Abstract
OBJECTIVE
Trans-apical beating-heart implantation of neo-chordae is yet an experimental procedure for mitral valve (MV) repair. We aimed to assess the performance of a new device in an acute animal study.
METHODS
A total of four domestic adolescent pigs were used as an acute model. The MV was assessed on the beating heart through a conventional trans-apical access. The NeoChord DS1000 device was used to implant polytetrafluoroethylene (PTFE) sutures to the MV leaflets. Procedural performance of the device was assessed and completed with surgical workflow analysis.
RESULTS
Overall 57 implantations using epicardial echocardiography guidance were performed (mean 14.3 implantations per animal). The MV leaflets were successfully grasped every second attempt (mean 2.3 ± 1.9) with no difference between the anterior and the posterior leaflet. A significant difference between an ‘expert’ surgeon (n > 20 implantations) and beginner surgeon was detected with regard to the duration for successful leaflet grasping (65 ± 73 vs 127 ± 105 s; p = 0.02) and the overall duration for implantation (130 ± 86 vs 230 ± 119 s; p = 0.002). Gross anatomy did not show major tear of leaflets. There were no device-related technical problems.
CONCLUSION
The NeoChord DS1000 device for trans-apical beating-heart implantation of neo-chordae to the MV valve showed a high procedural success. A significant difference between an expert and beginner surgeon was detected, which emphasizes the importance of training before introduction of this new technique into clinical practice. Surgical workflow analysis proved to be a valuable tool to assess the performance of this new technique.
Keywords: Mitral valve repair, Chordal replacement, Trans-apical
INTRODUCTION
Objective
Mitral valve (MV) repair surgery represents the gold standard for treatment of MV prolapse due to degenerative disease [1–4]. Chordal replacement with implantation of polytetrafluoroethylene (PTFE) sutures is an established repair technique in open-heart surgery with excellent short- and long-term results [5–7]. Thriving toward a trans-apical beating-heart approach, the NeoChord DS1000 (NeoChord Inc., Minnetonka, MN, USA) device has been developed to realize MV repair with implantation of neo-chordae [8]. However, when introducing new technologies, a certain amount of reliability and procedural success is required to allow subsequent successful application in humans. Here, surgical workflow analysis represents an innovative methodology to especially assess the performance and efficiency of new medical devices as well as surgeon training [9–11]. We herein aim to elucidate the concept and test the procedure of trans-apical beating-heart implantation of neo-chordae to the MV leaflets in an animal model as well as the procedural performance of the technique using workflow analysis.
MATERIAL AND METHODS
Concept
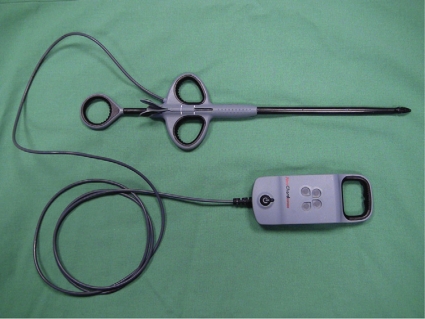
The concept of beating-heart trans-apical implantation of neo-chordae to the MV has been introduced and proven in acute and chronic animal studies by a group from the Mayo Clinic [8]. This group, in cooperation with NeoChord Inc. (Minnetonka, MN, USA), developed the DS1000 device (Fig. 1). The device is introduced into the left ventricle and left atrium, respectively, through a trans-apical access (Fig. 2). Mitral leaflet grasping is enabled with subsequent deployment of a PTFE suture (Fig. 3). This is achieved by an adjustable-gripper-like tip of the device with two jaws. After grasping the leaflet, the presence of tissue between the two jaws can be assessed using the device monitor (Fig. 1). When confirming a good (poor) grasp with enough tissue (no tissue) between the jaws, the four lights on the monitor reflect the white color of the leaflet tissue (red color of the blood). A needle to puncture the leaflet and retract the suture, as well as an exchangeable cartridge for loading of the PTFE suture, is installed within the device. After application of the PTFE neo-chordae to the leaflet by puncturing the leaflet and completely retracting the suture, the device is retracted, both ends of the suture are pulled exteriorly of the apex, and a girth hitch knot is tied and pulled tight to enable secure fixation of the suture onto the leaflet margin (Fig. 3). A French eye needle is then used to anchor the two ends of the suture individually over an additional felt pledget to the apex of the heart after adjusting the ideal length of the neo-chordae.
Figure 1:
The NeoChord DS1000 device for application of neochordae to the mitral valve. The device is shown including the device monitor, which is used to confirm sufficient grasp.
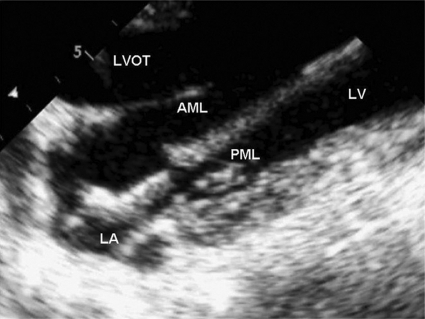
Figure 2:
The NeoChord DS1000 delivery system within the left atrium in an echocardiographic long axis view of an acute animal model. The device has been introduced through the apex of the heart and the jaws of the device are open toward the anterior mitral leaflet. When aiming for the posterior leaflet the device needs to be twisted for 180°. AML = anterior mitral leaflet; PML = posterior mitral leaflet; LA = left atrium; LV = left ventricle; LVOT = left ventricular outflow tract.
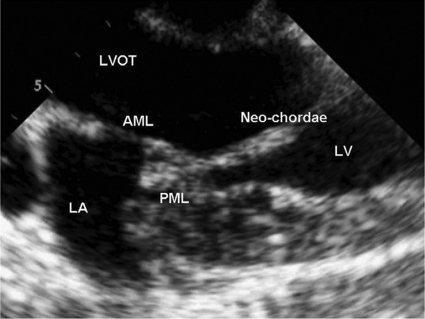
Figure 3:
After deploying the neo-chordae to the posterior leaflet the device is retracted through the apical access. The neo-chordae is then suspended between the PML and apex of the heart at the desired length. AML = anterior mitral leaflet; PML = posterior mitral leaflet; LA = left atrium; LV = left ventricle; LVOT = left ventricular outflow tract.
Animal model
Four domestic pigs underwent a medium sternotomy. Purse-string sutures were placed on the apex and a trans-apical access to the MV was created on the beating heart. Implantation of neo-chordae to the mitral leaflets using the NeoChord DS1000 device was repeatedly performed.
Surgical workflow analysis
Procedural success was assessed using surgical workflow analysis, as previously described [9,11]. All implantation procedures were videotaped and recorded. Workflow analysis of the overall procedure was performed and analysed regarding four aspects:
Duration of the procedure is defined as the time duration that is needed to successfully perform each phase. Phases are defined as follows: Phase I – introduction of the device from apex into the left atrium; Phase II – grasping of the mitral leaflet; Phase III – fixation of the neo-chordae to the leaflet and complete retraction of the device from the heart.
Success/attempts: An attempt was considered successful, if the leaflet was grasped with subsequent implantation of the neo-chordae (with regard to Phases II and III).
Anterior or posterior leaflet: Implantation of neo-chordae was performed either on the anterior or on the posterior leaflet.
Surgeon: All procedures were performed by two surgeons. Surgeon A was considered ‘expert’ with an overall implantation of more than 20 implantations, whereas surgeon B was considered ‘beginner’ surgeon (<20 implantations).
Statistical analysis
Continuous variables are expressed as mean and standard deviation throughout the article. Significance was tested with Student’s t-test at a level of p < 0.05. Analysis was performed using the SAS JMP7.0 program (SAS Institute, Cary, NC, USA).
RESULTS
Overall, 57 implantations using epicardial echocardiography guidance in four pigs were performed. Mean duration of phases were as follows: I = 46 ± 46 s; II = 78 ± 83 s; III = 25 ± 26 s. Successful grasping of leaflet was achieved roughly every second attempt (mean 2.3 ± 1.9; range 1–9; Table 1) and showed overall a high procedural success. The duration and success rate for grasping the anterior leaflet were not significantly different compared with the posterior leaflet (Table 1). A significant difference between an expert surgeon (n > 20 implantations) and a beginner surgeon was detected with regard to the duration for successful leaflet grasping (65 ± 73 vs 127 ± 105 s; p = 0.02), the overall duration for implantation (130 ± 86 vs 230 ± 119 s; p = 0.002) and the number of attempts to grasp the leaflet and securely implant a suture to the leaflet (1.9 ± 1.7 vs 3.7 ± 2.1; p = 0.005; Table 1). Surgical workflow analysis revealed no significant learning curve regarding the duration of the procedure from pig 1 to pig 4. Overall, there were no device-related technical problems.
Table 1:
Workflow analysis of trans-apical implantation of neo-chordae on the beating heart in an acute animal model. Comparison between AML and PMLas well as between expert and beginner surgeon
| Phase 1 | Phase II | Phase III | Phase l-lll | Attempts | |
|---|---|---|---|---|---|
| Overall | 46 ± 46 | 78 ± 83 | 25 ± 26 | 148 ± 100 | 2.3 ± 1.9 (range 1-9) |
| AML | 49.9 ± 50.5 | 76.5 ± 83.4 | 27.2 ± 29 | 153 ± 109 | 2.1 ± 1.6 (range 1-9) |
| PML | 32.7 ± 23.7 | 81.2 ± 56.7 | 17.6 ± 9.6 | 131 ± 63 | 2.8 ± 2.6 (range 2-9) |
| p-value | 0.2 | 0.8 | 0.2 | 0.5 | 0.3 |
| Expert surgeon | 40.2 ± 39.7 | 65.6 ± 72.9 | 23.3 ± 22.3 | 129 ± 86 | 1.9 ± 1.7 (range 1-7) |
| Beginner surgeon | 70.3 ± 63.8 | 127.2 ± 104.6 | 32.5 ± 39 | 230 ± 119 | 3.7 ± 2.1 (range 2-9) |
| p-value | 0.05 | 0.02 | 0.3 | 0.002 | 0.005 |
AML = anterior mitral leaflet; PML = posterior mitral leaflet.
DISCUSSION
Surgical MV repair with implantation of neo-chordae has reached a high level: it is associated with a low operative mortality, high freedom from re-operation, and long-term survival similar to the general population [1,2,4,6]. It may thus be postulated that trans-apical chordal replacement – as it follows a similar principle – may lead to similar results. Criticism, however, is eligible as not much is known about MV repair with chordal replacement as a stand-alone repair.
Clinical rationale
Clinical application of the NeoChord device has not yet been pursued. However, an ideal patient would be presenting with symptomatic MR due to an isolated prolapse of the posterior leaflet, preferably of the P2 segment, no dilatation of the left ventricle and mitral annulus. The potential benefits of this repair technique are considered to be significant as it would be performed without extracorporeal circulation on the beating heart through a minimal invasive approach, especially because current guidelines for patients with significant MR due to degenerative disease suggest performing MV repair at a reference center at the onset of symptoms and before structural cardiac changes, such as an increase in ventricular size, occur [12]. Eventually, in an ideal candidate, the procedure would aim to completely restore native valve anatomy and physiological valve function.
Despite the simplicity of the concept and the device, numerous challenges exist for potential future clinical application: (1) procedural failure with the need for conversion to open heart surgery; (2) incomplete MV repair without reduction of MR to at least grade 1+ or less; and (3) injury of MV leaflets or chordae tendinae, the aortic valve, left atrium, ventricle, or other structures. Apart from these potential drawbacks, several obstacles may emerge during the implantation procedure: (1) exact positioning of the neo-chordae to the prolapsing segment; (2) incomplete re-suspension of the leaflet without reduction of the MR; (3) length adjustment of the neo-chord to eliminate prolapse but, at the same time, prevent additional restriction of the leaflet; and (4) overall incomplete repair. Clarification of these aspects, however, is beyond the scope of this study and can at this moment only be assumed.
Animal study
With regard to future clinical application and the above-named challenges of this new procedure, the present animal study aimed to assess the practicability of the NeoChord device using a workflow analysis. End points were duration of the procedure, success rate for grasping MV leaflets and deploying a PTFE suture, respectively, as well as the surgeon’s learning curve.
This study shows that the NeoChord procedure is feasible and reproducible in an animal model. It can be performed successfully within a short time even by an inexperienced surgeon. Furthermore, it shows that grasping of the anterior and/or posterior MV leaflet is feasible with subsequent deployment of neo-chordae. Surgical workflow analysis showed an efficient procedural performance of the technique, which is promising regarding further application in humans. To our understanding, this pre-clinical evaluation of the NeoChord DS1000 has been valuable to test the repeatability of the procedure, to assess the general applicability of the device, to identify potential hazards, and, especially for training surgeons.
In conclusion, trans-apical beating-heart implantation of neo-chordae to MV leaflets is feasible and reproducible in an acute animal model and has proven a high procedural success. It may represent an alternative for MV repair in humans, however, clinical application has yet to be pursued.
Conflict of interest: none declared.
APPENDIX A. CONFERENCE DISCUSSION
Dr T. Folliguet (Paris, France): I just have a couple of comments and a question. As we know, with a dystrophic mitral valve, there is a wide range between thin dystrophic with an acute rupture and where the valve is very fragile and small, whereas when you have Barlow's disease, you have excess tissue with a thick valve and leaflet. So my question is very simple. It seems to me this would probably be a nice technique for Barlow's because it is probably easier to grasp the leaflet since it is thick, but then you would probably have to combine this with an annuloplasty ring or something like this, whereas in acute rupture of P2 or P3, some times you don't really need a ring because there is no enlargement and this would be a nice technique. However, I am just afraid that you may have some damage of the leaflet with this technique in fragile tissue, especially in older people. But aside from this, I think it is a technical issue, I think you have to pursue in this way.
So my question is very simple. If this work is now feasible, in which patients would you do it if you had a variety of patients?
Dr Seeburger: You are addressing a good point, and you have probably seen the presentation at the Techno College.This procedure has been done in humans, and we encountered one big problem, and that was because one of the patients had a very thin leaflet.We were able to place the chords but we were not able to tension them, because as soon as we started tensioning, then they came off the leaflet, and that was just because of the tissue quality.So we would not recommend this procedure for patients with very thin tissues. We would recommend it for patients with very thick and not Barlow type, but very big prolapsing tissue.That is one issue.
And the other point you are addressing is the concomitant annuloplasty ring. Of course, you cannot use an annuloplasty ring with this technique. There are other techniques that are being evaluated, but this technique would just be for patients with an isolated P2 prolapse and not much annular dilatation.
Dr G. Lutter (Kiel, Germany): I believe the fixation is done on the apex, and you are using an apical approach, specifically a trans-apical approach, meaning that you will have your device or delivery system introduced on the same side as the neochordae are fixed. Therefore you should bear in mind a reduction of the size during the following days because of scar tissue problems due to this trans-apical approach. Did you see mid-term in your pigs that this chord got longer due to the fact that there is remodeling on the apex?
In nature chords are connected to papillary muscles and you put them into the apex. Do you have any concerns or any experience on this?
Dr Seeburger: Well, certainly there are concerns but we don't have the answer from the study, because this trial was an acute animal study, sowe don't know anything about mid-term results. But it is a concern when you take this technique to humans, especially when the ventricle becomes smaller after reduction of MR that might be an issue, but we just don't know. It can potentially happen, but we just don't have data to support or refute this issue.
Dr Lutter: But do you think you could also apply your technique to the papillary muscle so that the needle comes from the apex through the papillary muscle to one of the leaflets and is connected there?
Dr Seeburger: Well, that is a good idea but not with this technique, because with this device the tip is 0.8 cm, so it would destroy the papillary muscle completely. If we or somebody else can come up with something that is much smaller, then this might be feasible, but not at this time,or let's say not at this stage of development.
Dr M. Antunes (Coimbra, Portugal): The discussant raised the aspect of fixation of the neochordae at the apex of the ventricle under echocardio- graphic guidance, which has been described a long time ago in surgical procedures. How did you fix the chordae to the apex of the ventricle? How did you measure the length?
Dr Seeburger: Well, it is very simple. You use a French eye needle and you go through the apex laterally through the myocardium, so you use it for both ends of the suture, fix them over an additional felt pledget and just tie it down.
Dr Antunes: But how did you judge the length?
Dr Seeburger: Judging the length is playing around, more or less. Judging the length is looking at the echo, letting the heart beat, and then pulling on the chords or make them loose, and then when you think you havea good length, correct tension and no more MR, then you tie the knots.
REFERENCES
- 1.Braunberger E, Deloche A, Berrei A, Abdallah F, Celestin JA, Meimoun P, Chatellier G, Chauvaud S, Fabiani JN, Carpentier A. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104:I8–11. [PubMed] [Google Scholar]
- 2.David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and BL prolapse. J Thorac Cardiovasc Surg. 2005;130:1242–9. doi: 10.1016/j.jtcvs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 3.Gillinov AM, Cosgrove DM, Blackstone EH, Diaz R, Arnold JH, Lytle BW, Smedira NG, Sabik JF, McCarthy PM, Loop FD. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998;116:734–43. doi: 10.1016/S0022-5223(98)00450-4. [DOI] [PubMed] [Google Scholar]
- 4.Seeburger J, Borger MA, Doll N, Walther T, Passage J, Falk V, Mohr FW. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior, and bileaflet prolapse. Eur J Cardiothorac Surg. 2009;36:532–8. doi: 10.1016/j.ejcts.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Frater RW, Vetter HO, Zussa C, Dahm M. Chordal replacement in mitral valve repair. Circulation. 1990;82:IV125–30. [PubMed] [Google Scholar]
- 6.David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg. 1991;101:495–501. [PubMed] [Google Scholar]
- 7.Seeburger J, Falk V, Borger MA, Passage J, Walther T, Doll N, Mohr FW. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: à ègalité. Ann Thorac Surg. 2009;87:1715–20. doi: 10.1016/j.athoracsur.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bajona P, Katz WE, Daly RC, Zehr KJ, Speziali G. Beating-heart, off-pump mitral valve repair by implantation of artificial chordae tendineae: an acute in vivo animal study. J Thorac Cardiovasc Surg. 2009;137:188–93. doi: 10.1016/j.jtcvs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Neumuth T, Trantakis C, Riffaud L, Strauss G, Meixensberger J, Burgert O. Assessment of technical needs for surgical equipment by surgical process models. Minim Invasive Ther Allied Technol. 2009;18:341–49. doi: 10.3109/13645700903384484. [DOI] [PubMed] [Google Scholar]
- 10.Krauss A, Muensterer OJ, Neumuth T, Wachowiak R, Donaubauer B, Korb W, Burgert O. Workflow analysis of laparoscopic Nissen fundoplication in infant pigs- a model for surgical feedback and training. J Laparoendosc Adv Surg Tech A. 2009;19(Suppl.):S117–22. doi: 10.1089/lap.2008.0198.supp. [DOI] [PubMed] [Google Scholar]
- 11.Neumuth T, Jannin P, Strauss G, Meixensberger J, Burgert O. Validation of knowledge acquisition for surgical process models. J Am Med Inform Assoc. 2009;16:72–80. doi: 10.1197/jamia.M2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B American College of Cardiology American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease), Society of Cardiovascular Anesthesiologists. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]