Abstract
OBJECTIVE
Remodeling of the left ventricle (LV) in ischemic cardiomyopathy frequently leads to functional mitral regurgitation (MR). The indication for correcting MR in patients undergoing LV reconstruction (LVR) is unclear. In this study, we evaluated our strategy of correcting MR ≥ grade 2+ by restrictive mitral annuloplasty (RMA) during LVR.
METHODS
We studied 92 consecutive patients (76 men, mean age 61 ± 10 years) who underwent LVR for ischemic heart failure (IHF). RMA was performed in all patients with MR ≥ grade 2+ on preoperative echocardiography and in patients who showed increased MR to ≥grade 2+ immediately after LVR. Patients were attributed to a RMA and no-RMA group, depending on whether or not concomitant RMA had been performed. Mean clinical and structured echocardiographic follow-up was 47 ± 20 months and was 100% complete.
RESULTS
In 38 out of 40 patients (95%) with preoperative MR ≥ grade 2+, concomitant RMA was planned and performed. In 17 out of 52 patients (33%) with MR < grade 2+ preoperatively, MR increased after LVR to ≥grade 2+ leading to additional RMA during a second period of aortic cross-clamping. Early mortality in the RMA group (n = 55) was 12.7% and survival at 36 months 78.2 ± 11.2%. Early mortality in the no-RMA group (n = 37) was 5.4% and survival at 36 months 81.1 ± 12.8%. Patients in the RMA group had significantly more reduced LV function with greater LV dimensions and volumes preoperatively. Echocardiography demonstrated sustained improvement in LVEF with reduction of LV volumes in both patient groups. Recurrence of MR at late follow-up was observed in 2 patients (1 patient per group).
CONCLUSIONS
Patients with IHF eligible for LV reconstruction have MR ≥ grade 2+ in 44% of cases. In one-third of IHF patients with MR < grade 2+ preoperatively, MR increases to ≥grade 2+ after LVR. Concomitant mitral valve repair for MR ≥ grade 2+, on either preoperative echocardiography or immediately after LVR, results in favorable late clinical and echocardiographic outcome that proved to be similar to patients without concomitant mitral valve repair, despite more advanced disease.
Keywords: Left ventricular reconstruction (LVR), Dor procedure, Mitral regurgitation, Restrictive mitral annuloplasty (RMA), Ischemic heart failure (IHF)
INTRODUCTION
Remodeling of the left ventricle (LV) in ischemic cardiomyopathy leads to systolic and diastolic dysfunction, and frequently to functional mitral regurgitation (MR) as a secondary phenomenon [1–5]. Surgical ventricular restoration or left ventricular reconstruction (LVR) restores LV shape, reduces LV volume, and improves pump function in patients with ischemic cardiomyopathy [6,7]. The impact of LVR on MR – both early and late – is unclear, as is the indication for concomitant correction of the MR during LVR. Our management of MR in patients undergoing LVR encompasses performing a restrictive mitral annuloplasty (RMA) when MR ≥ grade 2+, established either preoperatively or immediately post-LVR. In this study, we evaluated the results of this strategy in patients with ischemic heart failure (IHF), who underwent LVR, with or without concomitant RMA, with a focus on late clinical and echocardiographic outcome.
MATERIALS AND METHODS
Ninety-two consecutive patients with ischemic cardiomyopathy and heart failure (NYHA class III or IV and LV ejection fraction ≤ 35%) underwent LVR between April 2002 and April 2007. Patients were considered eligible for LV reconstructive surgery when they had LV dilatation following an antero-septal myocardial infarction with an echocardiographically derived Wall Motion Score Index (WMSI) ≤ 2.5, or with evidence of contractile reserve when WMSI exceeded 2.5, as described earlier [8]. Patients were attributed to an RMA and no-RMA group, depending on whether or not concomitant RMA had been performed.
Patient characteristics
There were 76 men and mean age was 61 ± 10 years. All patients presented with IHF, 76 patients (83%) were in NYHA class III. Mean LVEF was 25 ± 7% (range 12–35%). Median interval after myocardial infarction was 36 months (range 1–360). Logistic EuroSCORE averaged 10 (range 3–42). All patients underwent elective surgery. Preoperative moderate to severe (≥grade 2+) MR was present in 40 patients (43.7%) on transthoracic echocardiography (TTE). Patient characteristics are summarized in Table 1.
Table 1:
Preoperative patient characteristics (n = 92)
| RMA group (n = 55) | No-RMA group (n = 37) | |
|---|---|---|
| Age (years) (mean ± SD) | 60 ± 9 | 62 ± 11 |
| Gender, male/female (n) | 44/11 | 32/5 |
| Median interval after infarction (months, range) | 48 (1–228) | 84 (2–360) |
| ≤3months (n, %) | 4 (7.3%) | 1 (2.7%) |
| > 3 months (n, %) | 51 (92.7%) | 36 (97.3%) |
| No. of coronary vessels with stenosis of >70% (n, %) | ||
| One | 28 (50.9%) | 14 (37.8%) |
| Two | 18 (32.7%) | 13 (35.1%) |
| Three | 9 (16.4%) | 10 (27.0%) |
| Previous cardiac surgery (n, %) | 2 (3.6%) | 4 (10.8%) |
| Renal insufficiency (n, %) | 1 (1.8%) | 2 (5.4%) |
| Severe pulmonary hypertension (n, %) | 10 (18.2%) | 0 |
| Logistic EuroSCORE (mean ± SD) | 10 ± 10 | 9 ± 9 |
| NYHA class (mean ± SD) | 3.2 ± 0.4 | 3.1 ± 0.3 |
| III (n, %) | 44 (80%) | 32 (86.5%) |
| IV (n, %) | 11 (20%) | 5 (13.5%) |
| V02max (ml kg−1 min− 1, mean ± SD) | 16 ± 4 | 19 ± 6 |
| Clinical ventricular tachyarrhythmia (VT) (n, %) | 9 (16.4%) | 4 (10.8%) |
| Preoperative (biventricular) ICD implantation (n, %) | 14 (25.5%) | 7 (18.9%) |
NYHA: New York Heart Association; VT: ventricular tachyarrhythmia; ICD, implantable cardioverter defibrillator.
Preoperative echocardiography
A transthoracic echocardiogram was performed within 5 days prior to surgery. When significant mitral and/or tricuspid regurgitation was demonstrated on TTE, transesophageal echocardiography (TEE) was additionally performed to further evaluate the severity and mechanism of the regurgitation. The severity of mitral and/or tricuspid regurgitation was graded semi-quantitatively from color-flow Doppler acquisitions in the conventional parasternal long-axis and apical four-chamber images. Mitral and tricuspid regurgitation was characterized as: mild, 1+ (jet area/left or right atrial area <10%); moderate, 2+ (jet area/left or right atrial area 10–20%); moderately severe, 3+ (jet area/left or right atrial area 20–45%); and severe, 4+ (jet area/left or right atrial area >45%). LV volumes and LV ejection fraction were calculated from conventional apical two- and four-chamber images, using the biplane Simpson’s technique. LV dimensions (end-systolic and end-diastolic) were determined from parasternal M-mode acquisitions. Echocardiographically derived WMSI was used to evaluate LV function. As recommended by the American Society for Echocardiography, a 16-segment model was used for left ventricular segmentation [11]. WMSI was derived as the sum of all wall motion scores divided by the number of segments visualized.
Surgical technique
The surgical technique was described earlier [8]. In summary, all operations were performed using normothermic cardiopulmonary bypass, aortic cross-clamping, and intermittent antegrade warm-blood cardioplegia. LVR was carried out according to Dor using a shaping Fontan-stitch at the transitional zone between viable and scarred myocardium. Sizing of the residual ventricle was done using a saline-filled balloon or commercially available shaper (TRISVR, Chase Medical, Richardson, TX, USA) using a reference LV size of 55 ml m−2 body surface area as described by Menicanti et al. [9]. An endoventricular oval Dacron patch was used to close the residual opening after tightening the Fontan stitch around the balloon. To facilitate the creation of a neo-apex, one or two u-shaped stitches were placed in the inferior wall in patients with a ‘wrap-around’ left anterior descending coronary artery (11–15% of patients) [10]. Concomitant myocardial revascularization was performed whenever indicated, preferentially using all arterial grafts (single or bilateral mammary arteries) in patients ≤70 years of age. A concomitant tricuspid annuloplasty was performed using an MC3-ring (Edwards Lifesciences, Irvine, CA, USA) in patients with significant tricuspid regurgitation (>grade 2+) or when the tricuspid annular diameter exceeded 40 mm on TTE. In patients with documented preoperative ventricular arrhythmias, a cryo-ablation at the border zone between scar tissue and viable myocardium was performed. Since 2006 implantation of an epicardial LV lead for resynchronisation therapy formed a routine part of the procedure. After termination of extracorporeal circulation, TEE was repeated to assess LV shape and function. Mitral and tricuspid valve competency were assessed; transmitral diastolic gradient and length of coaptation of the mitral valve leaflets were measured. A summary of the surgical data is provided in Table 2.
Table 2:
Surgical data (n = 92)
| RMA group (n = 55) | No-RMA group (n = 37) | |
|---|---|---|
| LVR with patch (n, %) | 53 (96.4%) | 36 (97.3%) |
| Patch size (cm2) (mean ± SD) | 13 ± 7 | 12 ± 8 |
| Inferior wall plication (n, %) | 8 (14.5%) | 4 (10.8%) |
| Balloon/shaper size (ml) (mean ± SD) | 109 ± 13 | 110 ± 11 |
| Mitral valve annuloplasty (n, %) | 55 (100%) | 0 |
| Median ring size (range) | 26 (24–32) | – |
| Tricuspid valve annuloplasty (n, %) | 20 (36.4%) | 0 |
| Median ring size (range) | 28 (26–38) | – |
| CABG (n, %) | 32 (58.2%) | 26 (70.3%) |
| No. of distal anastomoses/patient (mean ± SD) | 2 ± 1 | 3 ± 1 |
| Use of bypass grafts | ||
| LIMA only (n, %) | 13 (40.6%) | 4 (15.4%) |
| RIMA only (n, %) | 0 | 2 (7.7%) |
| BIMA (n, %) | 7 (21.9%) | 7 (26.9%) |
| LIMA + vein (n, %) | 8 (25%) | 7 (26.9%) |
| Vein only (n, %) | 4 (12.5%) | 6 (23.1%) |
| Cryo-ablation (n, %) | 5 (9.1%) | 7 (18.9%) |
| Epicardial LV-lead (n, %) | 15 (27.3%) | 9 (24.3%) |
| ECC time (min.) (mean ± SD) | 220 ± 57 | 174 ± 56 |
| Aortic cross-clamping time (min) (mean ± SD) | 150 ± 48 | 122 ± 31 |
| IABP (n, %) | 18 (32.7%) | 2 (5.4%) |
LVR: left ventricular restoration; CABG: coronary artery bypass grafting; LIMA: left internal mammary artery; RIMA: right internal mammary artery; BIMA: bilateral internal mammary artery; LV: left ventricle; ECC: extra corporeal circulation; IABP: intra aortic balloon pump.
Management of MR
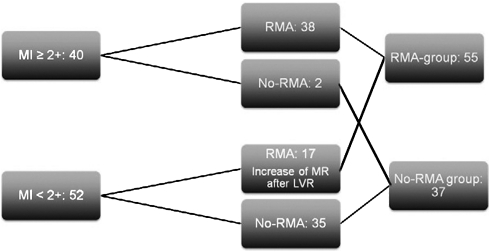
Our management of MR during LVR encompassed performing RMA in all patients with MR ≥ grade 2+ on preoperative echocardiography and in patients who showed increase of MR to ≥grade 2+ on intraoperative TEE, as routinely performed immediately after LVR after discontinuation of extracorporeal circulation. In these latter patients, additional RMA was performed during a second period of aortic cross-clamping. RMA was performed by transseptal approach with downsizing using a semi-rigid ring (Carpentier Edwards Physio Ring, Edwards Lifesciences, Irvine, CA, USA). For further analysis, patients were attributed to either the RMA group or the no-RMA group based on the procedure performed. A flowchart demonstrating MR management in all patients is shown in Fig. 1.
Figure 1:
Management chart of MR during LVR. MR: mitral regurgitation; RMA: restrictive mitral annuloplasty; no-RMA: no restrictive mitral annuloplasty; LVR: left ventricular restoration.
Clinical and echocardiographic follow-up
Patients were maintained on optimal medical treatment for heart failure after surgery. Functional status was assessed using the NYHA classification for symptoms of heart failure. An independent physician at the outpatient clinic evaluated the symptoms before surgery and at annual follow-up. Serial transthoracic echocardiograms were performed after surgery, starting just prior to hospital discharge and followed by annual examinations at the outpatient clinic. From these examinations, LV ejection fraction, LV dimensions and volumes, presence of MR, and transmitral diastolic gradient were assessed.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). Categorical variables are described as frequencies and percentages and compared using the chi-square test with Yates’ correction. Continuous data are expressed as mean ± standard deviation (SD) or median with ranges and compared using the Student’s t-test for paired data. The Kaplan–Meier method was used to model survival. Survival between two groups was compared by the Mantel–Cox log rank test. A P-value <0.05 was considered significant.
RESULTS
Intraoperative management of MR
Preoperative TTE demonstrated MR ≥ grade 2+ in 40 patients. In 38 patients (95%), concomitant RMA was performed. RMA was not performed in two patients, because of a completely calcified posterior mitral annulus in one patient and a complicated procedure in another patient, making additional mitral surgery inappropriate. Fifty-two patients had preoperative MR < grade 2+.
Eight patients had no MR preoperatively; in these patients MR did not appear after LVR. A total of 17 patients with MR grade 1+ on preoperative examination showed increasing MR to ≥grade 2+ immediately after LVR and underwent subsequent RMA. In the remaining 35 patients, MR stayed < grade 2+ immediately after LVR. The flowchart of MR management is shown in Fig. 1.
None of the patients had primary organic valvular disease; in all patients the mechanism underlying MR was systolic restriction of both leaflets with annular dilatation. Median RMA ring size was 26 (range 24–32). Apart from the patient with the accepted MR grade 2+, intraoperative TEE demonstrated absent or mild MR in all patients. In patients who had undergone concomitant RMA, mean length of leaflet coaptation after mitral valve repair was 8 ± 2 mm and mean transmitral diastolic gradient was 2.9 ± 1.7 mmHg.
Comparison of baseline echocardiographic characteristics between RMA and no-RMA group
Based on above-mentioned criteria for mitral valve repair, 55 patients were attributed to the RMA group and 37 to the no-RMA group. Comparing preoperative TTE data, WMSI in the RMA group proved to be significantly higher than in the no-RMA-group (2.6 ± 0.5 vs 2.3 ± 0.5, P < 0.01), indicating more and/or more severe regional LV wall-motion abnormalities and hence an overall greater deterioration of LV function. In addition, LV volumes and dimensions were significantly larger in the RMA group (P < 0.01 for left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD)). These data are summarized in Table 3.
Table 3:
Transthoracic echocardiographic data
| Baseline |
Early postop. |
P-value baseline versus early postop. |
1-year FU |
P-value baseline versus 1 -year FU |
2-year FU |
P-value baseline versus 2-year FU |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | |
| EF (%) | 24 ± 7 | 27 ± 7 | 35 ± 8 | 39 ± 11 | <0.01 | <0.01 | 33 ± 12 | 39 ± 9 | <0.01 | <0.01 | 30 ± 10 | 40 ± 6 | <0.01 | <0.01 |
| LVESV (ml) | 190 ± 88 | 146 ± 61 | 99 ± 36 | 87 ± 39 | <0.1 | <0.01 | 109 ± 55 | 95 ± 29 | <0.01 | <0.01 | 116 ± 41 | 87 ± 28 | <0.01 | <0.01 |
| LVEDV (ml) | 249 ± 96 | 196 ± 72 | 150 ± 47 | 136 ± 43 | <0.01 | <0.01 | 155 ± 56 | 151 ± 34 | <0.01 | 0.01 | 167 ± 45 | 144 ± 38 | <0.01 | <0.01 |
| LVESD (cm) | 5.3 ± 1.1 | 4.8 ± 1.1 | 5.0 ± 0.9 | 4.3 ± 1.0 | NS | 0.03 | 4.9 ± 1.1 | 4.7 ± 0.8 | 0.11 | NS | 4.9 ± 1.1 | 4.7 ± 0.8 | 0.21 | NS |
| LVEDD (cm) | 6.8 ± 0.9 | 6.1 ± 0.9 | 6.2 ± 0.9 | 5.7 ± 0.8 | <0.01 | <0.01 | 6.2 ± 0.9 | 6.0 ± 0.5 | <0.01 | NS | 6.1 ± 1.1 | 6.2 ± 0.5 | 0.01 | NS |
| WMSI | 2.6 ± 0.5 | 2.3 ± 0.5 | ||||||||||||
EF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; WMSI: wall motion score index; postop.: postoperative; FU: follow-up; SD: standard deviation; NS: not significant.
Early outcome
In-hospital mortality in the RMA group and no-RMA group was 12.7% (seven patients) and 5.4% (two patients), respectively. Causes of death in the RMA group were refractory heart failure in four patients (one following postoperative myocardial infarction), sepsis in two patients and acute respiratory distress syndrome (ARDS) in one patient. Patients in the RMA group who had MR < grade 2+ preoperatively (but who showed an increase of MR directly after LVR), early mortality was 5.8% (one patient). In this patient, the cause of death (sepsis) was unrelated to the concomitant mitral valve procedure. Postoperative inotropic support (inotropic support continued for ≥12 h postoperatively) was required in all patients – 18 patients (32.7%) also required intraaortic balloon counterpulsation (IABP) support. Four patients in this group required temporary postoperative hemodialysis. One patient developed an ischemic cerebral infarction. Mean postoperative stay in the intensive-care unit was 8 ± 9 days. Mean postoperative hospital stay was 18 ± 14 days.
Both patients in the no-RMA group died of heart failure. Postoperative inotropic support was also required in all patients in the no-RMA group – two patients (5.4%) required support by additional IABP. Mean postoperative stay in the intensive-care unit was 5 ± 7 days. Mean postoperative hospital stay was 15 ± 10 days.
TTE performed just prior to hospital discharge demonstrated absent or mild MR (grade 0 or 1+) in all patients in both patient groups. Serial results of echocardiographic examination of the mitral valve are presented in Table 4. Early postoperatively a significant improvement in LVEF occurred in both patient groups. In the RMA group, LVEF increased from 24 ± 7% to 35 ± 8% (P < 0.01). In the no-RMA group, LVEF improved from 27 ± 7% to 39 ± 11% (P < 0.01). In both groups a reduction in LV volumes was observed: LVESV decreased in the RMA group from 190 ± 88 ml to 99 ± 36 ml (P < 0.01), whereas LVEDV decreased from 249 ± 96 ml to 150 ± 47 ml (P < 0.01). In the no-RMA group, LVESV decreased from 146 ± 61 ml to 87 ± 39 ml (P < 0.01) and LVEDV decreased from 196 ± 72 ml to 136 ± 43 ml (P < 0.01). Results are summarized in Table 3.
Table 4:
Parameters of mitral valve function
| Baseline TTE |
Intraoperative TEE |
Early postoperative TTE |
Late follow-up TTE |
|||||
|---|---|---|---|---|---|---|---|---|
| RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | RMA group | No-RMA group | |
| MR (grade) | 2.2 ± 1.0 | 0.9 ± 0.6 | 0.0 ± 0.1 | 0.6 ± 0.6 | 0.3 ± 0.4 | 0.5 ± 0.5 | 0.5 ± 0.6 | 0.8 ± 0.6 |
| Coaptation (mm) | – | – | 8 ± 2 | – | – | – | – | – |
| Transmitral grade (mmHg) | – | – | 2.9 ± 1.7 | – | 5.3 ± 3.3 | – | 3.7 ± 6.5 | – |
| MR (n) | ||||||||
| Grade 0 | 1 | 8 | 54 | 18 | 35 | 18 | 19 | 7 |
| Grade 1 + | 16 | 27 | 1 | 18 | 13 | 17 | 17 | 22 |
| Grade 2 + | 17 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Grade 3 + | 15 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Grade 4 + | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
RMA: restrictive mitral annuloplasty; MR: mitral regurgitation; TEE: transesophageal echocardiogram; TTE: transthoracic echocardiogram; Transmitral grade, mean diastolic transmitral gradient.
Late outcome
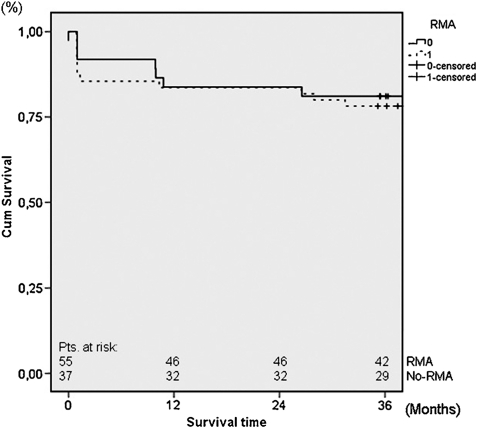
Follow-up extended to 94 months (mean 47 ± 20). Crude late mortality at 36 months in the RMA and no-RMA groups was 10.4% (five patients) and 14.3% (five patients), respectively. Overall Kaplan–Meier estimated survival at 36 months follow-up was 78.2% ± 11.2% in the RMA group and 81.1% ± 12.8% in the no-RMA group (Fig. 2). Comparing survival at 36 months between the RMA and no-RMA groups showed no significant difference (log rank P = 0.247).
Figure 2:
Thirty-six months survival in patients with and patients without concomitant RMA. RMA: restrictive mitral annuloplasty; no-RMA: no restrictive mitral annuloplasty.
Significant functional improvement was observed at late follow-up in both RMA and no-RMA groups with respectively 31 patients (83.8% of surviving patients) and 27 patients (90% of surviving patients in NYHA class I or II). Mean NYHA class decreased at late follow-up from 3.2 ± 0.4 preoperatively to 1.8 ± 0.9 (P < 0.01) and from 3.1 ± 0.3 preoperatively to 1.7 ± 0.8 (P < 0.01) in the RMA and no-RMA groups, respectively.
Echocardiography demonstrated a sustained improvement in LVEF with reduction of LV volumes in both patient groups at 1- and 2-year follow-up (Table 3). At late follow-up, recurrence of MR (≥grade 2+) was observed only in one patient in both groups (Table 4). The patient in the RMA group was functionally in NYHA class III and showed grade 2+ recurrent MR due to systolic restriction of both leaflets with limited coaptation. The patient in the no-RMA group was in NYHA functional class II and showed grade 3+ recurrent MR (with severe pulmonary hypertension) due to progressive tethering of the mitral valve leaflets with systolic restriction on TTE. LV volumes and dimensions in this patient were still smaller than preoperatively, but showed slight progression after the initial surgically induced reduction. Preoperatively, this patient had MR grade 1+ which remained stable after LVR. At discharge MR was still grade 1+. Despite increased dosages of diuretics and ace inhibitors, MR remained stable grade 3+ at late follow-up.
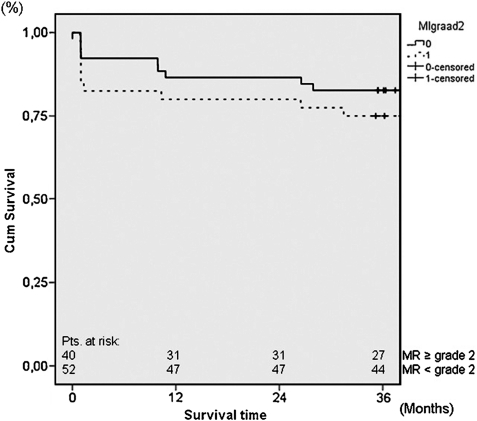
Survival analysis was also performed comparing 36 months survival between patients with preoperative MR ≥ grade 2+ and patients with preoperative MR < grade 2+ and demonstrated no significant difference between the two groups. Thirty-six months survival was 75.0 ± 13.6% and 82.7 ± 10.4% in patients with preoperative MR ≥ grade 2+ and patients with preoperative MR < grade 2+, respectively (log rank P = 0.628) (Fig. 3).
Figure 3:
Thirty-six months survival in patients with and patients without preoperative MR ≥ grade 2+. MR: mitral regurgitation.
DISCUSSION
Functional MR in patients with ischemic cardiomyopathy is a secondary phenomenon caused by remodeling of the LV [1–5]. MR is related to LV dilatation and is caused by geometrical changes at the annular, subannular, and ventricular level. Annular dilatation, increased distance between annulus and papillary muscles, and increased distance between the papillary muscles alter and reduce coaptation of the mitral valve leaflets [12]. MR leads to volume overload that promotes further LV remodeling and carries an excess mortality in post-infarction patients, which is unrelated to the underlying degree of LV dysfunction [13–16]. The presence of MR has been shown to be an independent marker of excess mortality, even when the potential artificial increase in LVEF was taken into account. LVR restores LV shape, reduces LV volume, and improves pump function in patients with ischemic cardiomyopathy [6,7]. Correcting functional MR by RMA results in excellent and durable results, as we have published before [23].
The impact of LVR on MR, both immediately and during longer follow-up, remains unclear, as is the indication for concomitant correction of MR during LVR. On the one hand, immediate decrease of LV volumes and diameters, with the reduction of the distances between annulus and papillary muscle and between the papillary muscles, can lead to improved mitral valve leaflet coaptation [12,18]. Reduction of wall stress by the decrease in LV volumes and dimensions contributes to improvement in ventricular and papillary muscle function [9]. On the other hand, it is possible that LVR leads to a distortion of the geometry of the LV and subvalvular apparatus, causing an increase in MR. Moreover, possible further LV remodeling over time with gradual increase of LV volumes and diameters might lead to the appearance or recurrence of MR at midterm follow-up if MR is left untreated [9].
There is little debate to treat functional MR when it is moderate–severe or severe (MR grade 3+ or 4+). However, there is no consensus on how to treat mild or moderate MR (MR grade 1+ and 2+). Di Donato et al. propose to leave MR grade 2+ untreated. They demonstrated an excellent survival; however, a substantial percentage of patients (29%) was found to have at least a moderate degree of MR (grade 2+) at follow-up [18]. Prucz et al. demonstrated an overall reduction in MR grade with good functional results and excellent survival in a group of patients who underwent LVR with untreated moderate MR. However, 76% of the patients still had MR > grade 2+ at follow-up [12]. As such, a conservative approach to functional MR grade 2+ will leave a significant proportion of patients at risk for the potentially deleterious effects of MR, which are further LV remodeling and increased mortality. As has been demonstrated, a moderate degree of MR proves to be of hemodynamic importance in patients with reduced LV function and imposes significant clinical implications in post-infarction patients, even in those with minimal symptoms [15,25]. In the setting of ischemic MR, even a regurgitant volume as little as 30 ml is associated with a limited 5-year survival of 47%.
A conservative approach to functional MR grade 2+ might be related to the idea of an increased perioperative mortality caused by the additional intervention on the valve. In our study, perioperative mortality and morbidity were indeed higher in the RMA group, but it should be noted that patients in that group had more advanced disease, as demonstrated by the higher preoperative WMSI (more wall-motion abnormalities) and larger LV volumes and dimensions. MR should be regarded as the result of ongoing LV remodeling, and the increased perioperative risk should be interpreted against that background and, in addition, be weighed against the increased complication rate at longer follow-up associated with untreated MR. It has also been shown by others that concomitant mitral annuloplasty does not add by itself to the risk of the operation [9,20].
Aggressive correction of MR ≥ grade 2+ by RMA during LVR results in excellent functional improvement, favorable 36 months survival, and very low recurrence of MR. Moreover, elimination of MR leads to a similar functional improvement and equal survival comparing patients with and without preoperative MR ≥ grade 2+ (mean NYHA class at late follow-up 1.8 ± 0.9 and 1.7 ± 0.8 in the RMA and no-RMA groups, respectively, P = NS; 3-year survival 78.2% vs 80.7%, P = NS). This comparable outcome occurs despite the fact that patients with MR ≥ grade 2+ undergoing LVR have a more severely damaged LV, as also reflected by the higher early mortality and more frequent need of IABP support. Similar results were found by Athanasuleas and the RESTORE group, who demonstrated an increased 30-day mortality by twofold from 4% to 8.7%, but the 5-year survival after LVR was not influenced [7,21]. In our previously published meta-analysis, we found however that concomitant mitral valve surgery was associated with both an increased risk for early (RR = 1.57, P = 0.001) and late mortality (RR = 4.28, P < 0.001) [22]. The discrepancy in late outcome may be explained by the fact that concomitant mitral valve surgery – in the studies that were entered into the meta-analysis – comprises both mitral valve repairs and replacements. Mitral valve repair is associated with a better survival than mitral valve replacement (especially without preservation of the subvalvular apparatus) because of better preservation of ventricular contraction and fewer complications related to prosthetic deterioration, malfunction, or hypocoagulation [24]. Moreover, patient selection, surgical techniques (myocardial protection), and peri-operative management have improved over time.
LV reverse remodeling in IHF is also influenced by myocardial revascularization. Revascularization of viable but dysfunctional myocardium because of ischemia may resolve functional MR; however, this has proved to be very unpredictable [19]. The recently published STICH-trial, reporting over 1000 patients, randomized for either coronary artery bypass grafting (CABG, n = 501) and CABG and LVR (n = 499), did not demonstrate any benefit of LVR over CABG [17]. Since patients with severe post-infarction heart failure were not included in this trial (only 49% of patients were in NYHA class III or IV), and patients who would clearly benefit from LVR were not randomized, we do not consider that study representative for the patients evaluated in the current study. Moreover, both the reduction in LV volume (19% in the STICH-trial vs 60–69% (LVEDV) in our study) and the type of LV reconstruction (in 59% of the LVR patients in the STICH-trial, an endoventricular patch was used compared to 96–97% of the patients in this study) were different. Finally, it should be noted that in our study 42% of the patients in RMA group did not have coronary vessels suitable for revascularization and thus could not benefit from revascularization alone.
As published by our group recently, the recurrence rate of MR in patients who underwent RMA for MR ≥ grade 2+ in ischemic and non-ischemic cardiomyopathy and heart failure was 19% at a mean follow-up of 2.6 year [16]. These patients had similarly dilated LVs and reduced LVEF as the patients in the current study. The combination of reduction in LV volumes and reduction in wall stress by LVR with RMA probably contributed to the low recurrence rate of MR in these patients.
The long-term clinical and echocardiographic results of this study support our strategy of managing MR in patients undergoing LVR: when MR is absent preoperatively, neither appearance of MR directly after LVR or at late follow-up is observed. Rightfully, no concomitant RMA is performed in these patients. In patients with preoperatively MR ≥ grade 2+ and in patients showing increase of MR ≥ grade 2+ immediately after LVR, concomitant RMA is performed with excellent functional improvement, favorable 36 months survival, and very low recurrence of MR. In patients with MR < 2+ after LVR, concomitant RMA is not performed, which is justified by the low occurrence rate of MR at late follow-up.
CONCLUSIONS
Patients with IHF eligible for LV reconstruction have MR ≥ grade 2+ in 44% of cases. In one-third of IHF patients with MR < grade 2+ preoperatively, MR increases to ≥grade 2+ after LVR. Concomitant mitral valve repair for MR ≥ grade 2+, on either preoperative echocardiography or immediately after LVR, results in favorable late clinical and echocardiographic outcome that proved to be similar to patients without concomitant mitral valve repair, despite more advanced disease.
LIMITATIONS
Although the present study includes a relatively large sample size, more patients need to be studied to confirm the current results. Also, longer follow-up data are needed to evaluate the long-term results. Possibly, in some patients MR would have decreased after LVR and CABG alone. Our proven strategy of treating functional or ischemic MR ≥ grade 2+ by RMA, however, precludes any comments on this potential effect.
Conflict of interest: none declared.
APPENDIX A. CONFERENCE DISCUSSION
Dr L. Menicanti (Milan, Italy): This paper deals with a very tough group of patients with mitral regurgitation after an acute myocardial infarction, low ejection fraction, and a large left ventricle, the type of patient that presents a very high mortality in all published series. The results you reported are different in some way, and you report the same survival in the two groups of patients with and without mitral regurgitation before the procedure. So it seems that with your techniques, you put a zero on the impact of the bad ventricle that is normally present with mitral regurgitation. I have two questions for you.
You have an incredibly low rate of recurrence of mitral regurgitation, around 2%, and I would like to ask if you have the same recurrence in the patients with mitral regurgitation that are treated, irrespective of the cause, ischemic or not, with the same dilatation of the ventricle?
Dr Klein: In a recently published paper in JACC in August of this year, we showed that the predictors of recurrence of MR in patients with ischemic and non-ischemic cardiomyopathy at 2.6 years is around 19%. So probably the left ventricular reconstruction combined with restrictive mitral annuloplasty, by the reduction of left ventricular volumes and reduction in wall stress, is the cause of the low recurrence rate of MR.
Dr Menicanti: And the other thing, in your manuscript you described a group of patients in whom, after the procedures, some degree of mitral regurgitation is still present, and in this group of patients you went back onto extracorporeal circulation and you corrected the mitral regurg. So I would like to ask you if this group of patients presents a more difficult postoperative period, higher mortality? How is it in the follow-up period?
Dr Klein: Mortality in this group of 17 patients is only one patient. He died of a sepsis in the ICU. So it is a low mortality of 5.4%. And both functional improvement and follow-up are essentially the same as in the other group of patients. So concomitant restrictive mitral annuloplasty in this patient group did not add to the surgical risk and did not pose a risk of reduced survival.
Dr Menicanti: Because we are always afraid to go back onto extracorporeal circulation with this type of patient, but it seems that there is no danger at all.
Dr Klein: We need a little bit more balloon pumping, of course, in these patients, but functional class improvement is the same, survival is the same, and mortality is low.
Dr M. Deja (Katowice, Poland): Your paper is very interesting, and I absolutely agree with the results you are presenting. I have, however, two questions to ask. Your group, and Professor Dion in particular, was always teaching that you should never assess mitral regurgitation while under anesthesia in the operating theatre. So how are you judging when it is appropriate to go back and do a repair on the patient that you actually did SVR on a minute ago? That is the first question.
And the other is less a question and more a remark. Although I agree with the results you are showing and I believe they are true, some kind of control group is missing. You are just making the assumption that if they both fail the same way, you improved something. Maybe if you did nothing they would fail the same way, too.
Dr Klein: Interesting questions. Answering your first question, we come off bypass and then we wait for a while to let the ventricle improve or resume its function and then we evaluate. In anesthesia you can underestimate but you cannot overestimate the degree of MR if the ventricle is performing well at the time. So we wait a while and then we assess the function.
Dr Deja: Do you perform any kind of loading or anything like this?
Dr Klein: Not after the reconstruction, no. And to answer your second question, you are right, of course, there is no control group, but our previous results in both ischemic and non-ischemic patients demonstrating the efficiency of restrictive mitral annuloplasty made it standard practice in our hospital. So we performed restrictive mitral annuloplasty in this group of patients. But of course you are right, I cannot draw any conclusions as to whether the MR has decreased in a certain small group of patients.
Dr S. Bolling (Ann Arbor, MI): I have a question for you to reflect on Dr Menicanti's comments. Clearly you thought those that needed annuloplasty and those that did not need annuloplasty were very different groups of patients, but in the ‘did not need annuloplasty’ group of those 52 patients, you had to go back on 17 or 33% of those. One question. Did that make you unhappy? And two, did you change your institutional policy of perhaps being more aggressive in performing an annuloplasty with lesser preoperative MR?
Dr Klein: Yes, you are right. First, we are very aggressive in performing restrictive mitral annuloplasty in these patients. We don't do restrictive mitral annuloplasty for grade 1 MR, because it is not supposed to influence the left ventricular function and outcome in the future.
And you also wanted to know —
Dr Bolling: Did it make you unhappy to have to go back on bypass one-third of the time? That would make me unhappy. That seems like a high rate.
Dr Klein: It is all about the end results. You have to give a good treatment to these patients, and we know that leaving moderate MR or more in these patients results in a suboptimal outcome. So you have to go back and repair the valve.
Dr Bolling: I agree.
Dr K. Vural (Ankara, Turkey): Do your Kaplan—Meier curves and the subsequent survival comparison include operative mortality? Otherwise the perception of the diagram may be misleading, and, in my opinion, the legend or footnote of the diagram should contain this information. As far as I could see from your slides, there was a considerable difference between the mortalities of the mitral intervention group and the other group.
Dr Klein: Of course, in our Kaplan—Meier curve operative mortality is included, and in the first part of the graph you see a sharp drop that shows the operative mortality. And, yes, both groups are different. The patients in the RMA group have a more severe degree of disease, they have much more enlarged ventricles, and they therefore have a higher or a different mortality rate.
REFERENCES
- 1.McKay RG, Pfeffer MA, Posternak RC, Markis JE, Come PC, Nakao S, Aldermen JD, Ferguson JJ, Safian RD, Grossman W. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer M, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Gaudron P, Eilles G, Ertl G, Kochsiek K. Compensatory and non-compensatory left ventricular dilatation after myocardial infarction: time course and hemodynamic consequences at rest and during exercise. Am Heart J. 1992;123:377–85. doi: 10.1016/0002-8703(92)90649-g. [DOI] [PubMed] [Google Scholar]
- 4.Levine RA, Swammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 5.Trichon BH, Felker, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–43. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 6.Dor V, Saab M, Coste P, Kornazewska M, Montiglio F. Left ventricular aneurysm: new surgical approach. J Thorac Cardiovasc Surg. 1989;37:11–9. doi: 10.1055/s-2007-1013899. [DOI] [PubMed] [Google Scholar]
- 7.Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di Donato M, Menicanti L, Almeida DE, Oliveira S, Beyersdorf F, Kron IL, Suma H, Kouchoukos NT, Moore W, McCarthy PM, Oz MC, Fontan F, Scott ML, Accola KA RESTORE group. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilatation. JACC. 2004;44:1439–45. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Klein P, Holman ER, Versteegh MI, Boersma E, Verwey HF, Bax JJ, Dion RA, Klautz RJ. Wall motion score index predicts mortality and functional results after surgical ventricular restoration for ischemic heart failure. Eur J Cardiothorac Surg. 2009;35:847–53. doi: 10.1016/j.ejcts.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Menicante L, Di Donato M, Castelvecchio S, Santambrogia C, Montericcio V, Frigriola A, Buckberg G the RESTORE group. Functional ischemic mitral regurgitation in anterior ventricular remodelling: results of surgical ventricular restoration with and without mitral repair. Heart Fail Rev. 2004;9:317–27. doi: 10.1007/s10741-005-6808-1. [DOI] [PubMed] [Google Scholar]
- 10.Buckberg G, Menicanti L, De Oliveira S, Athanasuleas C The Restore Team. Restoring an elliptical chamber during rebuilding a wrap around anterior infarction. Eur J Cardiothorac Surg. 2005;28:772–4. doi: 10.1016/j.ejcts.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL, Gibbons RI, Antman EM, Alpert JS, Hiratzka LF, Faxon DP, Jacobs AK, Fuster V, Smith SC., Jr ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Cardiovasc Electrophysiol. 2002;13:1183–99. doi: 10.1046/j.1540-8167.2002.01183.x. [DOI] [PubMed] [Google Scholar]
- 12.Prucz RB, Weiss ES, Patel ND, Nwakanma LU, Shah AS, Conte JV. The impact of surgical ventricular restoration on mitral valve regurgitation. Ann Thorac Surg. 2008;86:726–34. doi: 10.1016/j.athoracsur.2008.04.100. [DOI] [PubMed] [Google Scholar]
- 13.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 14.Harris KM, Sundt TM, 3rd, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg. 2002;74:1468–75. doi: 10.1016/s0003-4975(02)03920-6. [DOI] [PubMed] [Google Scholar]
- 15.Bolling SF. Mitral valve reconstruction in the patient with heart failure. Heart Fail Rev. 2001;6:177–85. doi: 10.1023/a:1011421014480. [DOI] [PubMed] [Google Scholar]
- 16.Ciarka A, Braun J, Delgado V, Versteegh M, Boersma E, Klautz R, Dion R, Bax JJ, Van de Veire N. Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol. 2010;106:395–401. doi: 10.1016/j.amjcard.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O’Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Donato M, Castelvecchio S, Brankovic J, Santambrogio C, Montericcio V, Menicanti L. Effectiveness of surgical ventricular restoration in patients with dilated ischemic cardiomyopathy and unrepaired mild mitral regurgitation. J Thorac Cardiovasc Surg. 2007;134:1548–53. doi: 10.1016/j.jtcvs.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Aklog L, Filsoufi F, Flores KQ, Chen RH, Cohn LH, Nathan NS, Byrne JG, Adams DH. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation. 2001;104(12 Suppl. 1):I68–75. doi: 10.1161/hc37t1.094706. [DOI] [PubMed] [Google Scholar]
- 20.Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, Bailey MS, Guthrie TJ, Meyers BF, Damiano RJ., Jr Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78:794–9. doi: 10.1016/j.athoracsur.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di Donato M, Menicanti L, Almeida DE, Oliveira S, Beyersdorf F, Kron IL, Suma H, Kouchoukos NT, Moore W, McCarthy PM, Oz MC, Fontan F, Scott ML, Accola KA RESTORE group. Surgical ventricular restoration: the RESTORE group experience. Heart Fail Rev. 2004;9:287–97. doi: 10.1007/s10741-005-6805-4. [DOI] [PubMed] [Google Scholar]
- 22.Klein P, Bax JJ, Shaw LJ, Feringa HH, Versteegh MI, Dion RA, Klautz RJ. Early and late outcome of left ventricular reconstruction surgery in ischemic heart disease: a systematic review of the literature. Eur J Cardiothorac Surg. 2008;34:1149–57. doi: 10.1016/j.ejcts.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 23.Braun J, Bax JJ, Versteegh MI, Voigt PG, Holman ER, Klautz RJ, Boersma E, Dion RA. Pre-operative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2005;27:847–53. doi: 10.1016/j.ejcts.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Gillinov AM, Wierup PN, Blackstone EH, Bishays ES, Cosgrove DM, White J, Lytle BW, McCarthy PM. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–41. doi: 10.1067/mtc.2001.116557. [DOI] [PubMed] [Google Scholar]
- 25.Grigioni F, Detaint D, Avierinos J-F, Scott C, Tajik J, Enriquez-Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. JACC. 2005;45:260–7. doi: 10.1016/j.jacc.2004.10.030. [DOI] [PubMed] [Google Scholar]