |
| Pneumonectomy | 49 | 42.9 | 29.0–56.8 | |
| Postoperative chemotherapy | ||||
| No | 609 | 65.7 | 61.9–69.5 | 0.172 |
| Yes | 42 | 77.8 | 66.7–88.9 | |
| Postoperative radiotherapy | ||||
| No | 612 | 67.3 | 63.6–71.0 | 0.035 |
| Yes | 39 | 57.5 | 42.2–72.8 | |
| Current NC | ||||
| NO | 437 | 76.0 | 72.0–80.0 | <0.001 |
| N1 | 113 | 52.2 | 43.0–61.4 | |
| N2 | 101 | 42.6 | 33.0–52.2 | |
| SS | 60 | 45.0 | 32.4–57.6 | |
| MS | 41 | 39.0 | 24.1–53.9 | |
| Number NC | ||||
| nNO | 437 | 76.0 | 72.0–80.0 | <0.001 |
| nN1 (1-2) | 116 | 54.3 | 45.2–63.4 | |
| nN2 (>3) | 98 | 39.8 | 30.1–49.5 | |
| nN2a (3-5) | 57 | 45.6 | 32.7–58.5 | |
| nN2b (>5) | 41 | 31.7 | 17.5–45.9 | |
| LNR NC | ||||
| rNO | 437 | 76.0 | 72.0–80.0 | <0.001 |
| rN1 (<12%) | 114 | 58.8 | 49.8–67.8 | |
| rN2 (>12%) | 100 | 35.0 | 25.7–44.3 | |
| rN2a (12-26%) | 60 | 40.0 | 27.6–52.4 | |
| rN2b (>26%) | 40 | 27.5 | 13.7–41.3 | |
CI, confidence interval; Current NC, current nodal classification; LNR NC, nodal classification based on the ratio of metastatic to examined lymph nodes; Number NC, nodal classification based on the number of metastatic lymph nodes; OS, overall survival; SS, single station metastasis; MS, multiple stations metastasis.
Preoperative evaluation
All patients underwent physical examination, chest radiography, bone scintigraphy, bronchoscopic examination, and computed tomography (CT) or magnetic resonance imaging of the brain, as well as CT of the chest and upper part of the abdomen for the staging and evaluation of resectability before surgery. Positron emission tomography (PET) was rarely implemented during this study period. An LN >1 cm in its short axis on CT was determined as metastatic. We did not consider patients with clinical mediastinal LN metastasis to be a contraindication for surgery unless the swollen LNs appeared unresectable. Therefore, mediastinoscopy or transbronchial needle aspiration to confirm N2 disease has been rarely conducted in patients not included in clinical trials.
Treatment
Major anatomical lung resection with systematic nodal dissection was the standard operation during the study period in our hospital. Systematic nodal dissection involves the complete resection of ipsilateral mediastino-hilar LNs. However, prevascular and post-tracheal LNs in any location of primary lung tumor, paraesophageal and pulmonary ligament LNs in upper lobe lung tumor, and highest mediastinal, upper mediastinal, and lower paratracheal LNs in left lung tumors were usually not resected. About 15% of patients underwent hilar LN dissection with or without selective mediastinal nodal dissection. Hilar, interlobar, and lobar LNs were resected with affected lung lobes in the hilar LN dissection, and remote mediastinal LN stations, such as subcarinal LNs in the upper lobe tumor, were not resected in the selective mediastinal nodal dissection [9,10].
Follow-up
The patients were scheduled for checkups, chest radiography, and measurement of the serum tumor marker levels every 1–3 months for 2 years after the operation and every 6 months thereafter. A total of 649 patients were followed up until death or the last day of follow-up (31 December 2007). The median length of follow-up for surviving patients was 116 months (range: 58–261 months).
Pathological examination
Hilar and interlobar LNs were resected in combination with the lung, and then these nodes were classified into each nodal station according to the Naruke map [11] after the operation by a thoracic surgeon. The right upper mediastinal LNs, consisting of #1, #2, #3, and #4, or the left aortopulmonary window LNs, consisting of #5 and #6, were dissected en bloc. These nodes were immediately divided into each station. LNs #7, #8, #9, and left #4 were individually removed. Each LN was fixed in formalin and cut at its equator, stained with hematoxylin and eosin, and examined by means of light microscopy. The pathological stage was classified according to the current 7th edition of the TNM classification. When only one mediastinal LN station was involved, it was defined as a single-station metastasis, and the others were defined as multiple-station metastases.
Statistical analysis
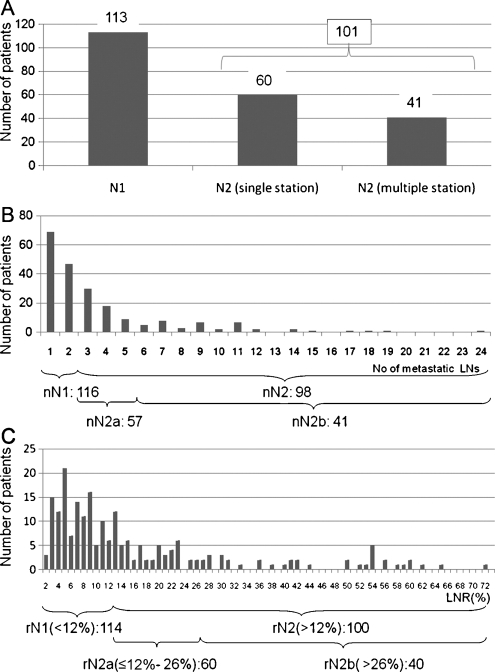
The cutoff numbers for each category in the two new nodal classifications (Number NC and LNR NC) were defined so that the numbers corresponded with paired categories within the Current NC (Fig. 1A). This was done in order to compare the prognosis of each category in the two new nodal classifications. The number of metastatic LNs ranged from 1 to 24, and the median was 2 for node-positive patients. In the Number NC, we classified the patients into three categories according to the number of metastatic LNs: nN0, the number of metastatic LNs was 0; nN1, 1–2; and nN2, >2. In addition, nN2 was subdivided into two categories: nN2a, the number of metastatic LNs was 3–5; and nN2b, >5 (Fig. 1B). The LNR was calculated by dividing the total number of metastatic LNs by the total number of LNs examined and multiplied by 100. The LNR ranged from 2.5% to 72.7%, and the median was 11.1% for node-positive patients (Fig. 1C). In the LNR NC, we classified the patients into three categories: rN0, the ratio of metastatic to resected LNs was 0; rN1, 1 to ≤12%; rN2, >12%. In addition, rN2 was subdivided into two categories, rN2a, the LNR was from 12% to 26%; rN2b, >26%.
Figure 1:
(A) This figure shows the number of patients in each category in Current NC with the subdivision of N2 into single and multiple stations. (B) This figure shows the distribution of patients according to the number of metastatic lymph nodes. The cutoff numbers of metastatic lymph nodes in Number NC were defined so that the numbers corresponded with paired categories within Current NC. (C) The distribution of patients according to the LNR is shown. The cutoff numbers of the LNR in the LNR NC were defined so that the numbers corresponded with paired categories within Current NC.
Survival curves were calculated using the Kaplan–Meier method, and differences in survival were determined by the log-rank test. Multivariate analysis of several prognostic factors was performed with Cox’s proportional hazards regression model using Statistical Package for the Social Sciences (SPSS) ver. 17 (SPSS Inc., Chicago, IL, USA).
RESULTS
The patients’ characteristics are shown in Table 1. The operative procedure employed in the majority of patients was a lobectomy with systematic nodal dissection. Pneumonectomy was carried out in only 49 patients. Postoperative chemo- and radiotherapy were applied in 13 (11.5%) patients and in one (0.9%) with N1 disease, respectively, and in 18 (17.8%) and 38 (37.6%) patients with N2 disease, respectively.
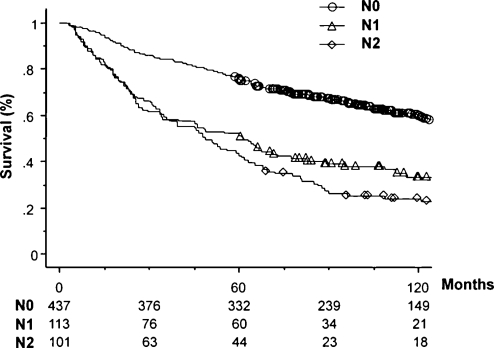
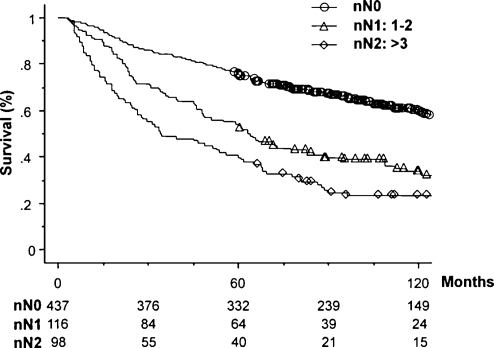
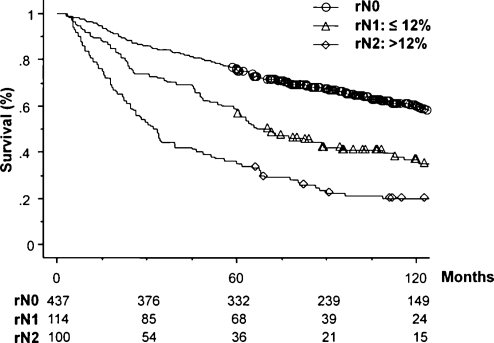
The number of patients and 5-year survival rates with a 95% confidence interval for each category in the three nodal classifications are also shown in Table 1. The numbers of patients in each category at the same level of the three nodal classifications were similar, for example, 101 patients in the Current NC, 98 in Number NC, and 100 in LNR NC in the N2 categories. This was because the cutoff numbers for each category in the two new nodal classifications were defined so that the numbers corresponded with paired categories within the Current NC. In addition, the number of patients within the subcategories of N2 was also similar, for example, 41 patients with multiple station N2 in the Current NC, 41 with nN2b in Number NC, and 40 with rN2b in the LNR NC. Figs. 2–4 show overall survival curves according to the Current NC, Number NC, and LNR NC, respectively. In the Current NC, the difference in survival between patients with N1 and N2 disease was relatively small compared with that between patients with N0 and N1 disease (Fig. 2). On the contrary, this difference in survival between patients classified in the N1 and N2 category was greater in Number NC (Fig. 3) and LNR NC (Fig. 4); that is, the survival rates of patients in the N1 and N2 category were 54.3% and 39.8% in Number NC, respectively, and 58.8% and 35.0% in LNR NC, respectively, although the patient numbers in the same N2 category were similar (Table 1). Regarding the subclassification in the N2 category, the patients with rN2b in LNR NC showed the lowest 5-year survival rate of 27.5%, compared with 31.7% of nN2b in Number NC, and 39.0% of multiple-station N2 in Current NC, although the numbers of patients in each category were similar. Table 2 shows multivariate analyses of overall survival. All factors analyzed in the univariate analysis (Table 1) were evaluated using multivariate analysis. Due to the fact that Current NC, Number NC, and LNR NC were strongly correlated with each other, we entered each nodal classification into multivariate analysis individually, and compared their hazard ratios. Multivariate analysis revealed that the age, pathological T status, postoperative chemotherapy, Current NC, Number NC, and LNR NC were significant independent prognostic factors. Among the N2 categories, the hazard ratio of rN2 in LNR NC was the largest, at 3.29. The hazard ratio of rN1 was 1.53, which was about half of that of rN2.
Figure 2:
Survival curves according to Current NC.
Figure 3:
Survival curves according to number of NC.
Figure 4:
Survival curves according to LNR NC.
Table 2:
Multivariate analysis of overall survival in all 651 patients
| Variable | Hazard ratio3 | P-value |
|---|---|---|
| Current NC | ||
| NO | 1 | – |
| N1 | 1.62 | 0.001 |
| N2 | 2.75 | <0.001 |
| Number NC | ||
| nNO | 1 | – |
| nN1 (1–2) | 1.62 | <0.001 |
| nN2 (>3) | 3.17 | <0.001 |
| LNR NC | ||
| rNO | 1 | – |
| rN1 (<12%) | 1.53 | 0.003 |
| rN2 (>12%) | 3.29 | <0.001 |
a Adjusted for age, gender, histology, pathological T status, surgical type, postoperative chemotherapy, and postoperative radiotherapy. Current NC, current nodal classification; LNR NC, nodal classification based on the ratio of metastatic to examined lymph nodes; Number NC, nodal classification based on the number of metastatic lymph nodes.
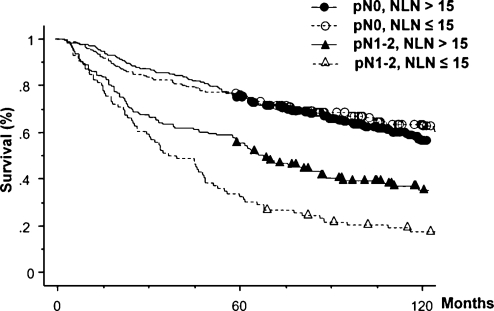
In addition to these analyses, we analyzed the impact of the number of negative LNs resected to understand why LNR NC was superior to Number NC. Fig. 5 shows survival curves according to the number of negative LNs resected and the presence or absence of LN metastasis. Among the patients showing no nodal metastasis, the survival curves were similar regardless of the number of negative LNs resected. However, among the patients with LN metastasis, the survival of patients for whom a larger number (>15) of negative LNs was resected was better than that of patients with a smaller number (≤15) resected (P = 0.002).
Figure 5:
Survival curves according to the number of negative lymph nodes resected and lymph node metastasis.
DISCUSSION
The nodal classification based on the location of LNs was established on the idea that LNs metastasis occurs in LNs neighboring the primary tumor, and then sequentially spreads to more distant nodes. However, recent studies on skip metastasis [5,12] and sentinel LNs [13,14] have revealed that metastasis may initially occur in the mediastinal LNs without N1 metastasis in about one-quarter of patients, who show a better prognosis than patients with non-skip N2 disease. From these results, we hypothesized that the generation of LN metastasis can be a more effective prognostic indicator than the metastatic location, and the number of metastatic LNs optimally represents the generation of LN metastasis. In other major malignancies such as colorectal carcinoma, breast cancer, and gastric carcinoma, nodal classifications in the current TNM staging system include the number of metastatic LNs [6]. Many studies have revealed that the number of metastatic LNs is a better prognostic indicator than their location in those cancers [15]. In lung cancer, two studies have also reported the usefulness of Number NC. Fukui et al. classified nodal categories according to the number of metastatic LNs as 1–3, 4–6, and >6 [16], and Lee et al. also classified them as 1–4, 4–14, and >14 [17]. Both studies demonstrated that Number NC was a stronger prognostic indicator than Current NC based on the location.
Recently, the LNR has been also investigated in esophageal [18], colorectal [7], and gastric cancers [19] as a more effective prognostic factor [8]. Frederique et al. compared the LNR with the number of metastatic LNs [7]. They demonstrated that the prognostic ability of the LNR was better than the number of metastatic LNs, and this ability was retained when patients with <12 LNs were analyzed. Inoue et al. also suggested that the LNR was more robust on examining the LN number. In our study, the LNR was a more effective prognostic indicator than the number of metastatic LNs. Why then is LNR NC better than Number NC? We investigated the effect of the number of resected LNs. We analyzed the number of negative LNs instead of the total number of LNs resected because the total number resected includes the number of metastatic LNs that adversely affect the survival. As a result, a larger number of resected negative LNs was found to be a significantly favorable prognostic factor in node-positive patients. Several studies in lung cancer and other malignancies have shown a larger number of resected LNs to be a favorable prognostic factor [20–22], and Johnson et al. reported that the number of negative nodes is also an independent prognostic factor for patients with stage IIIB and IIIC colon cancer [23]. They speculated that there were three possible mechanisms: stage migration, quality care, and tumor–host interaction. We consider that all three mechanisms contribute to the prognostic significance of LNR to some extent; however, we cannot specify which is the strongest. Regardless of the mechanisms, the number of LNs resected markedly affects the prognostic value of LNR, and this is considered the reason for the superiority of LNR NC compared with Number NC.
In this analysis, the cutoff numbers of the two new classifications trialed were defined so that the numbers of patients in each category corresponded with categories within the Current NC. This was done because our research objective was to compare the ability of the three classifications to classify patients into several categories according to their survival. If the cutoff numbers for the two new classifications are set in order to most effectively classify patients within the categories, this would have resulted in an unfair analysis when compared with the Current NC. Therefore, the cutoff numbers we used were not the best in terms of predicting survival. We believe that in order to determine the most effective cutoff numbers data accumulated from multiple institutions from several countries should be analyzed.
As the number of LNs resected can influence the accuracy of nodal staging, even in Current NC [22,24] but especially in Number NC and LNR NC, a minimum number of LNs to be examined should be defined. Although the present TNM classification proposed that more than five LNs should be resected to correctly define pN0, no relevant data have been shown. In other malignancies, 12 or more LNs should be resected for the nodal staging of colorectal carcinoma, 16 or more for gastric carcinoma, and six or more for breast cancer based on a number of reports [6]. All these staging systems include the number of metastatic LNs in recent editions. Ludwig et al. analyzed 16 800 patients with stage IA and IB in the Surveillance, Epidemiology, and End Results (SEER) database [22]. They concluded that a number between 11 and 16 may be the minimum required to examine based on the finding that a lower hazard ratio was observed in pN0 patients, and the survival difference was thought to be due to inaccurate staging. To establish the minimum number of LNs to be examined, a prospective multicenter study is needed.
A concern regarding Number NC and LNR NC is the fragmentation of LNs. LNs are sometimes resected as fragments, and the type of LN removal and examination can also influence the fragmentation; systematic nodal dissection, systematic or local sampling, or biopsy via mediastinoscopy or endobronchial ultrasonography. We consider that the number of metastatic LNs is effective only when systematic nodal dissection or systematic nodal sampling, not biopsy, is performed. Further, Number NC or LNR NC will be useful in a postsurgical setting to apply the best postsurgical adjuvant therapy, including chemotherapy and radiotherapy. We consider that LNR NC is robust against LNs fragmentation, because, if nodes are fragmented, both the numbers of metastatic LNs and total number of LNs will increase.
One limitation of our study was the length of the period of investigation. Therefore, we evaluated the time difference by dividing the patients into those in early and late periods, and found that our core results did not change (data not shown). Another limitation was the difference in the preoperative staging method and indication of surgery for clinical N2 disease. Recently, many institutions including ours have conducted PET as a staging modality. Mediastinoscopy was also more frequently applied in the USA than in Japan, which might be due to the difference in the indication for lung resection for clinical N2 patients. These differences may have influenced our results. Therefore, the present results should be confirmed in various situations.
In conclusion, the LNR as well as the number of metastatic LNs may be better prognostic indicators than Current NC. For the future revision of nodal classification, the number of metastatic LNs and LNR should be evaluated as indicators for the nodal classification of lung cancer.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- CT
computed tomography
- Current NC
current nodal classification based on the anatomical location of metastatic lymph nodes
- Number NC
new nodal classification based on the number of metastatic lymph nodes
- LNR
ratio of metastatic to examined lymph nodes
- LNR NC
new nodal classification based on the ratio of metastatic to examined lymph nodes
- LNs
lymph nodes.
REFERENCES
- 1.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto T, Cassivi SD, Yang P, Barnes SA, Nichols FC, Deschamps C, Allen MS, Pairolero PC. Completely resected N1 non-small cell lung cancer: factors affecting recurrence and long-term survival. J Thorac Cardiovasc Surg. 2006;132:499–506. doi: 10.1016/j.jtcvs.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, Watanabe Y, Mitsudomi T, Yoshimura M, Tsuboi M. Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg. 2001;122:803–8. doi: 10.1067/mtc.2001.116473. [DOI] [PubMed] [Google Scholar]
- 4.Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–9. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 5.Riquet M, Assouad J, Bagan P, Foucault C, Le Pimpec Barthes F, Dujon A, Danel C. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg. 2005;79:225–33. doi: 10.1016/j.athoracsur.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. 6th ed. New York: Springer; 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 7.Peschaud F, Benoist S, Julie C, Beauchet A, Penna C, Rougier P, Nordlinger B. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–73. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 8.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, De Santis F, Baiocchi L, Coniglio A, Nitti D. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–52. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117:1102–11. doi: 10.1016/s0022-5223(99)70246-1. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg. 2006;81:1028–32. doi: 10.1016/j.athoracsur.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 11.Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. 1978;76:832–9. [PubMed] [Google Scholar]
- 12.Prenzel KL, Monig SP, Sinning JM, Baldus SE, Gutschow CA, Grass G, Schneider PM, Holscher AH. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol. 2003;82:256–60. doi: 10.1002/jso.10219. [DOI] [PubMed] [Google Scholar]
- 13.Nomori H, Horio H, Naruke T, Orikasa H, Yamazaki K, Suemasu K. Use of technetium-99m tin colloid for sentinel lymph node identification in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;124:486–92. doi: 10.1067/mtc.2002.124496. [DOI] [PubMed] [Google Scholar]
- 14.Liptay MJ, Grondin SC, Fry WA, Pozdol C, Carson D, Knop C, Masters GA, Perlman RM, Watkin W. Intraoperative sentinel lymph node mapping in non-small-cell lung cancer improves detection of micrometastases. J Clin Oncol. 2002;20:1984–8. doi: 10.1200/JCO.2002.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes’ C class of colorectal cancer. An analysis of the NSABP clinical trials. Ann Surg. 1986;203:115–22. doi: 10.1097/00000658-198602000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5. [PubMed] [Google Scholar]
- 17.Lee JG, Lee CY, Park IK, Kim DJ, Park SY, Kim KD, Chung KY. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–5. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–71. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27–34. doi: 10.1245/aso.2002.9.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602–10. doi: 10.1097/01.sla.0000237655.11717.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–9. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–50. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570–5. doi: 10.1200/JCO.2006.06.8866. [DOI] [PubMed] [Google Scholar]
- 24.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–34. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]