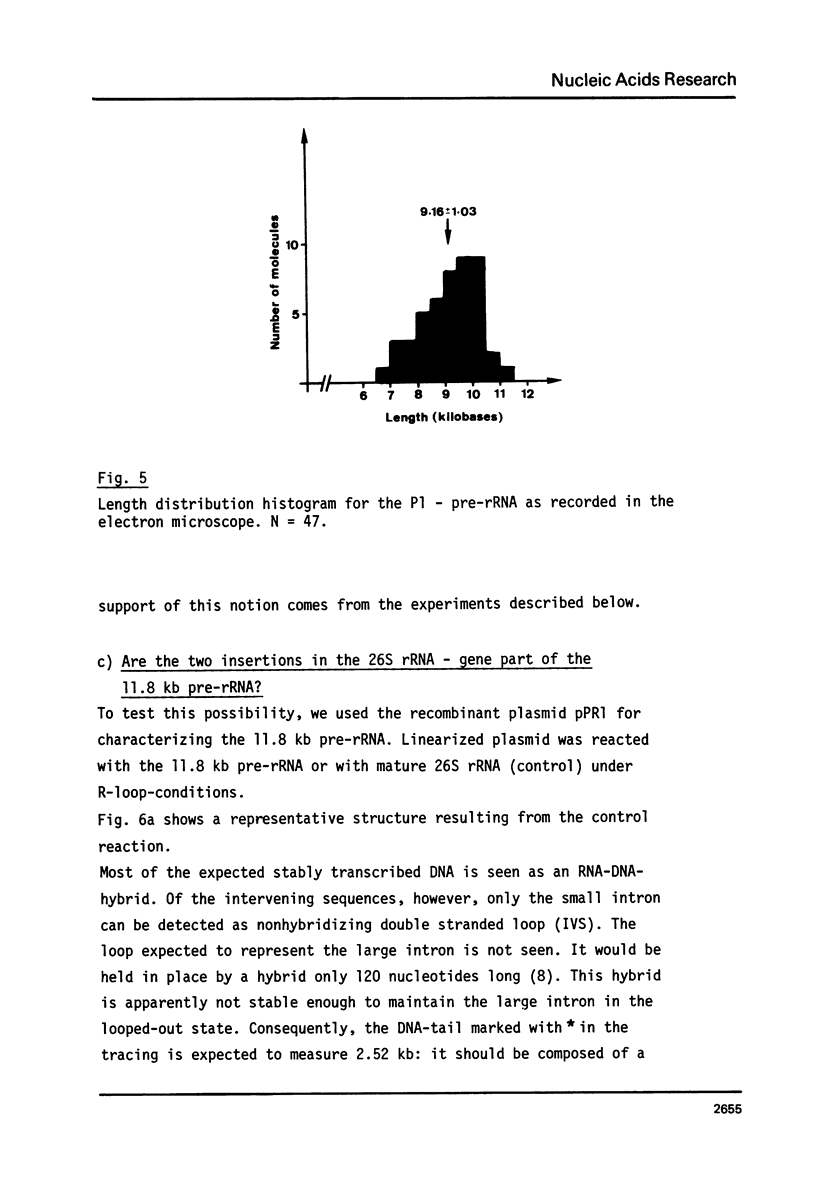
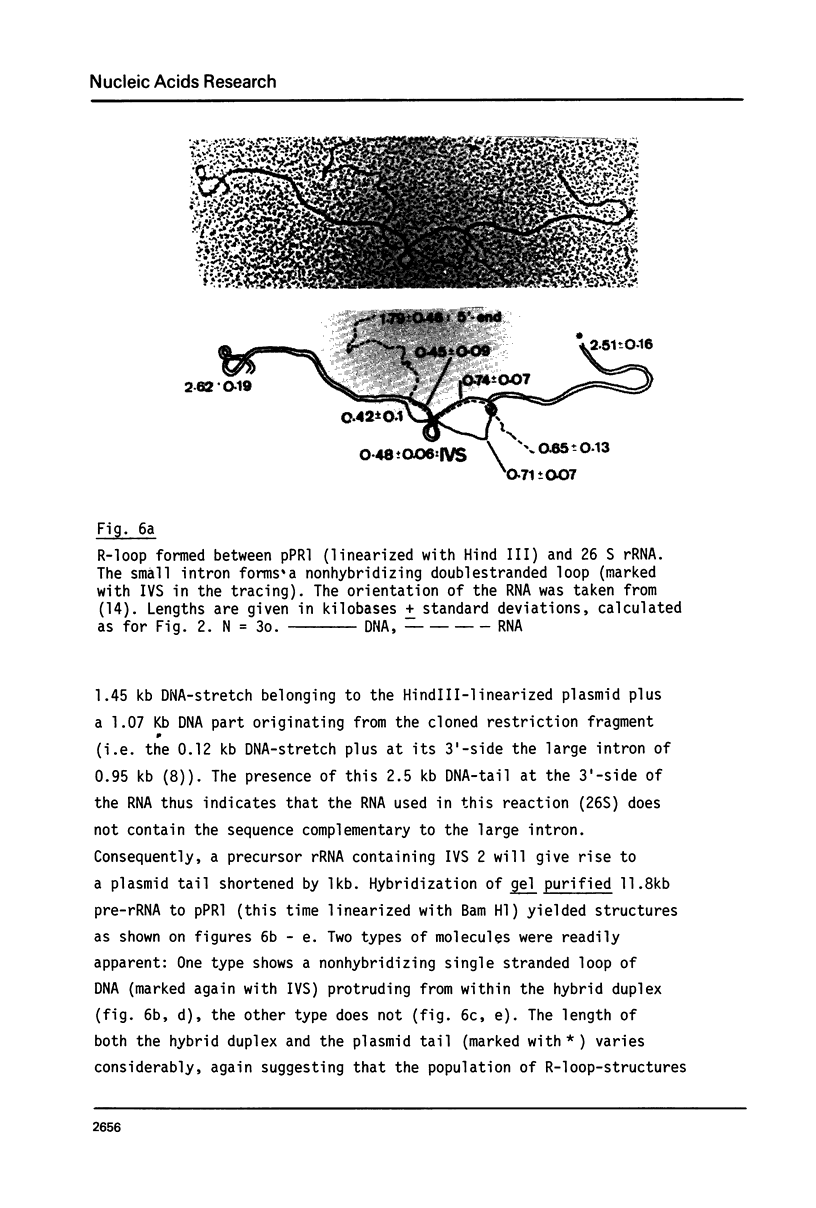
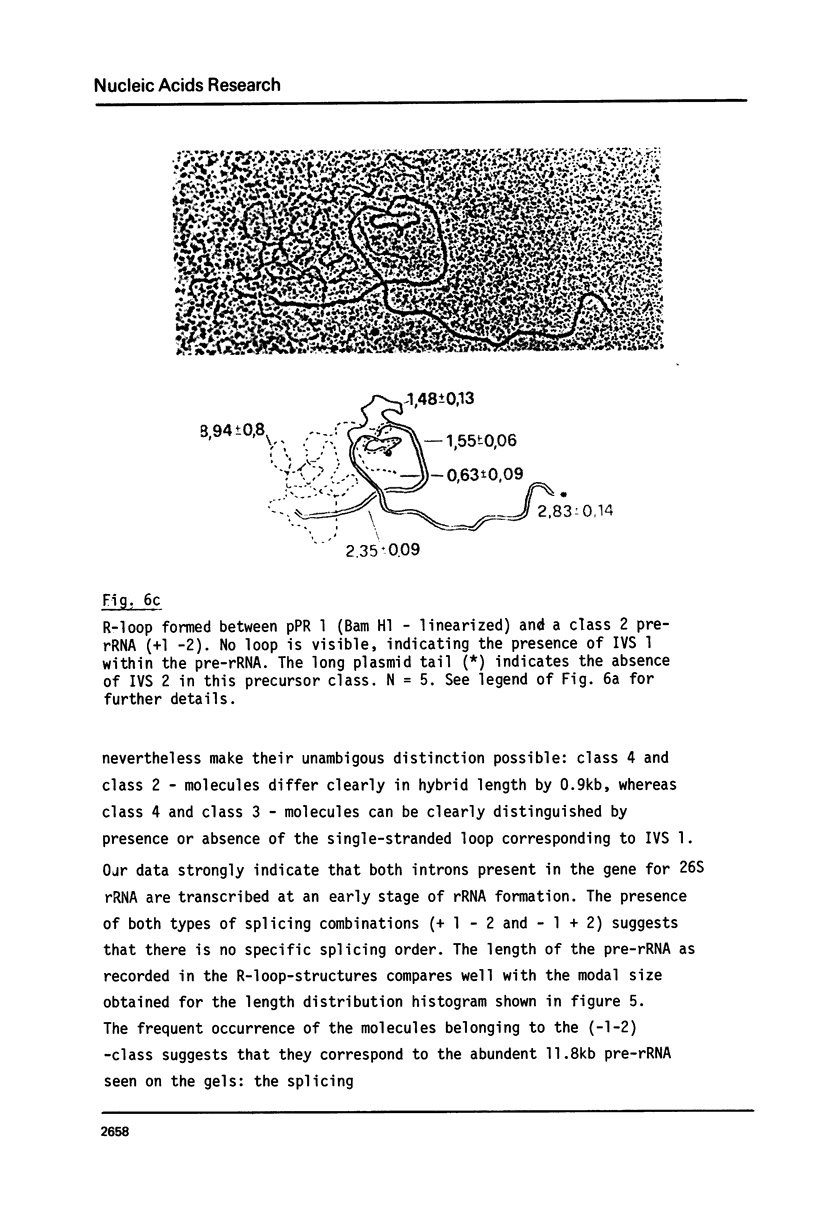
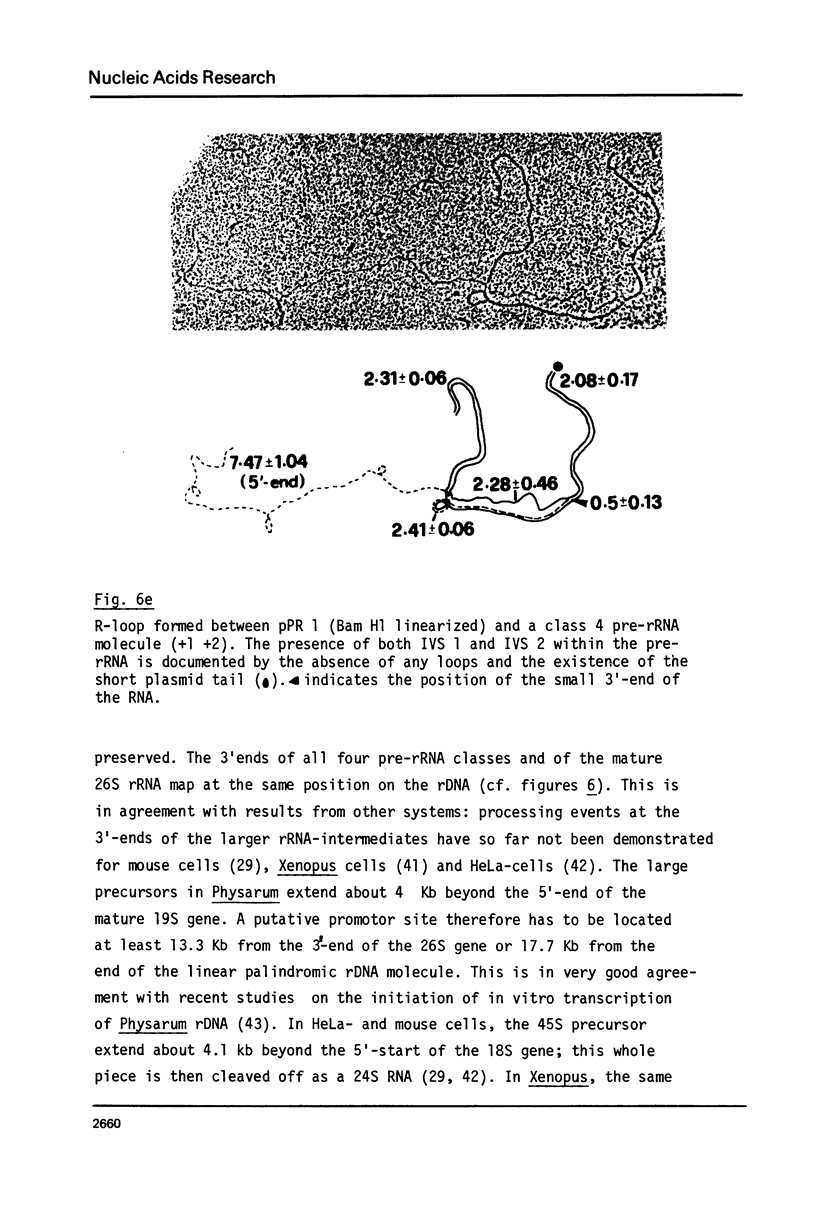
Abstract
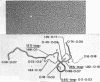
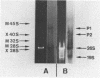
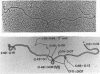
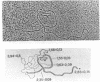
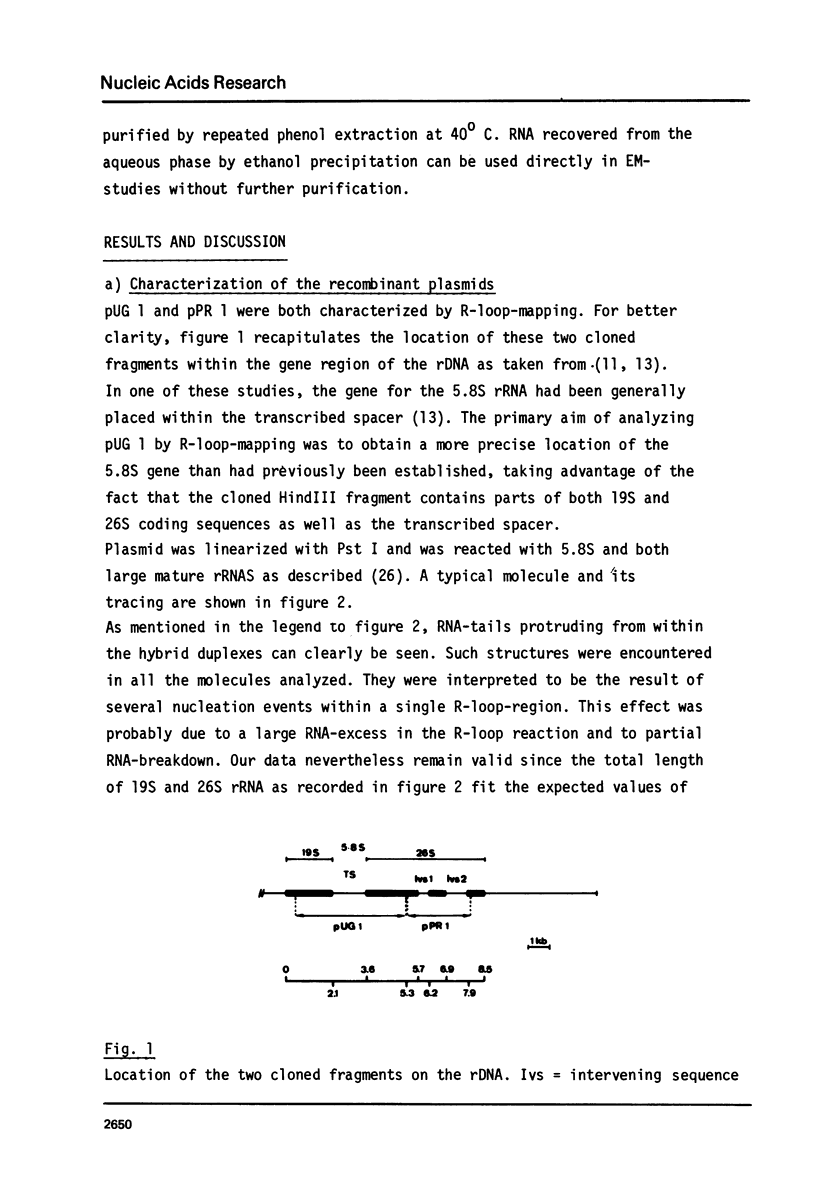
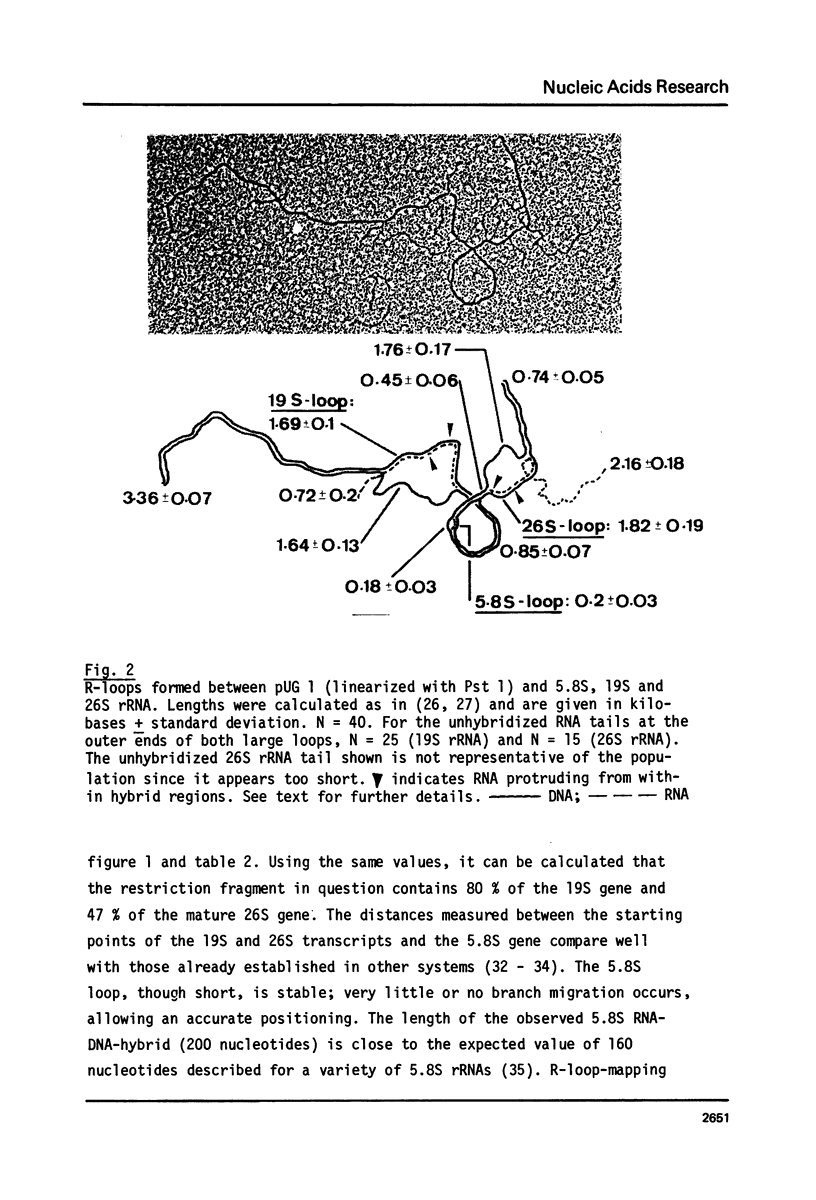
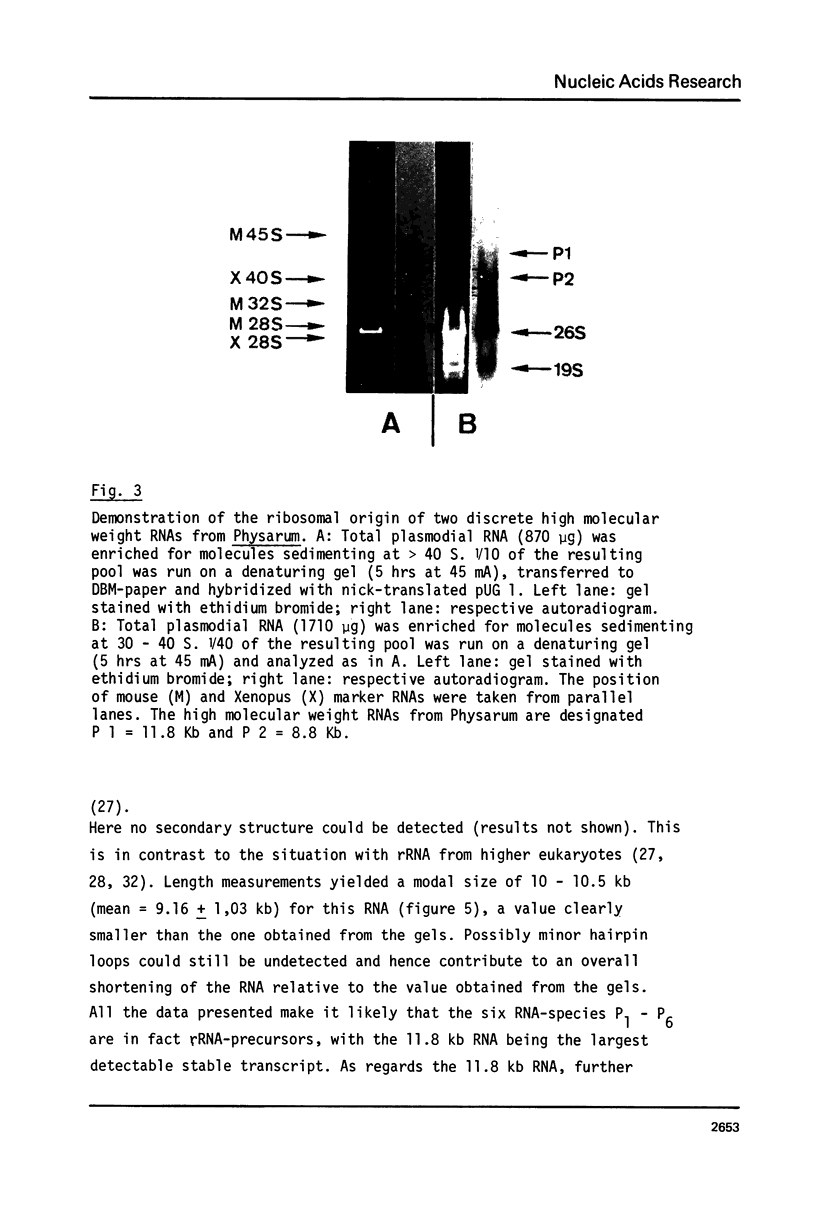
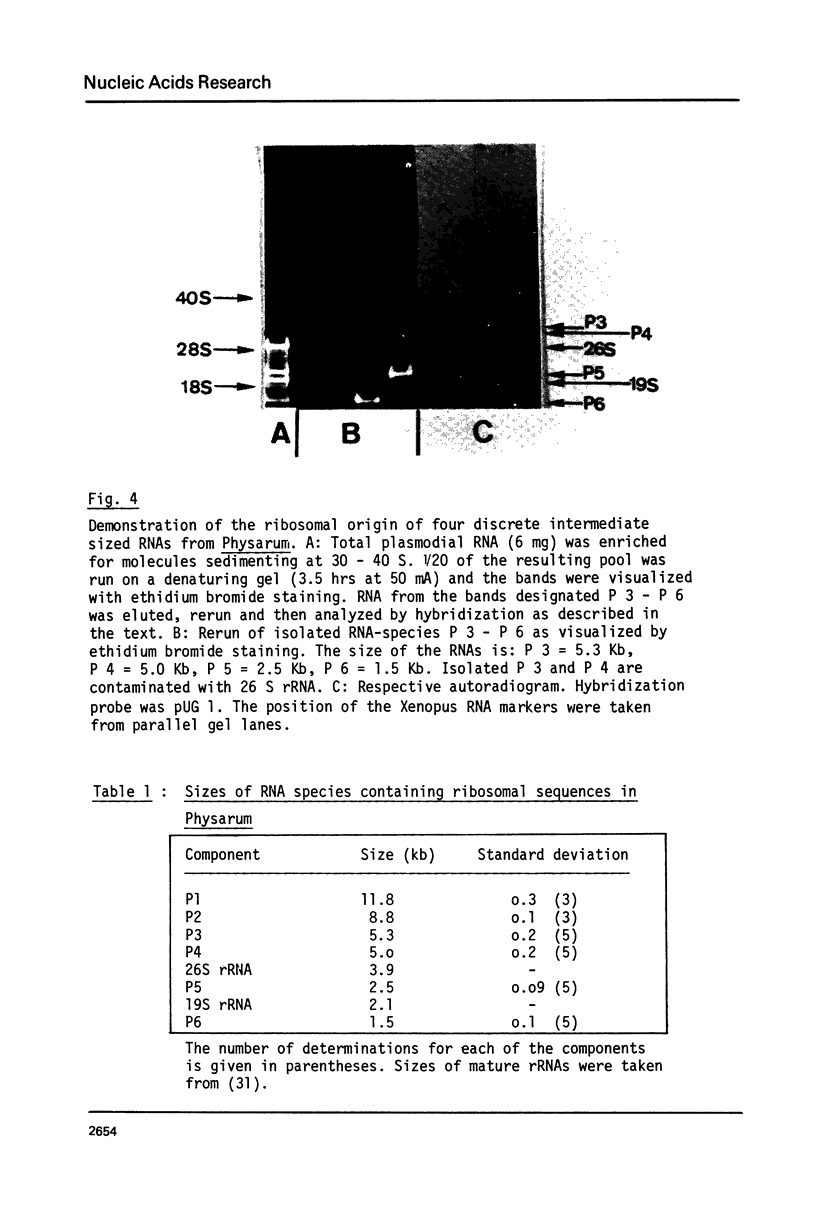
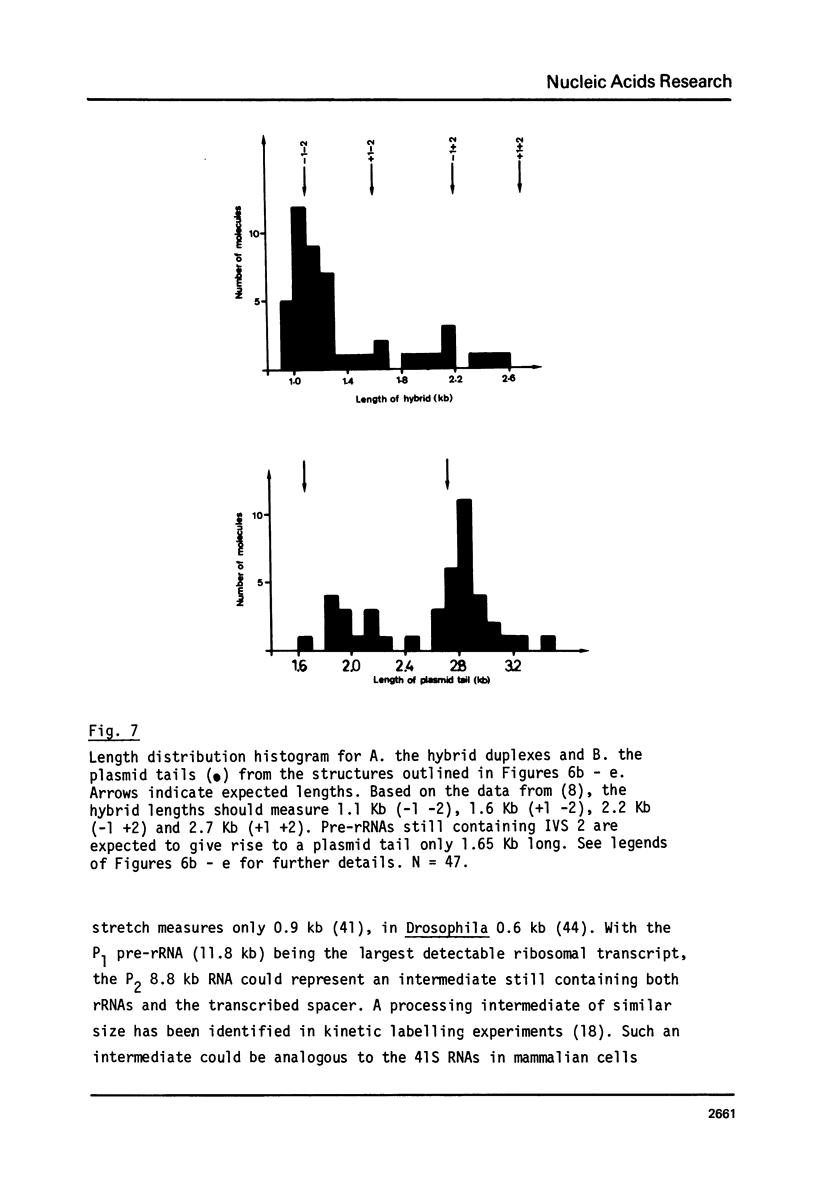
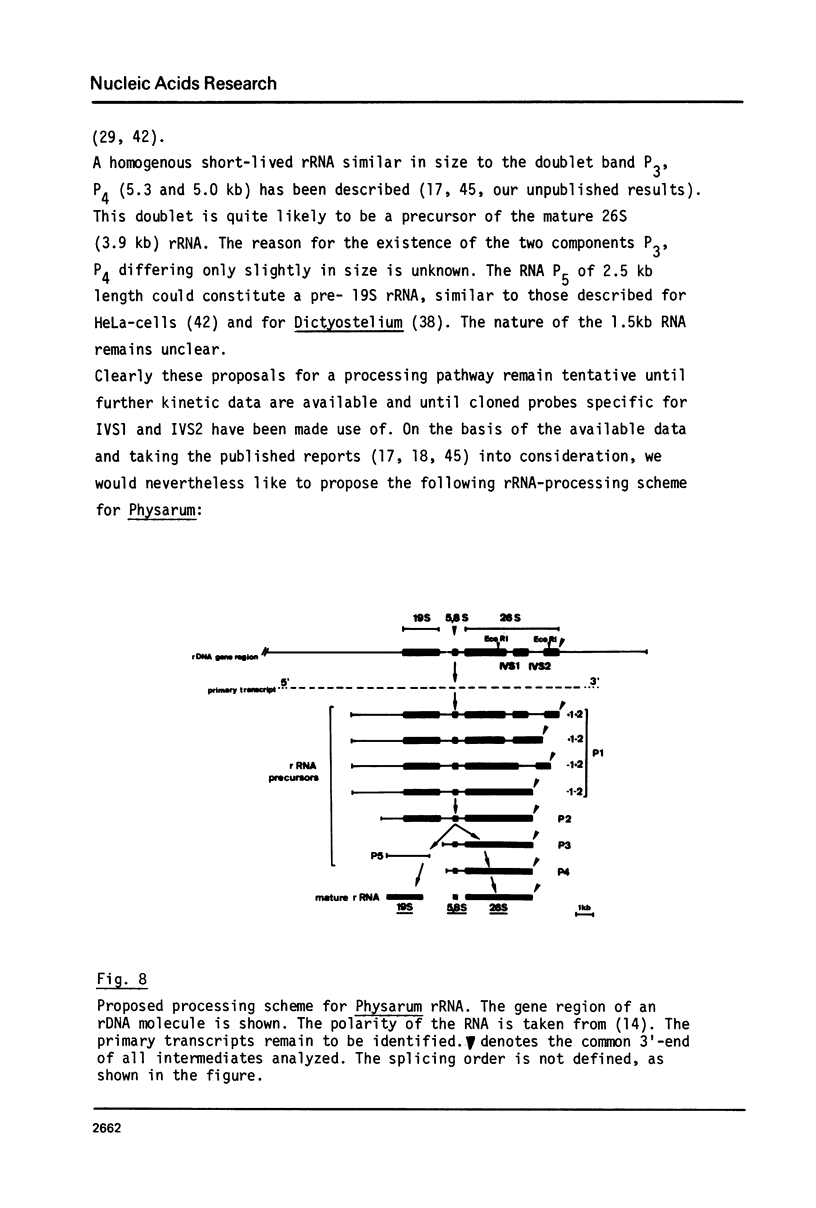
The processing of intermediates of Physarum rRNA has been investigated using two cloned rDNA restriction fragments as sequence specific probes. The size of the largest stable pre-rRNA detected is 11.8 Kb, as determined by denaturing gel electrophoresis. R-loop analysis revealed that this pre-rRNA fraction mainly consists of transcripts which do not contain the two insertion sequences present in the 26 S gene. However, a small number of molecules are found which still contain one or the other, or both, of these introns. These observations suggest that the two introns are transcribed but spliced out in a random order before RNA synthesis has reached the 3-end of the primary transcript. Transcription starts at about 17.7 Kb and stops at 4.4 Kb as measured from the ends of the linear palindromic rDNA molecule. Besides the 11.8 Kb transcript several further ribosomal RNA precursors have been identified, ranging in size from 2.5 to 8.8 Kb. In addition, the genes for 5.8 S RNA have been located by R-loop mapping.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts-Young B., Lodish H. F. Triphosphate residues at the 5' ends of rRNA precursor and 5S RNA from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1978 Feb;75(2):740–744. doi: 10.1073/pnas.75.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts-Young B., Maizels N., Lodish H. F. Precursors of ribosomal RNA in the cellular slime mold Dictyostelium discoideum. Isolation and characterization. J Biol Chem. 1977 Jun 10;252(11):3952–3960. [PubMed] [Google Scholar]
- Bos J. L., Heyting C., Borst P., Arnberg A. C., Van Bruggen E. F. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978 Sep 28;275(5678):336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. R., Littau V. C., Melera P. W., Allfrey V. G., Johnson E. M. Unique sequence arrangement of ribosomal genes in the palindromic rDNA molecule of Physarum polycephalum. Nucleic Acids Res. 1979 Apr;6(4):1433–1447. doi: 10.1093/nar/6.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din N., Engberg J., Kaffenberger W., Eckert W. A. The intervening sequence in the 26S rRNA coding region of T. thermophila is transcribed within the largest stable precursor for rRNA. Cell. 1979 Oct;18(2):525–532. doi: 10.1016/0092-8674(79)90069-2. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S RNA sequences and their precursors. Nucleic Acids Res. 1979 Jan;6(1):r29–r44. doi: 10.1093/nar/6.1.419-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Wyler T., Braun R. The gene for the 26 S rRNA in Physarum contains two insertions. FEBS Lett. 1979 Apr 15;100(2):347–350. doi: 10.1016/0014-5793(79)80366-x. [DOI] [PubMed] [Google Scholar]
- Hall L., Braun R. The organisation of genes for transfer RNA and ribosomal RNA in amoebae and plasmodia of Physarum polycephalum. Eur J Biochem. 1977 Jun 1;76(1):165–174. doi: 10.1111/j.1432-1033.1977.tb11582.x. [DOI] [PubMed] [Google Scholar]
- Harding J. D., Przybyla A. E., MacDonald R. J., Pictet R. L., Rutter W. J. Effects of dexamethasone and 5-bromodeoxyuridine on the synthesis of amylase mRNA during pancreatic development in vitro. J Biol Chem. 1978 Oct 25;253(20):7531–7537. [PubMed] [Google Scholar]
- Jacobson D. N., Holt C. E. Isolation of ribosomal RNA precursors from Physarum polycephalum. Arch Biochem Biophys. 1973 Nov;159(1):342–352. doi: 10.1016/0003-9861(73)90460-8. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., Affolter H. U., Atmar V. J., Seebeck T., Gubler U., Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Penman S. Processing steps and methylation in the formation of the ribosomal RNA of cultured Drosophila cells. J Mol Biol. 1978 May 15;121(2):219–238. doi: 10.1016/s0022-2836(78)80006-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Rusch H. P. A characterization of ribonucleic acid in the myxomycete Physarum polycephalum. Exp Cell Res. 1973 Nov;82(1):197–209. doi: 10.1016/0014-4827(73)90262-0. [DOI] [PubMed] [Google Scholar]
- Molgaard H. V., Matthews H. R., Bradbury E. M. Organisation of genes for ribosomal RNA in Physarum polycephalum. Eur J Biochem. 1976 Sep 15;68(2):541–549. doi: 10.1111/j.1432-1033.1976.tb10842.x. [DOI] [PubMed] [Google Scholar]
- Niles E. G. Isolation of a high specific activity 35S ribosomal RNA precursor from Tetrahymena pyriformis and identification of its 5' terminus, pppAp. Biochemistry. 1978 Oct 31;17(22):4839–4844. doi: 10.1021/bi00615a035. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Cheng T. Y., Freed J. J., Greenberg J. R., Kelley D. E., Tartof K. D. Evolution of the transcription unit of ribosomal RNA. Proc Natl Acad Sci U S A. 1970 Mar;65(3):609–616. doi: 10.1073/pnas.65.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Thomas M., Kramer R. A., Davis R. W. Unique arrangement of coding sequences for 5 S, 5.8 S, 18 S and 25 S ribosomal RNA in Saccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol. 1978 Aug 15;123(3):387–404. doi: 10.1016/0022-2836(78)90086-4. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Schibler U., Wyler T., Hagenbüchle O. Changes in size and secondary structure of the ribosomal transcription unit during vertebrate evolution. J Mol Biol. 1975 May 25;94(3):503–517. doi: 10.1016/0022-2836(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Stalder J., Braun R. Isolation of a minichromosome containing the ribosomal genes from Physarum polycephalum. Biochemistry. 1979 Feb 6;18(3):484–490. doi: 10.1021/bi00570a017. [DOI] [PubMed] [Google Scholar]
- Sun I. Y., Johnson E. M., Allfrey V. G. Initiation of transcription of ribosomal deoxyribonucleic acid sequences in isolated nuclei of Physarum polycephalum: studies using nucleoside 5'-[gamma-S]triphosphates and labeled precursors. Biochemistry. 1979 Oct 16;18(21):4572–4580. doi: 10.1021/bi00588a018. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Structure of ribosomal DNA in Physarum polycephalum. J Mol Biol. 1976 Sep 25;106(3):567–587. doi: 10.1016/0022-2836(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Wahli W., Ryffel G. U., Wyler T., Jaggi F. B., Weber R., Dawid I. B. Cloning and characterization of synthetic sequences from the Xenopus iaevis vitellogenin structural gene. Dev Biol. 1978 Dec;67(2):371–383. doi: 10.1016/0012-1606(78)90207-5. [DOI] [PubMed] [Google Scholar]
- Wahli W., Wyler T., Weber R., Ryffel G. U. Size, complexity and abundance of a specific poly(A)-containing RNA of liver from male Xenopus induced to vitellogenin synthesis by estrogen. Eur J Biochem. 1976 Jul 15;66(3):457–465. doi: 10.1111/j.1432-1033.1976.tb10570.x. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Kelley D. E., Perry R. P. Secondary structure maps of ribosomal RNA. II. Processing of mouse L-cell ribosomal RNA and variations in the processing pathway. J Mol Biol. 1974 Oct 25;89(2):397–407. doi: 10.1016/0022-2836(74)90527-0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of ribosomal RNA and DNA. I. Processing of Xenopus laevis ribosomal RNA and structure of single-stranded ribosomal DNA. J Mol Biol. 1974 Oct 25;89(2):379–395. doi: 10.1016/0022-2836(74)90526-9. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Wild M. A., Gall J. G. An intervening sequence in the gene coding for 25S ribosomal RNA of Tetrahymena pigmentosa. Cell. 1979 Mar;16(3):565–573. doi: 10.1016/0092-8674(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Zellweger A., Braun R. RNA of Physarum. II. Template replication and transcription in the mitotic cycle. Exp Cell Res. 1971 Apr;65(2):424–432. doi: 10.1016/0014-4827(71)90022-x. [DOI] [PubMed] [Google Scholar]