Abstract
OBJECTIVE
Acute right ventricular (RV) failure is a life-threatening condition with a poor prognosis, and sometimes the use of mechanical circulatory support is inevitable. In this article, we describe our experience using a centrifugal pump as a temporary percutaneous right ventricular assist device (RVAD) in patients with postoperative acute refractory RV failure after left ventricular assist device (LVAD) implantation.
METHODS
We retrospectively reviewed eight consecutive patients with acute RV failure who underwent temporary percutaneous RVAD implantation using a centrifugal pump after LVAD implantation between April 2008 and February 2011. A Dacron graft was attached to the main pulmonary artery and passed through a subxiphoid exit, where the outflow cannula was inserted. The inflow cannula was percutaneously cannulated using Seldinger’s technique in the femoral vein. The chest was definitely closed. The technique allowed bedside removal, avoiding chest re-opening.
RESULTS
The median patient age was 52 years (range: 41–58). The median duration of support was 14 days (range: 12–14). RV systolic function improved; central venous pressure and mean pulmonary artery pressure decreased significantly after RVAD support. In three patients, an oxygenator was integrated into the RVAD due to impaired pulmonary function. Six patients were successfully weaned. Five patients survived to hospital discharge. Technical problems or serious complications concerning decannulation were not observed.
CONCLUSION
This report suggests that implantation of temporary percutaneous RVAD using a centrifugal pump is a safe alternative in the treatment of postoperative acute refractory RV failure. Ease of device implantation, weaning, explantation, and limited number of complications justify a liberal use.
Keywords: Right heart failure, Temporary circulatory assistance, RVAD
INTRODUCTION
Acute right ventricular (RV) failure is a serious complication that frequently results in adverse outcomes with high mortality rate that may reach 70%. It occurs in approximately 0.1% of patients following conventional cardiac surgery, in 2–3% of patients following heart transplantation, and in 20–30% of patients requiring left ventricular assist device (LVAD) insert [1–3]. Postcardiotomy RV failure can be caused by prolonged cardioplegic arrest, inadequate myocardial protection and right coronary occlusion due to coronary vasospasm, air embolization, and thrombus. In transplant recipients, donor organ ischemia and preexistent or perioperative pulmonary hypertension mainly contribute to the development of acute refractory RV failure. Many factors may contribute to acute RV failure after LVAD insertion. During LVAD support, unloading of the left ventricle causes a leftward shift of the septum, and thus the interventricular balance is altered. Furthermore, preexisting biventricular failure or RV dysfunction with impaired RV geometry and moderate-to-severe tricuspid valve incompetence together with intra-operative bleeding and postoperative mismanagement are often factors contributing to the onset of RV failure. This complication has increased with both the expansion of the donor pool to include marginal donors and the increasing use of LVADs as a bridge to transplantation, unveiling a growing incidence of associated perioperative RV failure [1–6].
Although some patients recover successfully on medical right heart supporting therapies, including inotropes, phosphodiesterase inhibitors, or inhaled nitric oxide, others require mechanical circulatory support [3, 7]. The most effective therapy for these patients is an upgrade to biventricular mechanical support. However, it is well reco`gnized that primary as well as secondary biventricular ventricular assist device implantation is associated with higher mortality rates [8, 9].
Patients with RV failure are extremely ill, require inotropic and vasopressor support, and are coagulopathic due to hepatic congestion. In addition, most cases of refractory RV failure require short-term mechanical support. Hence, development of a quick and simple means to support the pulmonary circulation during periods of transient RV dysfunction is needed.
This article describes our successful experience in eight consecutive patients with postoperative acute RV failure after LVAD implantation supported with a temporary percutaneous RVAD using a centrifugal pump.
METHODS
After approval by the local Ethics committee, we retrospectively reviewed eight patients with postoperative acute RV failure after LVAD implantation (either the implantable INCOR or the paracorporeal EXCOR, Berlin Heart, Berlin, Germany), who underwent temporary percutaneous RVAD implantation using a centrifugal pump between April 2008 and February 2011.
The indications for RV failure were determined clinically and included inadequate cardiac output and systemic pressure despite large doses of inotropes and vasopressor agents, increased central venous pressure (CVP) and significant RV dysfunction seen by transesophageal echocardiography. The following echocardiographic parameters were defined as predictors for RV dysfunction after LVAD implantation: tricuspid valve incompetence, RV enddiastolic diameter (RVEDD) >35 mm, RV ejection fraction (RVEF) <30%, and right atrial dimension >50 mm. The same criteria were used by the Berlin group [10]. Variables analyzed included total time of RVAD support, hemodynamic parameters (mean pulmonary artery pressures, CVP, and cardiac output), and parameters of endorgan perfusion (levels of serum glutamic-oxaloacetic transaminase, serum glutamate pyruvate transaminase, total and direct bilirubin, and serum creatinine).
Our patients were supported with a percutaneous RVAD. The inflow cannula (23–25 Fr) was percutaneously cannulated in the femoral vein using Seldinger’s technique. An 8-mm Dacron graft (Vascutek-Gelweave, Inchinan, UK) was anastomosed end to side to the main pulmonary artery with a 5/0 polypropylene running suture using a Satinsky side clamp and passed through a subxiphoid exit, where the outflow cannula (21–23 Fr) was inserted. The graft was tied tightly around the cannula outside the chest with umbilical tapes and secured firmly to the chest wall with multiple heavy sutures. For mechanical circulatory support, cannulae were connected to a centrifugal pump (Rotaflow, Maquet CP, Hirrlingen, Germany). An integration of an oxygenator into the RVAD was possible, when the pulmonary function was impaired. The chest was definitely closed. As all blood contact surfaces of the system were heparin coated with the BIOLINE technique (Maquet CP), systemic anticoagulation could be kept at a minimum. After 6 h without bleeding, we started anticoagulation with heparin (activated partial thromboplastin time, aPTT 50–60 s), and we maintained this anticoagulation protocol until RVAD explantation. Usually, 100 mg day−1 acetylsalicylic acid was administered to inhibit platelet aggregation. After successful treatment of the underlying respiratory disease, and when lung function improved at moderate ventilator settings, oxygenator was weaned. After an uneventful recovery, the RVAD was weaned under echocardiography control and surveillance of CVP and LVAD flow. RV recovery included improved RV systolic function on echocardiography, no escalation of inotropic support, maintenance of a low CVP (<15 mmHg), and return of transaminases and creatinine to near-normal levels. Prior to explantation of the RVAD, its flow was decreased to 1 l min−1 and the hemodynamic status reassessed. The pump lines were clamped, and skin exit of the graft was widely prepared and draped. Gentle traction on the graft allowed the redundant portions from inside the chest to be exposed. The umbilical tapes were cut and the cannula was removed. The graft was then divided at the skin level and oversewn. By delivering the redundant portion, the graft was sterile at the point of division. The closed stump was allowed to retract into the oblique chest wall track, and the skin incision was loosely closed. Removal of the inflow femoral was simple with manual compression of the groin.
Statistical analysis
The statistical analysis was performed using the SPSS 16.0 software (SPSS, Chicago, IL, USA). Normal distribution was assessed by Lilliefors modification of the Kolmogorow–Smirnow test. Values of continuous data are presented as mean ± standard deviation (SD) or as median with interquartile range when appropriate. Categorical variables are displayed as frequency distributions (n) and simple percentages (%).
RESULTS
Patient characteristics are given in Table 1. The RVAD cohort consisted of two women and six men, with a median age was 52 years (range: 41–58). Acute RV failure developed after LVAD implantation (either the implantable INCOR or the paracorporeal EXCOR). In seven patients, the decision to proceed with device implantation was made after unsuccessful attempts to wean from cardiopulmonary bypass despite increased inotrope support. Device implantation was delayed in one patient after hemodynamic decompensation developed in the intensive care unit. In two patients, intra-aortic balloon pump was used before RVAD placement. In three patients, a percutaneous venoarterial extracorporeal membrane oxygenation (ECMO) was used prior to LVAD implantation. In the perioperative echocardiography examinations, six patients showed moderate-to-severe tricuspid valve incompetence. The postoperative mean blood loss was 600 ± 380 ml. The postoperative transfusion of packed red blood cell (PRBC) was 2 units (range: 1–4) and of fresh frozen plasma (FFPs) was 3 units (range: 2–5). Supported by substitution of FFPs and platelets, no postoperative exploration was required.
Table 1:
Patient data, characteristics and outcome
| No. | Gender | Age (years) | Oxygenator | Duration (days) | Weaned | Outcome |
|---|---|---|---|---|---|---|
| 1 | Male | 61 | – | 13 | + | + |
| 2 | Male | 31 | – | 16 | – | – |
| 3 | Male | 42 | – | 14 | – | – |
| 4 | Female | 52 | + | 3 | + | + |
| 5 | Male | 58 | – | 15 | + | – |
| 6 | Male | 51 | + | 7 | + | + |
| 7 | Male | 38 | + | 14 | + | + |
| 8 | Female | 58 | – | 13 | + | + |
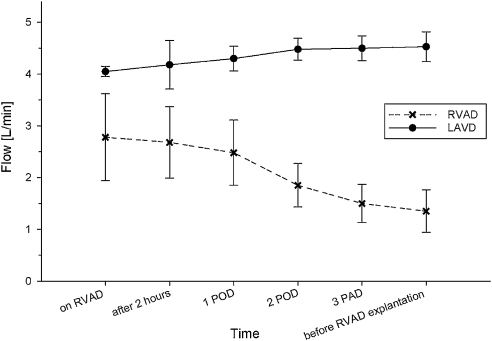
The median duration of RVAD support was 14 days (range: 12–14). In three patients, an oxygenator was integrated into the RVAD due to impaired pulmonary function. The oxygenator support duration was 6 days (3–8). Effective support with pump flows of 2.9 ± 1.2 l min−1 was achieved in all patients. The LVAD flow increased from 4.0 ± 0.2 l min−1 intra-operatively to 4.5 ± 0.5 l min−1 after RVAD support (p < 0.05) (Fig. 1).
Figure 1:
The blood flow in the patients with left ventricular assist device (LVAD) and temporary right ventricular assist device (RVAD).
Hemodynamic and echocardiographic parameters as measured at four different time points throughout RVAD support are shown in Table 2. The mean pulmonary artery pressure decreased from 42 ± 11 mmHg before implantation to 32 ± 12 mmHg on RVAD support and 26 ± 13 mmHg after RVAD support (p < 0.05). The CVP similarly decreased from 29 ± 8 to 17 ± 8 mmHg on RVAD support and 11 ± 8 mmHg after RVAD support (p < 0.05). Also, the cardiac output increased from 3.9 ± 0.8 to 5.4 ± 1.4 l min−1 after RVAD support (p < 0.05). In the echocardiography examinations, progressive improvement of RV function was documented. The RVEF increased from 24 ± 12% to 41 ± 8%, the RVEDD decreased from 39 ± 9 to 29 ± 9 mm, and the right atrial dimension decreased from 54 ± 13 to 39 ± 10 mm after RVAD support (p < 0.05). The parameters of end-organ perfusion decreased among the time points of RVAD support.
Table 2:
Hemodynamic parameters
| Time | mPAP (mmHg) | CVP (mmHg) | CO (l min−1) | RVEF (%) | RVEDD (mm) | RAD (mm) |
|---|---|---|---|---|---|---|
| Before implantation (n = 8) | 42 ± 11 | 29 ± 8 | 3.9 ± 0.8 | 24 ± 12 | 39 ± 9 | 54 ± 13 |
| On RVAD support (n = 8) | 32 ± 12* | 17 ± 8* | 4.3 ± 0.9* | 31 ± 15* | 31 ± 11* | 43 ± 12* |
| Before explantation (n = 6) | 24 ± 13* | 11 ± 9* | 5.1 ± 1.1* | 43 ± 13* | 28 ± 11 * | 39 ± 13* |
| Off RVAD (n = 6) | 26 ± 13* | 11 ± 9* | 5.4 ±1.5* | 41 ± 8* | 29 ± 9* | 39 ± 10* |
CO: cardiac output; CVP: central venous pressure; mPAP: mean pulmonary artery pressures; RAD: right atrial dimension; RVAD: right ventricular assist device; RVEF: right ventricular ejection fraction; RVEDD: right ventricular enddiastolic diameter.
*p < 0.05 to prior to right ventricular assist device support.
Six patients were successfully weaned from RVAD support, and two patients died while on RVAD support. After removal of the RVAD, no signs of right heart failure occurred. Causes of death were multiorgan failure and intracerebral bleeding. Five patients were discharged from the hospital. The cause of death in the patient who was weaned successfully but eventually died was multiorgan failure. In this patient, no signs of right heart failure occurred after weaning. The duration between weaning and death was 35 days after RVAD explantation.
The BIOLINE-coated Rotaflow pump is approved for 14 days. In two cases, the support duration was more than 14 days. Device-related complications that could not be controlled were not observed; hence, an exchange of pump head was not necessary. Other serious complications with an adverse effect on outcome were not observed (vascular complications, cannula thrombosis, bleeding, or hemolysis). Also, infections associated with the Dacron prosthesis during or after support were not observed.
DISCUSSION
Implantation of LVAD as a bridge to recovery or transplantation is a widely accepted treatment modality [8,11]. Despite the clinical benefit of LVAD usage, RV failure after LVAD implantation continues to be a serious complication with a poor prognosis [12].
Standard therapy for severe right heart failure consists of pharmacological inotropic support, volume unloading, and application of pulmonary vasodilators (prostaglandin and nitric oxide). When pharmacological agents are unable to improve RV function, surgeons must rely on mechanical means to restore blood flow to the pulmonary circulation and left ventricle. Several devices have been evaluated for this indication. In the early 1980s, pulmonary artery balloon pumps were used to support the failing RV. Several authors have reported successfully weaning patients off this treatment, when they presented with acute refractory RV failure following routine cardiac surgical procedures [13,14]. The pulmonary artery balloon pump support is best suited for a shorter duration in patients with up to a 50% reduction in optimal RV performance. This method is unsuitable for extended use and is not as reliable as the RVAD support [15].
Peripheral venoarterial ECMO for RV support is another alternative [3]. However, ECMO, although suitable for cardiopulmonary support in some instances, does not unload the ventricles to the degree possible with a ventricular assist device and has a high rate of device-related complications (thromboembolism, hemolysis, and bleeding) with increasing duration of support. Most commercially available ECMO devices have drawbacks, such as large priming requirements, lack of portability, and device size [16].
The right atrium to pulmonary artery bypass using an ECMO circuit or paracorporeal devices is a widely accepted modality. Early in our experience, centrifugal pumps were used with open-chest cannulation of the right atrium for venous return and of the pulmonary artery for arterial outflow. The catheters were brought through the chest wall through separate stab wounds. The chest was covered but not closed. Reports in the literature regarding the temporary use of the Levitronix CentriMag systems as an RVAD for RV failure have described different findings. A recently published multicenter study in which the CentriMag system was used for RV support in a therapy for 38 patients demonstrated a survival rate of 47%. The survival rate for the 12 patients with RV failure after LVAD implantation was 58% [17]. Bhama et al. reported similar results with a survival rate of 58% in 12 patients with temporary RVAD using the CentriMag system for RV failure after LVAD implantation [18]. By contrast, a report by Shuhaiber et al. described the use of the CentriMag system in five patients with RV failure after LVAD insertion with an early mortality rate of 100% [19]. The disadvantage of this technique is the reoperation to remove the right atrial and the pulmonary artery cannulae with additional risk for bleeding, wound infection, and device contamination. Hence, the ideal device for providing RV support should be one that is easy to implant and explant, provides adequate RV support, requires minimal anticoagulation, and is relatively inexpensive.
Previous reports have demonstrated the feasibility of minimally invasive RVAD insertion using different approaches. Cohn et al. described RVAD implantation through vessel grafts with bedside removal, while Minami et al. described cannulation of the outflow graft through the right pulmonary artery between the ascending aorta and the superior vena cava using Seldinger technique [20, 21]. Stepanenko et al. described implantation of temporary RVAD via a left lateral thoracotomy [22]. The basic idea of the transcutaneous RVAD via sternotomy was devised by Strauch et al. in 2004 [23]. The concept was seen particularly useful for patients with RV failure after LVAD implantation.
In this article, we describe our successful experience in eight consecutive patients with postoperative acute RV failure after LVAD implantation supported with a temporary percutaneous RVAD using a centrifugal pump. Our cases are of particular interest because the duration of RVAD support was relatively long (up to 16 days) and also because the survival rate was high (75%) in comparison with previously reported cases. An RV was recovered to an extent that adequate cardiac output can be generated despite significant RV dysfunction. Another advantage is that an integration of an oxygenator into the RVAD was possible, when perioperative an attempt to improve oxygenation with conventional ventilation was undertaken.
Bleeding and infections are the most frequent complications after RVAD and ECMO implantation [24, 25]. Most of the currently available devices require full-dose systemic anticoagulation. By contrast, our RVAD system (BIOLINE coating) runs with a ‘low-dose’ heparin infusion that does not exceed normal antithrombotic anticoagulation of the intensive care patient. Our approach diminishes the risk of bleeding, because this less invasive technique does not require extensive dissection of cardiac adhesions. During weaning from RVAD, the venous inflow cannula is easy to remove; no right atrial manipulation is needed. The outflow cannula is pulled out of the tube graft; the latter is ligated and buried in the abdominal wall. The prosthesis can be removed on further surgery as at the time of transplantation. The technique allowed bedside removal, avoiding chest re-opening. In our cases, none of the typical complications related to RVAD, such as cannula thrombosis, bleeding, or hemolysis, was observed. Also, infections associated with the Dacron prosthesis during or after support were not observed.
Although the device is licensed for only 14 days of continuous use, device-related complications that could not be controlled were not observed. Hence, an exchange of pump head was not necessary.
Nevertheless, our report has some limitations. The retrospective data collection is a major limitation. The report is a single-center experience and the number of patients is small.
In conclusion, our experience suggests that with a short period of mechanical support, bridge to recovery can be accomplished in a substantial number of patients with postoperative acute RV failure after LVAD implantation. We believe that, in many instances, the RV will recover to an extent that adequate cardiac output can be generated. Also, this report suggests that implantation of temporary percutaneous RVAD is a safe alternative in the treatment of postoperative acute refractory RV failure. The ease of device implantation, weaning, explantation, low need for anticoagulation, and limited number of complications justify a liberal use.
Conflict of interest: none declared.
REFERENCES
- 1.Kaul TK, Fields BL. Postoperative acute refractory right ventricular failure: incidence, pathogenesis, management and prognosis. Cardiovasc Surg. 2000;8:1–9. doi: 10.1016/s0967-2109(99)00089-7. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 3.Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: pathophysiology, clinical importance, and management. Anesth Analg. 2009;108:422–33. doi: 10.1213/ane.0b013e31818d8b92. [DOI] [PubMed] [Google Scholar]
- 4.Chen JM, Levin HR, Rose EA, Addonizio LJ, Landry DW, Sistino JJ, Michler RE, Oz MC. Experience with right ventricular assist devices for perioperative right-sided circulatory failure. Ann Thorac Surg. 1996;61:305–10. doi: 10.1016/0003-4975(95)01010-6. [DOI] [PubMed] [Google Scholar]
- 5.Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, Naka Y. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002;73:745–50. doi: 10.1016/s0003-4975(01)03406-3. [DOI] [PubMed] [Google Scholar]
- 6.Moazami N, Pasque MK, Moon MR, Herren RL, Bailey MS, Lawton JS, Damiano RJ., Jr Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004;23:1371–5. doi: 10.1016/j.healun.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Mebazaa A, Karpati P, Renaud E, Algotsson L. Acute right ventricular failure-from pathophysiology to new treatments. Intensive Care Med. 2004;30:185–96. doi: 10.1007/s00134-003-2025-3. [DOI] [PubMed] [Google Scholar]
- 8.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos R, Naftel DC, Kirklin JK, Taylor DO. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report 2005. J Heart Lung Transplant. 2005;24:1182–7. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–24. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y, Hennig F, Krabatsch T, Hetzer R. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008;27:1275–81. doi: 10.1016/j.healun.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Potapov EV, Loforte A, Weng Y, Jurmann M, Pasic M, Drews T, Loebe M, Hennig E, Krabatsch T, Koster A, Lehmkuhl HB, Hetzer R. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008;23:185–94. doi: 10.1111/j.1540-8191.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 12.Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, Horton K, Haddad F, Li DY, Renlund DG, Fisher PW. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–5. doi: 10.1016/j.amjcard.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Moreno-Cabral RJ, Stinson EB, Shinn JA, Shumway NE. Pulmonary artery balloon counterpulsation for acute right ventricular failure. J Thorac Cardiovasc Surg. 1980;80:760–3. [PubMed] [Google Scholar]
- 14.Jett GK, Siwek LG, Picone AL, Applebaum RE, Jones M. Pulmonary artery balloon counterpulsation for right ventricular failure. An experimental evaluation. J Thorac Cardiovasc Surg. 1983;86:364–72. [PubMed] [Google Scholar]
- 15.Spence PA, Weisel RD, Salerno TA. Right ventricular failure. Pathophysiology and treatment. Surg Clin North Am. 1985;65:689–97. doi: 10.1016/s0039-6109(16)43644-3. [DOI] [PubMed] [Google Scholar]
- 16.Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove 3rd DM. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001;122:92–102. doi: 10.1067/mtc.2001.114351. [DOI] [PubMed] [Google Scholar]
- 17.John R, Long JW, Massey HT, Griffith BP, Sun BC, Tector AJ, Frazier OH, Joyce LD. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg. 2011;141:932–9. doi: 10.1016/j.jtcvs.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Bhama JK, Kormos RL, Toyoda Y, Teuteberg JJ, McCurry KR, Siegenthaler MP. Clinical experience using the Levitronix CentriMag system for temporary right ventricular mechanical circulatory support. J Heart Lung Transplant. 2009;28:971–6. doi: 10.1016/j.healun.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Shuhaiber JH, Jenkins D, Berman M, Parameshwar J, Dhital K, Tsui S, Large SR. The Papworth experience with the Levitronix CentriMag ventricular assist device. J Heart Lung Transplant. 2008;27:158–64. doi: 10.1016/j.healun.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Cohn WE, Gregoric ID, La Francesca S, Frazier OH. Bedside right ventricular assist device removal in the conscious patient. Ann Thorac Surg. 2007;83:1556–7. doi: 10.1016/j.athoracsur.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Minami K, Bonkohara Y, Arusoglu L, El-Banayosy A, Körfer R. New technique for the outflow cannulation of right ventricular assist device. Ann Thorac Surg. 1999;68:1092–3. doi: 10.1016/s0003-4975(99)00789-4. [DOI] [PubMed] [Google Scholar]
- 22.Stepanenko A, Potapov EV, Krabatsch T, Hetzer R. Simple implantation of a temporary right ventricular device for right ventricular failure after left ventricular device implantation via a left lateral thoracotomy. ASAIO J. 2011;57:17–8. doi: 10.1097/MAT.0b013e318201a583. [DOI] [PubMed] [Google Scholar]
- 23.Strauch JT, Franke UF, Madershahian N, Wahlers T. Right ventricular assist device implantation – a new transcutaneous approach. Thorac Cardiovasc Surg. 2004;52:378–9. doi: 10.1055/s-2004-821321. [DOI] [PubMed] [Google Scholar]
- 24.McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical experience with 111 thoratec ventricular assist devices. Ann Thorac Surg. 1999;67:1233–8. doi: 10.1016/s0003-4975(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 25.Samuels LE, Holmes EC, Thomas MP, Entwistle 3rd JC, Morris RJ, Narula J, Wechsler AS. Management of acute cardiac failure with mechanical assist: experience with the ABIOMED BVS 5000. Ann Thorac Surg. 2001;71:67–72. doi: 10.1016/s0003-4975(00)02644-8. [DOI] [PubMed] [Google Scholar]