Abstract
OBJECTIVES
The prognosis of non-small-cell lung cancer (NSCLC) patients with malignant pleural disease (MPD), characterized by malignant pleural effusion and/or malignant pleural nodules, is reported to be poor, and patients with MPD are generally not subjected to surgery. However, whether or not the primary tumor should be resected, when MPD is first detected at thoracotomy, is controversial.
METHODS
The clinical records of 1623 consecutive NSCLC patients, who underwent surgery between 1990 and 2007, were retrospectively reviewed. A hundred patients (6.2%) were classified with pathological stage IV disease according to the seventh edition of the Union for International Cancer Control (UICC) staging system. There were 73 patients with MPD, which included 32 with effusion without nodules (MPE) and 41 with nodules with or without effusion (MPN). Intra- or postoperative pleural chemotherapy was administered to 37 MPD patients.
RESULTS
The median survival time, the 3-year survival rate and the 5-year survival rate for MPD patients were 25.9 months, 41.4%, and 23.7%, respectively, which are better outcomes than those for M1b patients (8.7 months, 18% and 18%, respectively) (log-lank test: p = 0.014). Among MPD patients, N0–1 disease was determined to be a favorable prognostic factor (p = 0.01). MPD status (MPE or MPN) was not prognostically significant (p = 0.40). MPE patients with N0–1 disease had a significantly better prognosis with a 5-year survival rate of 63.6% compared to MPE patients with N2–3 disease (p = 0.003). Twenty-seven percent of MPN patients with N0–1 disease achieved 5-year survival, whereas none of the MPD patients with N2–3 disease survived longer than 5 years after surgery.
CONCLUSIONS
The prognosis of patients with surgically detected MPD, who underwent resection, was better than that of M1b patients. MPE patients with N0–1 disease may be candidates for resection.
Keywords: Lung cancer surgery, Pleural space
INTRODUCTION
Non-small-cell lung cancer (NSCLC) patients with malignant pleural disease (MPD), which includes malignant pleural effusion and/or malignant pleural nodules, are reported to have poor outcomes and are generally considered to be inoperable [1–3]. Analyses of a large patient database from the International Association for the Study of Lung Cancer (IASLC) Staging Project revealed that the median survival time (MST) of patients with MPD discovered during clinical staging was 8 months, and the 5-year survival rate was only 2% [3]. Based on these results, lung cancer patients with malignant pleural effusion/nodules were reclassified to stage IV (M1a) in the new staging system of the Union for International Cancer Control (UICC) [4].
However, NSCLC patients with MPD present with a broad range of disease stages, from minimal disease with little or no pleural effusion that is only detected by examination of pleural lavage fluid, to massive disease with multiple pleural nodules and a large amount of pleural effusion [5]. In clinical practice, pleural disease is sometimes only discovered at thoracotomy, and then it is often controversial whether or not the primary cancer lesion should be removed [6–9]. Generally, patients with minimal pleural dissemination detected only on lavage cytology have been subjected to surgery [4,10]. The treatment of patients with MPD plus sufficient effusion for standard cytological examination or macroscopically detected pleural nodules at thoracotomy is most controversial, because data on these patients are limited [6–10]. The survival data from the IASLC Staging Project for patients, who were pathologically diagnosed with MPD, revealed an MST and a 5-year survival rate of 18 months and 11%, respectively, which is better than the survival data reported for patients with clinically diagnosed MPD [3]. Other researchers have also reported similar favorable results for patients with pathologically diagnosed MPD [6–9]. On the other hand, poor postoperative outcomes have also been demonstrated in patients with malignant pleural effusion [1,10].
In our institution, pleural lavage cytology (PLC) has not been routinely performed. In this study, we reviewed the clinical records of patients with stage IV disease according to the seventh edition of the UICC staging system, to assess the relevance of pulmonary resection for patients with MPD first detected at thoracotomy by standard histological examination.
PATIENTS AND METHODS
Patients
The clinical records of 1623 consecutive NSCLC patients, who underwent surgery at Chiba University Hospital between 1990 and 2007, were retrospectively reviewed. Among them, 100 patients (6.2%) were classified with pathological stage IV disease according to the seventh edition of the UICC staging system [4]. The mean age at the time of surgery was 62.7 ± 10.9 years. Fifty patients (50%) were male. The median follow-up time was 29.5 months (3.2–120 months). Histological diagnoses of tumor cell types were based on the World Health Organization histological classification of lung tumors [11]. As many as 80 patients were with adenocarcinoma, 14 with squamous cell carcinoma, 3 with large cell carcinoma, and 3 with adenosquamous cell carcinoma. Standard lobectomies, bilobectomies, pneumonectomies, and partial resections were performed for 74, 11, 5, and 10 patients, respectively. Seventy-five patients (4.6%) had M1a disease, with 73 having MPD and two contralateral pulmonary metastases. We divided MPD into two groups as follows: malignant pleural effusion without nodules (MPE) and malignant pleural nodules with or without effusion (MPN). As many as 32 patients were with MPE and 41 with MPN. A total of 25 patients had M1b disease, with 16 patients having brain metastasis, two bone metastasis, two lymph node metastasis in the neck, and 5 having others. Intra- or postoperative pleural chemotherapy was administered to 37 MPD patients. Intraoperative pleural chemotherapy was administered before closing the chest, and/or postoperative chemotherapy was administered once or twice after operation through the chest tube. During the early study period, mitomycin-C was primarily used for pleural chemotherapy [9], and thereafter cisplatin was used. A total of 25 patients received postoperative adjuvant cisplatin-based chemotherapy. This observational study was approved by the Institutional Review Board of Chiba University Hospital, Chiba, Japan.
Statistical analysis
Intergroup differences in patient characteristics, including age, gender, cell type, tumor size, and procedure, were assessed using the Student’s t-test and χ2-test. The Kruskal–Wallis test was used to compare the nodal status of the three patient groups (MPE, MPN, and M1b). Survival curves were estimated by the Kaplan–Meier method, and significance was assessed by the log-rank test. The terminal event was death from any cause. Univariate survival analysis was performed using the Cox proportional hazards model. For multivariate survival analysis, variables, including age, gender, and procedure, were further analyzed in a stepwise manner. Statistical differences were considered to be significant for p values less than 0.05. All data were analyzed using Abacus Concepts, Survival Tools for StatView version 5.0 (Abacus Concepts, Inc., Berkeley, CA, USA).
RESULTS
Patient characteristics and survival in stage IV patients
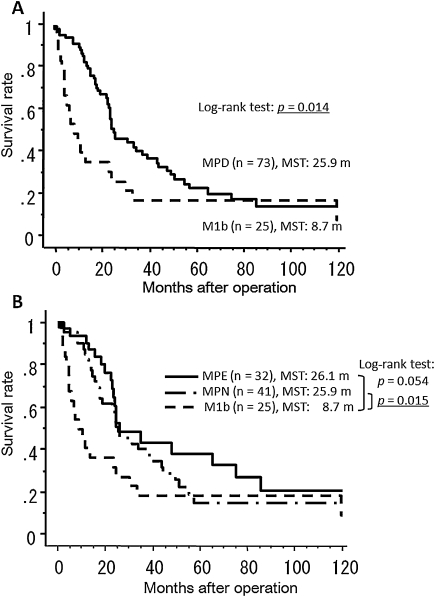
Patient characteristics are described in Table 1. Patients with contralateral lung metastases, who were included in the M1a group, were excluded from this analysis because of the low number of these patients (n = 2). There were no significant differences between the three patient groups, except for gender. Significantly more male patients existed in the M1b group compared to the other groups (p = 0.0001). Most of the MPD cancers were adenocarcinomas (90.6% MPE and 80.5% MPN), whereas 24% of the M1b cancers were squamous cell carcinomas. Approximately half of the MPD patients had tumors less than 3 cm in size (50% of MPE and 51.2% of MPN patients). The proportions of MPE and MPN patients with N0–1 status were 43.8% and 48.7%, respectively. The MST of all patients (n = 100) with pathological stage IV disease according to the seventh edition of the UICC staging system was 24.3 months. The 3-year and 5-year survival rates were 25.2% and 22.2%, respectively. The survival curves of patients with MPD (n = 73) and M1b (n = 25) disease are shown in Fig. 1A. The MST, the 3-year survival rate, and the 5-year survival rate were 25.9 months, 41.4%, and 23.7%, respectively, in the MPD patients, and 8.7 months, 18%, and 18%, respectively, in the M1b patients. The difference in overall survival was significant (long-rank test: p = 0.014), although there appeared to be little difference in long-term survival.
Table 1:
Clinical and pathological characteristics in patients with malignant pleural disease or M1b disease
| MPE |
MPN |
M1b |
p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Number | 32 | 41 | 25 | |||||
| Age (y) | Mean | 63.7 | (SD 9.6) | 62.4 | (SD 10.9) | 61.5 | (SD 12.5) | 0.51 |
| Gender | Male | 9 | 28.1 | 19 | 46.3 | 21 | 84 | 0.0001 |
| Female | 23 | 71.9 | 22 | 53.7 | 4 | 16 | ||
| Histology | Adenocarcinoma | 29 | 90.6 | 33 | 80.5 | 16 | 64 | 0.096 |
| Squamous cell | 3 | 9.4 | 5 | 12.2 | 6 | 24 | ||
| Large cell | 0 | 0 | 1 | 2.4 | 2 | 8 | ||
| Adenosquamous | 0 | 0 | 2 | 4.9 | 1 | 4 | ||
| Tumor size | <3 cm | 16 | 50 | 21 | 51.2 | 8 | 32 | 0.1 |
| >3 cm | 16 | 50 | 20 | 48.8 | 17 | 68 | ||
| Nodal status | N0 | 11 | 34.4 | 14 | 34.1 | 7 | 28 | 0.12 |
| N1 | 3 | 9.4 | 6 | 14.6 | 1 | 4 | ||
| N2 | 16 | 50 | 19 | 46.3 | 12 | 48 | ||
| N3 | 2 | 6.3 | 2 | 4.9 | 5 | 20 | ||
| Procedure | Partial resection | 2 | 6.3 | 6 | 14.6 | 1 | 4 | 0.46 |
| Lobectomy | 26 | 81.3 | 29 | 70.7 | 18 | 72 | ||
| Bilobectomy | 3 | 9.4 | 4 | 9.8 | 4 | 16 | ||
| Pneumonectomy | 1 | 3.1 | 2 | 4.9 | 2 | 8 | ||
MPE = malignant pleural effusion without nodules; MPN = malignant pleural nodules with or without effusion.
Figure 1:
(A) Postoperative survival curves of patients with malignant pleural disease (MPD) and patients with distant metastasis (M1b); (B) postoperative survival curves of patients with malignant pleural effusion without nodules (MPE), patients with malignant pleural nodules with or without effusion (MPN) and patients with distant metastasis (M1b). (MST = median survival time).
Survival of patients with MPD
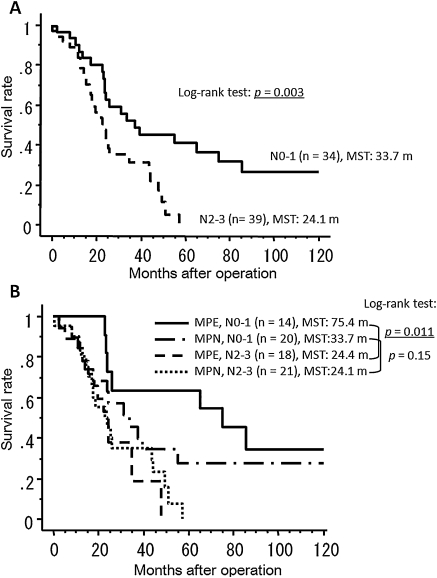
Results of univariate survival analyses using the Cox proportional hazards model are shown in Table 2. Although MPD status (MPE or MPN) was not significant, the N status (N0–1 vs N2–3) and type of surgical procedure (pneumonectomy vs other procedures) were found to be significant prognostic factors, and these results were confirmed by multivariate analysis (Table 3). In comparison with stage IV M1b patients, MPE patients had significantly better survival (p = 0.015) and MPN patients had a more favorable prognosis (p = 0.054) (Fig. 1B). The MST, the 3-year survival rate and the 5-year survival rate were 26.1 months, 43.1%, and 37.7%, respectively, in the MPE patients, and 25.9 months, 39.9%, and 14.8%, respectively, in the MPN patients. The survival curves of MPD patients stratified by N status (N0–1 vs N2–3) are shown in Fig. 2A and demonstrate a significant difference between the two categories (log-rank test: p = 0.003). We further divided these groups into four categories according to MPD status (Fig. 2B). MPE patients with N0–1 status (n = 14) had the best survival, with a 5-year survival of 63.6%, which is significantly better than MPE patients with N2–3 status (p = 0.011). Although the outcomes of MPN patients with N0–1 status were not as good as the outcomes of the MPE N0–1 group, 27.3% of these MPN patients achieved long-term survival whereas none of the MPD patients with N2–3 status survived longer than 5 years after surgery.
Table 2:
Univariate analysis for prognostic factors in patients with malignant pleural disease
| n | MST | HR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| Age (y) | <65 | 40 | 24.1 | 1 | ||
| >65 | 33 | 31.6 | 0.95 | 0.54–1.67 | 0.85 | |
| Gender | Male | 28 | 25.6 | 1 | ||
| Female | 45 | 26.1 | 1.14 | 0.64–2.04 | 0.65 | |
| Histology | Adenocarcinoma | 62 | 25.9 | 1 | ||
| Non-adeno | 11 | 24.4 | 0.96 | 0.43–2.15 | 0.93 | |
| Tumor size | ≤3 cm | 37 | 26.1 | 1 | ||
| >3 cm | 36 | 25.9 | 1.16 | 0.66–2.03 | 0.61 | |
| Nodal status | N01 | 34 | 37.7 | 1 | ||
| N23 | 39 | 24.1 | 2.47 | 1.33–4.60 | 0.0044 | |
| Procedure | Other | 70 | 26.1 | 1 | ||
| Pneumonectomy | 3 | 12.8 | 7.35 | 2.10–25.6 | 0.0018 | |
| Pleural disease | MPE | 32 | 26.1 | 1 | ||
| MPN | 41 | 25.6 | 1.4 | 0.78–2.50 | 0.26 |
CI = confidence interval, HR = hazard ratio. MST = median survival time, MPE = malignant pleural effusion without nodules, MPN = malignant pleural nodules with or without effusion.
Table 3:
Multivariate analysis for prognostic factors in patients with malignant pleural disease
| HR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Age (y) | <65 | 1 | ||
| >65 | 0.92 | 0.51–1.66 | 0.79 | |
| Gender | Male | 1 | ||
| Female | 1.1 | 0.59–2.05 | 0.77 | |
| Nodal status | NO—1 | 1 | ||
| N2–3 | 2.39 | 1.21–4.74 | 0.01 | |
| Procedure | Others | 1 | ||
| Pneumonectomy | 5.29 | 1.48–18.9 | 0.013 | |
| Pleural disease | MPE | 1 | ||
| MPN | 1.29 | 0.71–2.32 | 0.4 |
CI = confidence interval, HR = hazard ratio. MST = median survival time, MPE = malignant pleural effusion without nodules, MPN = malignant pleural nodules with or without effusion.
Figure 2:
(A) Postoperative survival curves of patients with malignant pleural disease (MPD) according to N status; (B) postoperative survival curves of patients with malignant pleural effusion without nodules (MPE) and patients with malignant pleural nodules with or without effusion (MPN) stratified by N status. (MST = median survival time).
DISCUSSION
In the seventh edition of the UICC lung cancer staging system, patients with MPD, which includes malignant pleural effusion and/or malignant pleural nodules, were reclassified from stage IIIB (T4) to stage IV (M1a). The IASLC Lung Cancer Staging Project reported that patients with clinical MPD had a dismal prognosis, with an MST of 8 months and a 2% 5-year survival rate [3]. Although clinically diagnosed MPD patients generally present with a fair amount of malignant pleural effusion [5], MPD that is first encountered at thoracotomy usually has less effusion or fewer pleural nodules, both of which were not definitively detected by preoperative examinations [8]. Therefore, a subgroup of patients with minimal pleural disease may be at an earlier stage of cancer progression in the pleural cavity. There have been a series of papers investigating the survival of patients with pleural disease detected by PLC. Although this group was reported to have a worse outcome than those patients without pleural disease [12–14], they have not been classified as MPD patients and surgical treatment has been generally undertaken even if the cytology is confirmed positive before resection. Recently, the International Pleural Lavage Cytology Collaborators performed a pooled data analysis on the survival of surgical patients with positive PLC [15]. In this analysis, the overall 1- and 5-year survival rates of positive PLC patients were 80% and 31%, respectively. They concluded that the impact of positive PLC on survival was equivalent to a single increase in the T category.
The IASLC Staging Project showed that pathologically staged MPD patients, most of whom were surgically treated, had an MST and 5-year survival rate of 18 months and 11%, respectively [3]. These outcomes were better than those for the clinically staged MPD patients mentioned previously. Since 2000, there have been five relatively large studies, including a former study at our institution, investigating more than 43 surgical MPD patients (Table 4). Ichinose et al. [6] gathered data on 227 surgically treated MPD patients from 21 institutions registered with the Japan Clinical Oncology Group (JCOG). The survival results of this study are very similar to the results from the IASLC. In addition, none of the 34 patients, who did not undergo resection of the primary lesion, survived for more than 5 years after thoracotomy. Similar results were reported by Fukuse et al. [8]. By contrast, unfavorable survival was demonstrated in the study reported by Sawabata et al. They investigated patients with minor malignant pleural effusion, with volumes less than 300 ml [10]. They did not observe any survival benefits for resection of the primary lesion compared to incomplete resection or exploratory thoracotomy. In the present study, the outcomes of MPD patients were better than the historical outcomes, with an MST of 25.9 months, and 3-year and 5-year survival rates of 41.4% and 23.7%, respectively. These outcomes were also significantly better than those of the surgically treated M1b patients. Pleural chemotherapy was performed in half of our patients, and one-third of the patients underwent postoperative adjuvant chemotherapy. These postoperative treatments may have played a role in these favorable outcomes. In addition, patients who did not undergo pulmonary resection were not included in this series. Preoperative selection of surgical patients may have contributed in part to the results, although pulmonary resection was primarily undertaken after encountering MPD at thoracotomy.
Table 4:
Summary of studies since 2000 of surgically treated patients with malignant pleural disease
| Author | Year | Patients | n | Survival |
Prognostic factors | ||
|---|---|---|---|---|---|---|---|
| MST (months) | 3-Y (%) | 5-Y (%) | |||||
| Ichinose | 2000 | MPD | 227 | 16.9 | 26.2 | 13.6 | N factor |
| Macroscopic residual tumor | |||||||
| Ohta | 2000 | MPN | 43 | 17 | 31.4 | 13.1 | Cell type |
| Shiba | 2001 | MPD | 65 | NA | NA | 14.3 | Ki-67 labeling index |
| N factor | |||||||
| Tumor differentiation | |||||||
| Fukuse | 2001 | MPD | 49 | 15 | 26.7 | 15.6 | Pleural nodules |
| T factor | |||||||
| Sawabata | 2002 | MPE | 43 | 13 | NA | 5 | NA |
| This study | MPD | 75 | 29.5 | 41.4 | 23.7 | N factor | |
MPD = malignant pleural disease; MPE = malignant pleural effusion without nodules, MPN #= malignant pleural nodules with or without effusion; MST = Median survival time; NA = not available; Y = year.
In the JCOG study [6], findings of adenocarcinoma, no clinical lymph node metastases, and no macroscopic residual tumors were demonstrated to be prognostic factors for better outcomes. Patients with these three factors had a promising 5-year survival rate of 24%. In another JCOG study, 100 patients with minimal MPD consisting of MPE of less than 300 ml or few malignant pleural nodules were investigated [16]. The 3-year and 5-year survival rates were 31.8% and 22.8%, respectively, results similar to those for resected patients with positive PLC. The subset analysis from Fukuse et al. [8] demonstrated that patients with MPE had significantly better outcomes than patients with malignant pleural nodules (hazard ratio HR 3.24, 95% confidence interval 1.26–8.35, p = 0.015), and they suggested that selected patients with small MPE volumes may have significant benefits from surgical resection. In the present study, multivariate analysis revealed that pneumonectomy and N status were significant prognostic factors in MPD patients, although no differences were seen between the two types of MPD (MPE vs MPN). Regarding surgical procedures, patients who underwent pneumonectomy had much worse outcomes than those who underwent less resection. Because only three patients underwent pneumonectomy, the significance of this prognostic factor should be regarded with caution. One reason for the poor outcomes may be that patients who underwent pneumonectomy had more advanced disease than the other patients; two out of three patients actually had N2 disease. No prognostic difference was seen for the other surgical procedures, including lobectomy, which was performed for the majority of our MPD patients (75%).
Regarding lymph node status in our study, for all MPD patients, N0–1 patients had significantly better outcomes than N2–3 patients. Similar results were reported from two previous studies [6,9]; however, they demonstrated a survival advantage for node-negative patients (N0) compared with node-positive patients (N1–3). In the present study, the prognostic significance was larger for the combined N0 and N1 group than for N0 alone (data not shown). In the JCOG study [6], the percent of patients undergoing lobectomy was 64%, which is lower than the percent of our patients undergoing lobectomy and bilobectomy (85%), and this may account for the different study results. If this result is confirmed, it may be reasonable to choose lobectomy as the surgical treatment for MPD patients.
When MPD patients were divided into MPE and MPN groups, which were further divided according to nodal status, the outcomes of N0–1 patients with MPE were much better than the outcomes of the other groups, with an MST of longer than 5 years. One possible explanation for this unexpected, good outcome is that there may have been patients who had malignant cells only in the pleural effusion but not on the parietal pleura. In such patients, especially those with early N status, cancer cells could have been completely removed by the surgical procedure. In the MPN patients, no significant difference was seen between the two nodal-status groups; however, the 5-year survival rate for N0–1 patients was greater than 20%, which appears to be a better outcome compared to the outcome for N2–3 patients with MPN where no patient was alive 5 years after operation.
There are limitations to our study, because it is a retrospective analysis based on assessable historical data. A randomized prospective study is needed to confirm the effect of surgery for MPD patients; however, the results of this study suggest that resection of the primary site can be considered for MPE patients with N0–1 status. In addition, among MPN patients with N0–1 status, there are patients who also achieve long-term survival after pulmonary resection.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors thank Professor Makoto Suzuki of the Department of Thoracic Surgery, Graduate school of Medicine, Kumamoto University, for his kind review of this article.
REFERENCES
- 1.Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res. 1997;3:47–50. [PubMed] [Google Scholar]
- 2.Jett JR, Scott WJ, Rivera MP, Sause WT American College of Chest Physicians. Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest. 2003;123:221S–5S. doi: 10.1378/chest.123.1_suppl.221s. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Sobin LH, Gospodarowicz MK, Wittekind C, International Union Against Cancer . 7th ed. Oxford, UK: Wiley-Blackwell; 2009. TNM classification of malignant tumours. p. 138–46. [Google Scholar]
- 5.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–50. doi: 10.4065/83.2.235. [DOI] [PubMed] [Google Scholar]
- 6.Ichinose Y, Tsuchiya R, Koike T, Kuwahara O, Nakagawa K, Yamato Y, Kobayashi K, Watanabe Y, Kase M, Yokoi K Japan Clinical Oncology Group. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today. 2000;30:1062–6. doi: 10.1007/s005950070002. [DOI] [PubMed] [Google Scholar]
- 7.Ohta Y, Tanaka Y, Hara T, Oda M, Watanabe S, Shimizu J, Watanabe Y. Clinicopathological and biological assessment of lung cancers with pleural dissemination. Ann Thorac Surg. 2000;69:1025–9. doi: 10.1016/s0003-4975(99)01579-9. [DOI] [PubMed] [Google Scholar]
- 8.Fukuse T, Hirata T, Tanaka F, Wada H. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer. 2001;34:75–81. doi: 10.1016/s0169-5002(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 9.Shiba M, Kakizawa K, Kohno H, Shibuya K, Yamakawa H, Hiroshima K, Fujisawa T. Prognostic implication of Ki-67 immunostaining in treating subclinical pleural cancer found at thoracotomy in lung cancer patients. Ann Thorac Surg. 2001;71:1765–71. doi: 10.1016/s0003-4975(01)02589-9. [DOI] [PubMed] [Google Scholar]
- 10.Sawabata N, Matsumura A, Motohiro A, Osaka Y, Gennga K, Fukai S, Mori T Japanese National Chest Hospital Study group for Lung Cancer. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: is tumor resection beneficial for prognosis? Ann Thorac Surg. 2002;73:412–5. doi: 10.1016/s0003-4975(01)03426-9. [DOI] [PubMed] [Google Scholar]
- 11.Travis WB, Brambilla E, Muller-Hermelink HK, Harris CC World Health Organization Classification of Tumours. Lyon: IARC Press; 2004. Pathology and Genetics of Tumours of the Lung. Pleura, Thymus and Heart. [Google Scholar]
- 12.Okada M, Sakamoto T, Nishio W, Uchino K, Tsuboshima K, Tsubota N. Pleural lavage cytology in non-small cell lung cancer: lessons from 1000 consecutive resections. J Thorac Cardiovasc Surg. 2003;126:1911–5. doi: 10.1016/s0022-5223(03)00715-3. [DOI] [PubMed] [Google Scholar]
- 13.Lim E, Ali A, Theodorou P, Nicholson AG, Ladas G, Goldstraw P. Intraoperative pleural lavage cytology is an independent prognostic indicator for staging non-small cell lung cancer. J Thorac Cardiovasc Surg. 2004;127:1113–8. doi: 10.1016/j.jtcvs.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Vicidomini G, Santini M, Fiorello A, Parascandolo V, Calabrò B, Pastore V. Intraoperative pleural lavage: is it a valid prognostic factor in lung cancer? Ann Thorac Surg. 2005;79:254–7. doi: 10.1016/j.athoracsur.2004.06.115. [DOI] [PubMed] [Google Scholar]
- 15.International Pleural Lavage Cytology Collaborators. Impact of positive pleural lavage cytology on survival in patients having lung resection for non-small-cell lung cancer: an international individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2010;139:1441–6. doi: 10.1016/j.jtcvs.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose Y, Tsuchiya R, Koike T, Kuwahara O, Nakagawa K, Yamato Y, Kobayashi K, Watanabe Y, Kase M, Yokoi K Japan Oncology Group. Prognosis of resected non-small cell lung cancer patients with carcinomatous pleuritis of minimal disease. Lung Cancer. 2001;32:55–60. doi: 10.1016/s0169-5002(00)00206-3. [DOI] [PubMed] [Google Scholar]