Abstract
OBJECTIVE
The aim of the present study was to observe the changes of hemodynamics, stereology in pulmonary vascular remodeling and messenger RNA (mRNA) expressions of transforming growth factor beta 1, and receptors in carotid artery-jugular vein (CA-JV) shunt pulmonary artery hypertension model of rats.
METHODS
Thirty-six Sprague-Dawley rats were randomized into three groups: CA-JV group, monocrotaline (MCT) administration group, and control group. Left CA-JV shunts were established in CA-JV group. Dorsal subcutaneous injections of MCT (60 mg kg−1 ) were received in MCT group. Ligations of left common carotid artery and external jugular vein were performed in control group. Right ventricular systolic pressure (RVSP) measurement, histological evaluation of the pulmonary tissue, and mRNA levels of transforming growth factor beta 1 (TGFß1), receptor 1 and receptor 2, were investigated after 6 weeks on MCT group, and after 12 weeks on both control and CA-JV groups.
RESULTS
Compared with control group, RVSP, percentage of fibrous tissue (F%) in pulmonary arterioles, mRNA levels of TGFß1, and receptors of CA-JVand MCT groups increased significantly. Severe hemodynamics change was found in MCT groups. On the other hand, CA-JV group demonstrated more obvious fibrogenesis and TGFß1 signals' upregulation in two pulmonary artery hypertension (PAH) models.
CONCLUSIONS
CA-JV shunt model of rats was a well-established PAH animal model simulating congenital heart disease with systemic-pulmonary shunt.
Keywords: Fibrosis hypertension, Pulmonary arterioles, Transforming growth factor beta 1 rat
INTRODUCTION
Pulmonary artery hypertension (PAH) is a syndrome characterized by proliferative and obstructive remodeling of pulmonary arterioles [1], together with progressive increase in pulmonary vascular resistance and, ultimately, right ventricular failure and death [2]. PAH is a multi- pathogenesis disease. Some are idiopathic; other etiological factors include hypoxia, pulmonary, and pulmonary vascular diseases; heart diseases; connective tissue diseases; and inflammation and drugs [3-6]. Congenital heart disease (CHD) with systemic-pulmonary shunt is common type in cardiac surgery.
The common animal models used in PAH research include monocrotaline (MCT)-induced and hypoxia-induced PAH rat models [7,8]. However, PAH formation mechanisms of these models are totally different to that of CHD. Carotid artery- jugular vein (CA-JV) shunt PAH model [9] is one model which induces PAH formation depending on increasing blood flow of pulmonary artery. The advantages of this model include simple operation, fewer traumas to animals, similar hemo- dynamics, and pathophysiological change with CHD.
Idiopathic and other types of PAH have very similar histopathologic changes, including vascular and interstitial lesions, the former of which is also called pulmonary vascular remodeling (PVR) [10]. It is commonly recognized that excessive vasoconstriction, PVR, and microvessels' thrombosis are pathophysiological basis of PAH formation [11]. In early researches, vasoconstriction is emphatically concerned. Recently, more and more researchers become aware of the importance of PVR [12]. The participation of cellular proliferation, including endothelial cells (EC), smooth muscle cells (SMC) and fibroblasts, and extracellular matrix (ECM) oversynthesis in the mechanism of PVR was also studied [13].
Today, the widely adopted evaluation standard of PVR is Heath-Edwards six grades scale and its revisions [14,15].
However, the disadvantages of this scale are overly dependence of subjective assessment of pathologists and lacking quantitative basis of evaluation. With the help of the combination of pathology and image analysis system, absolute value to describe the degrees of histopathological changes of PVR could be assessed. The aim of the present study was to observe the changes of hemodynamics, stereology in PVR and messenger RNA (mRNA) expressions of transforming growth factor beta 1 and receptors in CA-JV shunt PAH model of rats, to investigate the PAH formation mechanism and its differences to other models.
MATERIALS AND METHODS
Animal treatments and groups
Thirty-six male Sprague—Dawley rats weighing between 250 and 300 g were included in the present study. All animal care and experiments were performed in accordance with the guide for the care and use of animals of Laboratory Animals Center of Sun Yat-Sen University. Experiments were performed with permission of the local committee on animal experiment. All rats were equally randomly divided into three groups, including the control group, the operation (CA- JV) group, and the drug (MCT) group. Animals were housed three per cage. Food and water were freely available at all times, and the animals were maintained at a temperature- controlled room (21 °C; relative humidity 50—70%) on a normal light cycle.
Pulmonary hypertension animal models
Rats in the control group underwent ligations of left common carotid artery and external jugular vein. Left CA-JV shunts were established in the CA-JV group. Both artery and vein were skeletonized, ligated, cut distally, and occluded with vascular clips. The plastic coat of an artery indwelling catheter (20G, 1.1 mm χ 45 mm; BD, NJ, USA) was cut to an 8-mm-long tube and used as the shunt. The proximal artery end was everted after passing through the shunt, and then inserted into the proximal vein end. Clips were opened; blood flow and pulsation were observed. One dosage of 0.2 ml heparin (12,500 U/100 ml) was intraperitoneal injected for anticoagulation. A dose of 100,000 U penicillin was used before the operation for infection prevention. Rats of drug group received dorsal subcutaneous injections of monocrotaline (60 mg/kg of body weight (kgw); Sigma, St. Louis, MO, USA). All the animals survived to the procedure. Surgery and evaluation were performed after 6 weeks in MCT group, and after 12 weeks on both control and CA-JV groups.
Measurement of right ventricular systolic pressure (RVSP)
Rats were anesthetized with pentobarbital (40 mg/kg, intraperitoneal (i.p.) injection). At first oblique, incisions were made on the left neck; pulsation of artery-vein shunt was detected. Then the same incisions were made on the right neck; right common and external jugular veins were separated and ligated distally. A heparin-priming 3F catheter was inserted via right external jugular vein to right ventricle through superior vena cava and right atrium, to monitor RVSP.
Histological evaluation
After rats were sacrificed, the chests were opened via midline incisions. Marginal left lower pulmonary lobes were sampled for histological evaluation. Tissues were fixed in 10% formalin liquid and processed according to routine procedures. After dehydration and embedding in paraffin, serial transverse sections of lung were obtained. Total fibrous tissue accumulation was determined by preparing tissue sections with the connective tissue-specific Masson stain. The analysis used an OLYMPUS BX51 microscopic system (Olympus Co., Tokyo, Japan). Pulmonary arterioles of external diameter between 30 and 100 mm were chosen for each section. At least eight random arterioles and five sections for each specimen were analyzed in image analysis system (Image-Pro Plus 6.0, Media Cybernetics Inc., Bethesda, MD, USA). Fibrosis of pulmonary arterioles was recorded in a quantitative way by stereologic analysis. Color segmentation was used to evaluate the percentage of fibrous tissue (F%). It was determined as follows: F% = 100 ∑ai/ ∑Aì. ∑ai was the total area of fibrous tissue and ∑Ai was the total test area. All histological examinations were performed by two different pathologists without prior knowledge of the treatment given to the animals of which the specimens were taken.
Real-time PCR analysis
Lung tissue was used for total RNA isolation to study the changes in transcription of transforming growth factor beta 1 (TGFß1), transforming growth factor beta receptor 1 (TGFßRI), and transforming growth factor beta receptor 2 (TGFßRII) involved in the initiation of the processes leading to fibrosis. Lungs were removed from rats, and left lower lobe tissues were dissected with thickness <0.5 cm, soaking in 1 — 2 ml RNAlater solution (Ambion Inc., Austin, TX, USA) and stored at 4°C until analysis. The total mRNA had been isolated from the tissue samples according to the manufacturer's instructions (Omega Bio-TekInc., Norcross, GA, USAE.Z.N.A. Total RNA kit). mRNA concentrations in tissue samples were evaluated using the real-time polymer chain reaction (PCR) assay. The specific primers for the target genes and glyseraldehyde-3-phosphate dehydrogenase (GAPDH) (synthesized by Biolink Bio-technologies Co., Guangzhou, China) used are described in Table 1. Totally 2 mg RNA sample of each group was reversely transcripted into cDNA. Then 5 ml cDNA was subjected to PCR reaction as the template. PCR reaction condition was pre-denaturing at 94 °C for 3 min, 94 °C for 45 s, 52 °C (50 °C for TGFßRII) for 45 s, 72 °C for 1 min and 35 amplification cycles, then 72 °C for 10 min. The final products were identified by electrophoresis in 1.5% agarose gel. The calculation and analysis were performed by the computer detection system (GDS-8000 pc, UVP Inc., Upland, CA, USA).
Table 1:
Primers for the target genes and GAPDH used in real-time PCR
| Target genes | Primers | Target fragment length (bp) |
|---|---|---|
| TGFβ1 | FP: 5′-CCGCAACAACGCAATCTA-3′ | 437 |
| RP: 5′-TGAGGAGCAGGAAGGGTC-3′ | ||
| TGFβRI | FP: 5′-GGTCCAGTCTGCTTCGTC-3′ | 411 |
| RP: 5′-TTTGTTGTCTGCTGCTAT-3′ | ||
| TGFβRII | FP: 5′-CCCCAAGTTCACCTACCAC-3′ | 421 |
| RP: 5′-GGGCAGCAGTTCCGTATT-3′ | ||
| GAPDH | FP: 5′-AGACAGCCGCATCTTCTTGT-3′ | 343 |
| RP: 5′-CTTGCCGTGGGTAGAGTCAT-3′ |
Primers for the target genes were listed in Table 1.
Statistical methods
Data were analyzed using the SPSS 14.0 statistical package. Quantitative data were calculated as mean ± standard deviation. One way analysis of variance (ANOVA) was used to describe statistical differences and Bonferroni method was used for multiple comparison. A P < 0.05 was considered to be statistically significant.
RESULTS
The RVSP in rat models
RVSP increased significantly in CA-JV group (37.69 ± 3.00 mmHg) compared with control group (25.41 ± 2.08 mmHg, P < 0.0001). MCT administration (MCT group) also induced a more significant increase in RVSP (43.99 ± 4.60 mmHg, P < 0.0001 vs control group). There was also significant difference in RVSP between CA-JV and MCT groups (P =0.004).
Histopathology
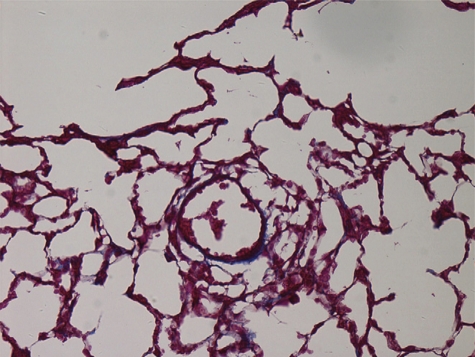
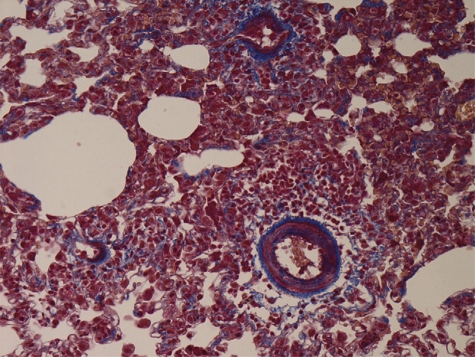
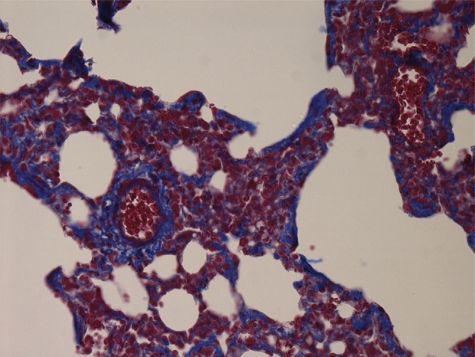
In the control group, walls of pulmonary arterioles were thin and even. Intima, media, and extima were difficult to be distinguished. Most tissues were in the Masson red staining. Fibrous tissues in blue staining could be observed rarely, dispersedly, and only extima distribution (Fig. 1). Conspicuous PVR could be observed in both CA-JV and MCT groups, characterized by pulmonary arterioles luminal stenosis and occlusion, media muscularization, intima and extima thickness, and fibrosis. Fibrous tissue hyperplasia in all layers demonstrated with the increase of the Masson blue staining. In some arterioles, intima and extima fibrosis manifested internal and external elastic lamina forming. Proliferative fibrous tissues in media distributed to concentric circles. Fibrous tissues also invaded in paravascular stroma (Figs. 2 and 3).
Figure 1:
control group. Masson 400×.
Figure 2:
MCT group. Masson 400×.
Figure 3:
CA-JV group. Masson 400×.
F% of pulmonary arterioles increased in two rat model groups (33.59 ± 7.58% in CA-JV group, 30.33 ± 7.24% in MCT group), which were significantly higher than control group (16.26 ± 7.27%, P < 0.0001 and P < 0.0001). There were significant differences in multiple comparisons of three groups (P = 0.002).
TGFß1 and receptors mRNA expressions
Compared with the control group, a sharp increase of TGFß1 mRNA level was already detected in CA-JV and MCT groups (1.17 ± 0.09 and 1.09 ± 0.07 vs 0.56 ± 0.06, P < 0.0001, P < 0.0001 vs the controls). There was significant difference between the CA-JV and MCT groups (P = 0.032).
The expression of TGFßRI mRNA level in CA-JV and MCT groups increased significantly compared with the control group (0.80 ± 0.06 and 0.93 ± 0.02 vs 0.20 ± 0.01, P < 0.0001, P < 0.0001). Difference between the CA-JV and MCT groups was also significant (P < 0.0001).
Significant increase of TGFßRIl mRNA level in CA-JV and MCT groups was also detected (1.15 ± 0.05 and 1.17 ± 0.23, respectively, vs 0.46 ± 0.28 in the controls, P < 0.0001 and P < 0.0001, respectively). There was no significant difference between CA-JV and MCT groups (P = 1.000; Table 2).
Table 2:
TGFβ1, TGFβRI and TGFβRIl mRNA expressions in two pulmonary hypertension models of rats versus control group
| Control | CA-JV | MCT | |
|---|---|---|---|
| Samples | 12 | 12 | 12 |
| TGFß1 | 0.56 ± 0.06 | 1.17± 0.09 | 1.09 ± 0.07 |
| TGFßRI | 0.20 ± 0.01 | 0.80 ± 0.06 | 0.93 ± 0.02 |
| TGFßRII | 0.46 ± 0.28 | 1.15 ± 0.05 | 1.17 ± 0.23 |
Bold and italic values mean three groups had significant difference. Only bold values mean CA-JV and MCT group had not significant difference, nevertheless had significant difference compared with control group.
DISCUSSION
In the present study, we demonstrated that both CA-JV shunt and MCT administration could increase RVSP and induce PVR in model rats. These two models are both well-established PAH animal models. Numerous researchers prefer to use MCT-PAH model because of its easy establishment and short observation period (3-6 weeks). The mechanisms of MCT-PAH establishment include arterial endothelial cell injuries, vascular permeability increasing, and inflammatory materials releasing, which trigger severe and progressing PAH [16]. This process has something close to idiopathic PAH in human. However, in the clinical practice of cardiac surgery clinical practice, most PAH patients are CHD-PAHs, which base on the increasing of pulmonary blood flow. CA-JV shunt PAH model simulates these pathophysiologic changes of systolic-pulmonary shunt. The model needs more time to observe the formation of PVR in animals (12 weeks). This model supplies another optional and different research choice for, at least, cardiac surgery. Therefore, it should be necessary to investigate the relevant mechanism of PVR in CA-JV shunt model.
Differences could be found between two PAH rat models in this study. RVSP in MCT group is significant higher than that in CA-JV group. On the other hand, F% of pulmonary arterioles in CA-JV group is significantly higher than that in MCT group. It is suggested that MCT could easily cause dynamic changes and CA-JV shunt led to more structural remodeling, especially fibrosis forming. MCT is a pyrrolidine alkaloid which is activated in liver and then transfers to lungs causing rapid and extensive inflammatory pulmonary artery injury and trauma. Pulmonary artery pressure (PAP) and RVSP increase dramatically followed by secondary amplified inflammatory responses subsequent to endothelial trauma. CA-JV shunt increases pulmonary circulation blood flow and involves in resistance pulmonary arteries at the beginning. The increasing blood flow causes constant, high shear stress to vessels. Shear stress can regulate function of endothelial nitric oxide synthetase (NOS) [17] and cytokines just as platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) [18,19]. This cytokines participate in the modulation of PVR. The results of the study suggested that the effect of constant high shear stress on inducing fibrous tissues proliferation, or fibrosis, was stronger than that of a single inflammatory trauma to endothelium. This would be the important characteristics for CA-JV shunt in the mechanism of PVR.
The definition of fibrosis is a condition in which fibrous connective tissue spreads over or replaces normal smooth muscle or other normal tissue. So the fibrotic proliferation of pulmonary arterioles could be defined as one kind of ‘fibrosis’, so-called vascular fibrosis. In the present study, the indicators of fibrosis activators, the mRNA level of TGFß1, and receptors were detected in the models, indicating the existence of observed pulmonary arteriole fibrosis. TGFß1 is one kind of multi-functional cytokines. It directly increases the synthesis, stimulates the formation of connective tissue, and inhibits degradation of extracellular matrix [20]. It is widely acknowledged as the most effective promoting factor for fibrogenesis and the key player in wound healing [21] and organ fibrosis, such as liver [22], kidney [23], and pancreas [24]. TGFß1 performs its fibrosis effect through TGFßRs, and the TGFßRs related with the fibrosis effect are mainly via TGFßRI and TGFßRII. The present results showed that TGFß1, TGFßRI, and TGFßRII mRNA levels in both two model groups were significantly increased compared with control group. The overexpression of TGFß1 and TGFßRI mRNA in CA-JV group was much more significant than that in MCT group. The result suggested that there were distinct underlying mechanisms of PVR in CA-JV shunt and MCT administration PAH models.
Most previous reports have described structural and functional abnormalities of cells, such as ECs and SMCs, in PVR [25]. Seldom researchers concern about interstitial lesions, as fibrosis, though it is also important part of PVR. One of the reasons may be that it is difficult to be evaluated. Frequency and severity of the microscopic fibrosis lesions were quantified by a graded scale of 0-3: 0, normal and minimal lesions; 1, mild lesions; 2, moderate lesions; and 3, severe lesions. With the help of combination of pathology and image analysis system, it is possible to use a stereological indicator to objectively evaluate the severities of fibrosis. Exactly, compared with cellular proliferation, fibrosis would be much more threatening to the PAH patients. Because when the induction of apoptosis becomes more and more realistic in anti-proliferation treatment, degradation of fibrous tissue is still an unsolved problem. There is no any confirming efficient therapy for organ and tissue fibrosis in clinical practice currently. Therefore, TGFß1 should be a therapeutic target in systemic-pulmonary shunt PAH.
CONCLUSION
The present study demonstrated that CA-JV shunt PAH rat model was a well-established PAH animal model. The hemodynamics changes in this model were not as prominent as the widely used MCT model, but much significant increasing fibrogenesis of PVR and TGFß1 signal mRNA overexpression were observed in CA-JV shunt PAH model than MCT model.
Funding
This work was supported by The National Science Foundation for Distinguished Youth Scholar of China (No. 30525020) and the National Basic Research Program of China (973 Program) (2010CB5295007).
Conflict of interest: none declared.
REFERENCES
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Hsu HH, Ko WJ, Hsu JY, Chen JS, Lee YC, Lai IR, Chen CF. Simvastatin ameliorates established pulmonary hypertension through a heme oxyge- nase-1 dependent pathway in rats. Respir Res. 2009;10:32. doi: 10.1186/1465-9921-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang IM, Klepetko W. Chronic thromboembolic pulmonary hypertension: an updated review. Curr Opin Cardiol. 2008;23:555–9. doi: 10.1097/HCO.0b013e328311f254. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Manes A, Farahani KV, Pelino F, Palazzini M, Negro L, Romanazzi S, Branzi A. Pulmonary arterial hypertension associated to connective tissue diseases. Lupus. 2005;14:713–7. doi: 10.1191/0961203305lu2206oa. [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Abenhaim L. Fatal pulmonary arterial hypertension associated with phenylpropanolamine exposure. Heart. 2004;90:e42. doi: 10.1136/hrt.2004.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–91. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 7.Kay JM, Harris P, Heath D. Pulmonary hypertension produced in rats by injection of crotalaria spectabilis seeds. Thorax. 1967;22:176–9. doi: 10.1136/thx.22.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boake WC, Daley R, McMillan IK. Observations on hypoxic pulmonary hypertension. Br Heart J. 1959;21:31–9. doi: 10.1136/hrt.21.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto T, Takeishi Y, Shishido T, Takahashi H, Itoh M, Kubota I, Tomoike H. Role of nitric oxide in progression of cardiovascular remodeling induced by carotid arterio-venous shunt in rabbits. Jpn Heart J. 2003;44:127–37. doi: 10.1536/jhj.44.127. [DOI] [PubMed] [Google Scholar]
- 10.Miniati D. Pulmonary vascular remodeling. Semin Pediatr Surg. 2007;16:80–7. doi: 10.1053/j.sempedsurg.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res. 2005;97:95–8. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med. 2001;22:433–49. doi: 10.1016/s0272-5231(05)70282-3. [DOI] [PubMed] [Google Scholar]
- 14.Heath D, Ewards J. A description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533–47. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 15.Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin. 2004;22:343–51. doi: 10.1016/j.ccl.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Reindel JF, Ganey PE, Wagner JG, Slocombe RF, Roth RA. Development of morphologic, hemodynamic, and biochemical changes in lungs of rats given monocrotaline pyrrole. Toxicol Appl Pharmacol. 1990;106:179–200. doi: 10.1016/0041-008x(90)90239-q. [DOI] [PubMed] [Google Scholar]
- 17.Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281:L490–8. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- 18.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005;115:2691–4. doi: 10.1172/JCI26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedgwood S, Devol JM, Grobe A, Benavidez E, Azakie A, Fineman JR, Black SM. Fibroblast growth factor-2 expression is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res. 2007;61:32–6. doi: 10.1203/01.pdr.0000250013.77008.28. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Wei HS, Lin S. Signal transduction of hepatic stellate cells in liver fibrosis. J Med Mol Biol. 2006;5:361–3. [Google Scholar]
- 21.Cordeiro Mf. Beyond mitomycin: TGF beta and wound healing. Prog Retin Eye Res. 2002;21:75–89. doi: 10.1016/s1350-9462(01)00021-0. [DOI] [PubMed] [Google Scholar]
- 22.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Cui Y, Fang L, Li F. Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas. 2008;37:e31–8. doi: 10.1097/MPA.0b013e3181744b50. [DOI] [PubMed] [Google Scholar]
- 24.Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000;58:1885–92. doi: 10.1111/j.1523-1755.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- 25.Hooper WC, Mensah GA, Haworth SG, Black SM, Garcia JG, Langleben D. Vascular endothelium summary statement V: pulmonary hypertension and acute lung injury: public health implications. Vascul Pharmacol. 2007;46:327–9. doi: 10.1016/j.vph.2006.10.017. [DOI] [PubMed] [Google Scholar]