Abstract
OBJECTIVE
Primary leiomyoma of the respiratory tract is a rare disease. Based on our experience, we investigated its clinical characteristics and outcomes of treatment.
METHODS
Between 1997 and 2008, 16 patients in our institution (nine male, seven female) were found to have primary leiomyoma of the respiratory tract. The median patient age was 46.5 years (range 17–66 years). The tumor was located in the trachea in four patients, in the carina and main bronchus in four, in the bronchus intermedius in four, in the lobar or segmental bronchus in two, and in the lung parenchyma in two.
RESULTS
Tumor removal through bronchoscopic intervention using Nd–YAG (neodymium–yttrium–aluminum–garnet) laser cauterization was attempted in 11 patients; it failed in two with wide-based tumors. Surgical resection was performed in seven patients. Operative procedures included tracheal resection and end-to-end anastomosis (n = 3), lobectomy (n = 3), and bilobectomy (n = 1). There were no in-hospital mortalities. During a median follow-up duration of 43.2 months, one patient who underwent bronchoscopic removal had recurrence, for which segmental resection of the trachea and main bronchus with carinal reconstruction was performed.
CONCLUSIONS
Bronchoscopic intervention can offer successful control of primary leiomyoma of the main airway stem; however, in cases of a wide-based tumor, bronchoscopic intervention can result in incomplete resection or recurrence. Surgical complete resection can yield satisfactory outcomes in patients with primary leiomyoma occurring in the respiratory tract.
Keywords: Primary leiomyoma of the respiratory tract, Rigid bronchoscopy, Surgery
INTRODUCTION
Leiomyoma of the respiratory tract is the rarest type of benign pulmonary neoplasm and accounts for <2% of benign tumors of the lower respiratory tract [1]. Tracheal leiomyoma represents approximately 1% of all tracheal tumors [2].
Pulmonary leiomyoma is thought to arise from the smooth muscle of the bronchi or bronchiole and can present as either parenchymal or endobronchial lesions [3]. Symptoms of leiomyoma of the respiratory tract manifest differently according to the location of the tumor. A parenchymal tumor is usually detected incidentally without symptoms [4], whereas tracheal leiomyoma can cause critical conditions, such as bronchial asthma, exertional dyspnea, or asphyxia due to upper airway obstruction. Bronchial lesions can manifest as obstructive pneumonia and atelectasis [1].
Through a review of our experiences, we examined the clinical characteristics of leiomyoma of the respiratory tract and evaluated the outcomes of treatment to establish an optimal strategy.
PATIENTS AND METHODS
Patient enrollment
Between 1997 and 2008, 16 patients at Samsung Medical Center were found to have primary leiomyoma of the respiratory tract. The patients’ medical records provided data regarding age, gender, smoking history, past medical history, symptoms at initial presentation, tumor location and size, and early and late outcomes of treatment, including recurrence.
The Institutional Review Board of Samsung Medical Center approved this retrospective study and waived the requirement for informed consent.
Preoperative evaluations
Patients had either flexible bronchoscopy, chest computed tomography (CT), or both before therapy of curative intent. Initial flexible bronchoscopy before the curative procedure was performed under local anesthesia in 14 patients. Among these, bronchoscopic biopsy was performed in nine patients, and seven were diagnosed with primary leiomyoma. In the remaining five patients, biopsy could not be obtained because of following reasons: (1) the tumor was too hyperemic for biopsy (n = 2), (2) tumor was located in the lung parenchyma (n = 2), and (3) emergency rigid bronchoscopy was performed due to large tumor in the trachea that might cause asphyxia (n = 1). Chest CT was performed in 14 patients before curative procedures. Pre-interventional or preoperative pulmonary function was tested in 15 patients. The appropriate treatment modality was selected based on the results of these examinations.
Bronchoscopic intervention
After pre-treatment evaluation, 11 patients underwent bronchoscopic intervention by an experienced bronchoscopist using neodymium–yttrium–aluminum–garnet (Nd–YAG) laser cauterization as the initial treatment. Laser ablation of the tumor stump, after mechanical removal of the tumor by snaring or forceps using rigid bronchoscopy under general anesthesia, was preferentially performed. If the lesion was too small for mechanical removal and laser ablation, it was precisely targeted and vaporized with an Nd–YAG laser using flexible bronchoscopy. The total amount of energy delivered by the Nd–YAG laser varied from 780 J to 2760 J at a 20-W intensity in continuous pulse mode. Vacuum suction was constantly applied to evacuate ablated materials and gas. Patients were carefully monitored for adequate ventilation/oxygenation during the procedure.
Surgical resection
Different surgical and anesthesiologic strategies were used, according to the location of the lesion. For tracheal tumors and main bronchial lesions that required carinal reconstruction, the operation was performed using an anterior approach via median sternotomy in a supine position, and ventilation was secured by inserting a flexible endotracheal tube into the distal airway using a separate ventilation circuit. For tumors located at the lobar bronchus or more distal locations, the usual segmentectomy or lobectomy was planned with a lateral decubitus position. We tried to preserve the lung parenchyma as much as possible, if a sufficient resection margin could be secured.
Statistics
Descriptive statistics for categorical variables are reported as frequency and percentage, and continuous variables are reported as mean or median, as appropriate. All calculations were performed using Statistical Package for Social Sciences (SPSS) 17.0 software (SPSS Inc, Chicago, IL, USA), and a p-value of <0.05 was considered significant.
RESULTS
Each patient was designated with a case number. Patient demographics, symptoms, and tumor size and location, and treatment outcomes are shown in Table 1. The median age of the patients was 46.5 years (range 17–66 years), nine patients (56.3%) were male, and 10 (62.5%) were smokers. Cough was the most common major complaint, manifested in 10 patients (62.5%), while six patients (37.5%) had dyspnea, and four patients (25.0%) had recurrent pneumonia with radiologic features of atelectasis. Three patients presented with no symptoms. The tumor was located in the trachea in four patients (25.0%), between the carina and the main bronchus in four (25.0%), in the bronchus intermedius in four (25.0%), in the lobar or segmental bronchus in two (12.5%), and in the lung parenchyma in two (12.5%).
Table 1:
Patient demographics and tumor data
| Case no. | Gender | Age | Smoking | Past history | Symptoms and duration | Location | Size (mm) | Initial treatment modality | Treatment outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 45 | 30 py | Bronchus intermedius | 7 × 5 | Laser vaporization | Cured | ||
| 2 | M | 51 | 3.3 py ex-smoker | - | Chronic cough, sputum | Right upper lobar bronchus | 15 × 15 | Laser ablation after snaring | Cured |
| 3 | M | 43 | 33 py | Dry cough | Bronchus intermedius | 15 × 15 | Laser ablation after snaring | Cured | |
| 4 | F | 46 | 0 | Bronchus intermedius | 10 × 10 | Laser ablation after snaring | Cured | ||
| 5 | M | 17 | 0 | Chronic cough, sputum | Right main bronchus | 40 30 | Laser ablation after snaring | Cured | |
| 6 | F | 47 | 0 | - | Dyspnea on exertion, wheezing | Trachea | 10 × 10 | Laser ablation after tumor removal | Cured |
| 7 | M | 66 | 46 py ex-smoker | DM, HTN | Chronic cough, sputum, dyspnea | Bronchus intermedius | 12 × 10 | Laser ablation after tumor removal | Cured |
| 8 | F | 66 | 0 | DM, HTN, uterine myoma | Dyspnea on exertion | Left main bronchus | 10 × 10 | Laser ablation after tumor removal | Cured |
| 9 | M | 21 | 0 | - | Chronic cough, sputum | Carina to left main bronchus | 25 × 20 | Laser ablation after snaring | Recurred after 17 months Carinal resection and reconstruction |
| 10 | M | 64 | 40 py | HTN | Dyspnea on exertion, wheezing | Trachea | 22 × 16 | Laser ablation after tumor removal | Incomplete resection: Tracheal resection and end-to-end anastomosis |
| 11 | F | 38 | 0 | Old TB | Recurrent pneumonia | Rt. main | 25 × 15 | Laser ablation after snaring | Incomplete resection: Rt. lower bilobectomy |
| 12 | F | 55 | 0 | HTN, asthma | Chronic cough, dyspnea on exertion | Trachea | 50 × 35 | Tracheal resection and end-to-end anastomosis | Cured |
| 13 | M | 63 | 80 py | DM, old TB | Chronic cough, dyspnea | Trachea | 18 12 | Tracheal resection and end-to-end anastomosis | Cured |
| 14 | F | 45 | 0 | Chronic cough, sputum | Lung parenchyma (LLL) | 15 × 12 | LLL lobectomy | Cured | |
| 15 | M | 47 | 0 | Lung parenchyma (RUL) | 27 25 | VATS RU Lobectomy | Cured | ||
| 16 | F | 28 | 0 | - | Chronic cough, sputum | RLL superior segmental bronchus | 32 × 25 | VATS RLLobectomy | Cured |
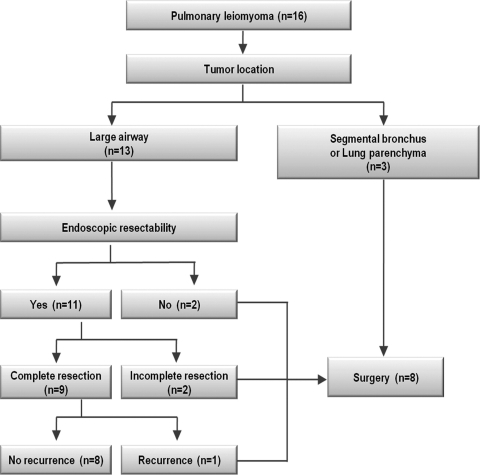
The algorithm for selection of treatment modalities is illustrated in Fig. 1. Bronchoscopic intervention was attempted in 11 patients, and complete resection was achieved in nine (cases 1–9). However, tumors with a wide base could not be resected completely, even under rigid bronchoscopy (cases 10 and 11), and subsequent surgical resection was performed in these patients. Five patients underwent surgical resection as the initial therapeutic modality. Two patients who had broad-based endotracheal tumors underwent tracheal resection and end-to-end anastomosis (cases 12 and 13). In three patients, the tumor was located at the segmental bronchus or lung parenchyma. However, these lesions were located centrally; therefore, lobectomy (including two cases of video-assisted thoracoscopic surgery (VATS) lobectomy) was performed instead of sublobar resection or bronchial sleeve resection in these patients.
Figure 1:
Algorithm for selection of treatment modalities in patients with pulmonary leiomyoma.
There was no in-hospital mortality. In one patient (case 9) who underwent tumor removal by snaring and laser ablation for a carinal tumor, a large bronchomediastinal fistula was found at the carina on the first bronchoscopic follow-up (a week after bronchoscopic intervention). A 12-mm Dumon stent (Axione, Paris, France) was inserted to obliterate the fistula. This stent was removed 40 days later via rigid bronchoscopy under general anesthesia.
Among the seven patients who underwent surgery, two had postoperative wound problems. One patient (case 10) had simple sternal-wound dehiscence and underwent delayed primary wound closure on postoperative day 21. The other patient (case 13) with known diabetes mellitus had sternal-wound infection and underwent omental flap transposition on postoperative day 24.
Chest CT was routinely performed every 6 months for at least 2 years, and yearly thereafter. Follow-up bronchoscopy was performed at least once. There was no follow-up loss over a median of 43.2 months (range 5.6–125.7 months).
One patient (case 9), who underwent bronchoscopic removal and suffered from bronchomediastinal fistula, developed a recurrence after 17 months. Segmental resection of the trachea and main bronchus with carinal reconstruction was performed without complication. There was no evidence of recurrence for 89 months after this operation.
DISCUSSION
Primary leiomyoma of the respiratory tract is a very rare disease and has been reported only as a case presentation [2,5–7], as part of a report on respiratory benign tumors [8–12], or in review articles that collected cases from multiple institutions [3,13,14]. Here, we present our clinical experience and treatment outcomes of primary leiomyoma occurring in the respiratory tract in a series of 16 patients at a single institution. Although a study population of 16 cases is not sufficient for the evaluation of tumor characteristics, biology, or statistical comparison of treatment modalities, it is one of the largest series at a single institution, to date.
Leiomyoma is predominant in the third and fifth decades of age, with a mean age of 35 years for bronchial and lung parenchymal lesions and 40.6 years for tracheal lesions [1]. In our study, the mean age of all patients was 46.25 years. The mean age for patients with bronchial and lung parenchymal lesions was 44.7 years, and the mean age for patients with tracheal and carinal lesions was 49.6 years. There was no statistically significant difference in patient age, according to the location of tumor (by Mann–Whitney test, p = 0.364). Leiomyoma of the lung parenchyma is known to occur twice as often in females as in males, whereas leiomyoma of the trachea occurs more often in males [1]. We had only two patients with lung parenchymal lesions, one of each gender. Further, we had five patients with tracheal and carinal lesions, three of whom were males. In addition, all the female patients had gynecologic examinations, which included trans-abdominal or transvaginal ultrasound within at least 2 years prior to surgery, but none had uterine leiomyoma.
Endotracheal or endobronchial leiomyoma is commonly associated with symptoms, which are manifested as a chronic cough, sputum, dyspnea, stridor, and fever due to obstructive pneumonia. Large tracheal tumors can even cause obstruction of the main airway, resulting in symptoms of asphyxia. These conditions are the main reasons for resection of this benign tumor.
The prognosis of leiomyoma is favorable if complete resection is achieved. Treatment of primary leiomyoma of the respiratory tract depends on the location, size, width of the base of the tumor, and the reversibility of the distal pulmonary change resulting from the procedure [3]. Various interventional techniques have been used to treat endotracheal or endobronchial lesions, such as Nd–YAG laser, electrocautery, and snaring. These can be performed using either fiberoptic bronchoscopy or rigid bronchoscopy [6]. For many benign tumors that are exclusively endoluminal and polypoid, total removal with these procedures is often possible [6,15], and relief of symptoms is immediate. Furthermore, loss of lung tissue can be minimized, and the early and late pains and complications related to thoracotomy or sternotomy can be avoided. In cases of tumors with a large base or tumors located in the distal airway or lung parenchyma, surgical removal of the tumor must be preferentially considered over bronchoscopic intervention to secure complete resection. In this series, bronchoscopic tumor removal failed to achieve complete resection in two cases with a wide-based tumor, and subsequent surgical resection successfully controlled the tumor. Furthermore, we experienced one case of recurrence after bronchoscopic tumor removal. To achieve as large of a safety margin as was possible, wide laser resection was performed, and post-resection pathologic examination had confirmed that the resection margin was negative for tumor cells. This patient suffered from a bronchomediastinal fistula after the procedure. However, the tumor recurred at the site of resection, and the patient underwent carinal resection and reconstruction. Although further laser resection might result in remission or effective palliation for these lesions in some patients, it can be technically difficult and related with significant complications or recurrence, and surgical resection should be considered first in such situations [16].
In conclusion, bronchoscopic intervention can offer successful control of primary leiomyoma of the main airway stem. However, in cases of a wide-based tumor, bronchoscopic intervention may result in incomplete resection or recurrence. Surgery must be preferentially considered for large tumors with a wide base and for tumors located in the distal airway or lung parenchyma to achieve complete resection.
Conflict of interest: none declared.
REFERENCES
- 1.White SH, Ibrahim NB, Forrester-Wood CP, Jeyasingham K. Leiomyomas of the lower respiratory tract. Thorax. 1985;40:306–11. doi: 10.1136/thx.40.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiyama M, Yoshino I, Shoji F, Hamatake M, Yohena T, Osoegawa A, Maehara Y. Endotracheal surgery for leiomyoma of the trachea. Ann Thorac Cardiovasc Surg. 2009;15:206–8. [PubMed] [Google Scholar]
- 3.Ayabe H, Tsuji H, Tagawa Y, Tomita M, Tsuda N, Chen J. Endobronchial leiomyoma: report of a case treated by bronchoplasty and a review of the literature. Surg Today. 1995;25:1057–60. doi: 10.1007/BF00311694. [DOI] [PubMed] [Google Scholar]
- 4.Kim YK, Kim H, Lee KS, Han J, Yi CA, Kim J, Chung MJ. Airway leiomyoma: imaging findings and histopathologic comparisons in 13 patients. AJR Am J Roentgenol. 2007;189:393–9. doi: 10.2214/AJR.07.2079. [DOI] [PubMed] [Google Scholar]
- 5.Xiaogang Z, Huasheng W, Xingtao J. Carinal leiomyoma: report of a case treated by carinal resection and reconstruction. Thorac Cardiovasc Surg. 2001;49:235–7. doi: 10.1055/s-2001-16106. [DOI] [PubMed] [Google Scholar]
- 6.Tamura M, Murata T, Kurumaya H, Ohta Y. Leiomyoma of an accessory tracheal bronchus. Ann Thorac Surg. 2004;78:2163–5. doi: 10.1016/S0003-4975(03)01500-5. [DOI] [PubMed] [Google Scholar]
- 7.Ozcelik U, Kotiloglu E, Gocmen A, Senocak ME, Kiper N. Endobronchial leiomyoma: a case report. Thorax. 1995;50:101–2. doi: 10.1136/thx.50.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn Y, Chang H, Lim YS, Hah JH, Kwon TK, Sung MW, Kim KH. Primary tracheal tumors: review of 37 cases. J Thorac Oncol. 2009;4:635–8. doi: 10.1097/JTO.0b013e31819d18f9. [DOI] [PubMed] [Google Scholar]
- 9.Gaissert HA, Grillo HC, Shadmehr MB, Wright CD, Gokhale M, Wain JC, Mathisen DJ. Uncommon primary tracheal tumors. Ann Thorac Surg. 2006;82:268–72. doi: 10.1016/j.athoracsur.2006.01.065. discussion 272–3. [DOI] [PubMed] [Google Scholar]
- 10.Scott KJ, Greinwald JH, Jr., Darrow D, Smith RJ. Endobronchial tumors in children: an uncommon clinical entity. Ann Otol Rhinol Laryngol. 2001;110:63–9. doi: 10.1177/000348940111000112. [DOI] [PubMed] [Google Scholar]
- 11.Refaely Y, Weissberg D. Surgical management of tracheal tumors. Ann Thorac Surg. 1997;64:1429–32. doi: 10.1016/S0003-4975(97)00818-7. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 12.Shah H, Garbe L, Nussbaum E, Dumon JF, Chiodera PL, Cavaliere S. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest. 1995;107:1744–51. doi: 10.1378/chest.107.6.1744. [DOI] [PubMed] [Google Scholar]
- 13.Vera-Roman JM, Sobonya RE, Gomez-Garcia JL, Sanz-Bondia JR, Paris-Romeu F. Leiomyoma of the lung. Literature review and case report. Cancer. 1983;52:936–41. doi: 10.1002/1097-0142(19830901)52:5<936::aid-cncr2820520533>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Orlowski TM, Stasiak K, Kolodziej J. Leiomyoma of the lung. J Thorac Cardiovasc Surg. 1978;76:257–61. [PubMed] [Google Scholar]
- 15.Laksanabunsong P, Wongbunnate S, Charoenratanakul S. Leiomyomas of the lower respiratory tract: a case report. J Med Assoc Thai. 1993;76:465–9. [PubMed] [Google Scholar]
- 16.Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7:83–91. doi: 10.1016/S1470-2045(05)70541-6. [DOI] [PubMed] [Google Scholar]