Abstract
OBJECTIVES:
Reduced glutathione (GSH) has been shown to improve pulmonary graft preservation. Mitochondrial dysfunction is regarded to be the motor of ischemia–reperfusion injury (IR) in solid organs. We have shown previously that IR induces pulmonary mitochondrial damage. This study elucidates the impact of GSH preconditioning on the integrity and function of pulmonary mitochondria in the setting of warm pulmonary IR.
METHODS:
Wistar rats were subjected to control, sham, and to two-study-group conditions (IR30/60 and GSH-IR30/60) receiving IR with or without GSH preconditioning. Rats were anesthetized and received mechanical ventilation. Pulmonary in situ clamping followed by reperfusion generated IR. Mitochondria were isolated from pulmonary tissue. Respiratory chain complexes activities (I–IV) were analyzed by polarography. Mitochondrial viability (Ca2+-induced swelling) and membrane integrity (citrate synthase assay) were determined. Subcellular-fractional cytochrome C-content (Cyt C) was quantified by enzyme-linked immunosorbent assay (ELISA). Mitochondrial membrane potential (ΔΨm) was analyzed by fluorescence-activated cell sorting (FACS) after energizing and uncoupling. Inflammatory activation was determined by myeloperoxidase activity (MPO), matrix-metalloproteinase 9 (MMP-9) activity by gel zymography.
RESULTS:
Pulmonary IR significantly reduced mitochondrial viability in combination with ΔΨm hyper-polarization. GSH preconditioning improved mitochondrial viability and normalized ΔΨm. Cyt C was reduced after IR; GSH protected from Cyt C liberation. Respiratory chain complex activities (I, II, III) declined during IR; GSH protected complex II function. GSH also protected from MMP-9 and neutrophil sequestration (P > .05).
CONCLUSIONS:
GSH preconditioning is effective to prevent mitochondrial death and improves complex II function during IR, but not mitochondrial membrane stability. GSH-mediated amelioration of ΔΨm hyper-polarization appears to be the key factor of mitochondrial protection.
Keywords: Ischemia–reperfusion injury, Mitochondria, Respiratory chain, ΔΨm, Glutathione
INTRODUCTION
Since 1989, approximately 30 000 pulmonary transplantations were performed worldwide [1]. Mortality remains high, with acute graft dysfunction as the major risk factor for in-hospital death. Hence, improved graft preservation appears to be the key factor to avoid ischemia–reperfusion injury (IR) associated with early graft failure following pulmonary transplantation. Recently, we have demonstrated, in a rat model, that IR induces pulmonary mitochondrial damage [2]. Earlier, we have observed improved graft function using Celsior® solution in a large animal model [3]. We assumed reduced glutathione (GSH) to be the responsible component for superior graft preservation. In a following experiment, we have demonstrated protective effects of GSH-supplemented Perfadex® solution on early allograft function [4].
It is well established that mitochondria are highly susceptible to ischemia followed by reperfusion. Mitochondrial dysfunction resulting from IR jeopardizes cellular integrity of graft tissue: generation of reactive oxygen species (ROS), induction of apoptosis, and cellular depletion of high energetic triphosphates due to inhibition of the respiratory chain are the most detrimental results of IR-related mitochondrial injury [5–8]. IR-induced GSH decay appears to be pivotal in the development of mitochondrial dysfunction followed by allograft failure. Renner et al. [9] documented loss of GSH followed by lipid per oxidation in cardiomyocytes undergoing IR. Brown et al. [10] clearly evidenced association between GSH decay and destabilization of the mitochondrial membrane potential (ΔΨm) resulting in the development of cardiac arrhythmia. In IR, GSH functions as a reducing agent for detoxifying free hydrogen peroxide by the GSH peroxidase I enzyme enabling graft protection against oxygen-derived radicals [11]. Obviously, stabilization of the cellular pool of GSH might avoid mitochondria-derived radical-dependent cell injury. In addition, the degree of mitochondrial injury determines the cellular fate [12]. After ischemia and during reperfusion, increasing intracellular Ca2+ concentration in association with rapid equilibrium of an acidotic pH induces mitochondrial transition followed by induction of apoptosis [6]. To some degree, existing data suggest direct GSH dependency of mitochondrial survival [13,14]. Experimental depletion of the mitochondrial GSH pool also activates the mitochondrial transition pore (mMTP) and subsequent induction of apoptosis.
Complex II (succinate–ubiquinone oxidoreductase, SQR) catalyzes the oxidation of succinate to fumarate in the mitochondrial matrix. Following succinate oxidation, ubiquinone is reduced at the inner mitochondrial membrane. Catalysis at SQR contributes to mitochondrial superoxide generation [15]. For defense and signaling, SQR hosts relevant amount of reactive thiol groups such as GSH. Hence, GSH decay results in uncontrolled superoxide production and blocking of respiratory complex enzymes. We propose beneficial effects of GSH preconditioning on mitochondrial integrity and function during warm pulmonary IR followed by ameliorated tissue damage. To elucidate GSH-dependent mitochondrial protection, we performed comprehensive mitochondrial analysis in the setting of warm pulmonary ischemia of 30 min followed by 60 min of reperfusion in Wistar rats. The aim of this study was to demonstrate: (1) GSH-related protection of the respiratory chain during pulmonary IR; (2) protection of mitochondrial viability by GSH preconditioning; (3) inhibition of mitochondria-dependent induction of apoptosis by GSH preconditioning; and (4) GSH-related amelioration of tissue degradation, inflammation, and edema formation during IR.
MATERIALS AND METHODS
Experimental set-up
Comprehensive mitochondrial analysis consisted of photometric mitochondrial viability testing, fluorescence-activated cell sorting (FACS) analysis of mitochondrial membrane potential (ΔΨm), photometric citrate synthase assay, and respiratory chain analysis by polarography. Tissue degradation was reflected by matrix-metalloproteinase 9 (MMP-9) gelatin in vitro zymography, and pulmonary reperfusion edema was determined by tissue wet to dry weight ratio. Inflammation due to neutrophil granulocyte sequestration was assessed by tissue myeloperoxidase activity (MPO).
Animals
All animals received humane care in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animalsprepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised in 1996 as well as in compliance with the European Convention on Animal Care. Local authorities approved all animal experiments. Male Wistar rats (180–250 g) were obtained from Harlan-Winkelmann (Borchen, Germany).
Reagents and buffers
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich GmbH (Munich, Germany). Mitochondrial isolation buffer (Buffer 1a) contained 0.225 M mannitol, 0.005 M 3-(N-morpholino)propanesulfonic acid (MOPS), 0.075 M sucrose, 0.002 M Ethylenediaminetetraacetic acid (EDTA), and 1 mg ml−1 Bovine serum albumin (BSA) at pH 7.4. Mitochondrial washing buffer (Buffer 1b) contained 0.225 M mannitol, 0.075 M sucrose, and 20 mM Tris/HCl at pH 7.4 respiration buffer (Buffer 2) consisted of 0.3 M mannitol, 10 mM KH2PO4, 10 mM KCl, and 5 mM MgCl2 at pH 7.2. Mitochondria swelling buffer (Buffer 3) contained 250 mM sucrose, 5 mM KH2PO4, and 3 μM rotenone. Citrate synthase buffer (Buffer 4) contained 0.01 M Tris–HCL, 1 mM 5,5′-dithiobis-2-nitrobenzoic acid, 5 mM acetyl-CoA, and 5% Triton X in H2O. FACS buffer (Buffer 5) consisted of 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 250 mM sucrose, 10 mM MgCl2, and 12.5 mM KH2PO4. Zymography homogenization buffer (Buffer 6) contained 25 mM Tris/HCl, 150 mM NaCl, 1% Na-deoxycholate, 1% NP 40%, 0.1% Na-dodecylsulfate (SDS), and Complete Protease Inhibitor Cocktail Set, EDTA-free (Roche Diagnostics Grenzach, Germany) at pH 7.6.
Surgery and isolation of mitochondria
Animals were subjected to controls, sham, ischemia followed by reperfusion (IR30/60) and to IR after preconditioning with GSH (GSH-IR30/60) with n = 6 animals in each group. After induction of anesthesia using Isoflurane®, tracheal intubation using a 16G cannula was performed via surgical tracheotomy. Volume-controlled ventilation was started at a fiO2 of 1.0 with a tidal volume of 1.0–3.0 ml. Anesthesia was maintained by 1.5–3.0% of Isoflurane®. The left lung was accessed via left-lateral thoracotomy and 30 min of pulmonary hilum clamping-induced ischemia, and reperfusion was carried out for 60 min. Animals were sacrificed and lungs were harvested. Sham animals received 90 min of mechanical ventilation after thoracotomy and pulmonary hilum preparation without ischemia. Healthy animals without undergoing any surgery served as controls. For mitochondria isolation, lung tissue was immersed in 4 °C phosphate-buffered saline (PBS). Tissue was minced followed by dispersion in Buffer 1a using a Potter homogenizer with glass pistil (Potter-Elvehjem). The suspension was centrifuged for 5 min at 1500 × g at 4 °C. The pellet was discarded and the supernatant was filtered twice. The filtrate was centrifuged at 13,000 × g for 10 min at 4 °C. The subcellular fraction containing supernatant was stored at −80 °C. The pellet was washed twice using Buffer 1b. Protein content was measured using the BCA-test (Thermo Fischer Scientific, Germany) according to the manufacturer’s instruction. Mitochondria were suspended in Buffer 2 with the protein concentration adjusted to 150 μg/400 μl. In preparation for MPO assay and MMP9 gelatin zymography, lung tissue was snap frozen in liquid nitrogen and stored at −80 °C.
Assay of mitochondrial oxygen consumption and analysis of respiratory chain complexes
Mitochondrial oxygen consumption was measured using the Oxytherm® Clark-type electrode (Hansatech Instruments Ltd., Norfolk, UK). A modified protocol according to Zini et al. [16] was applied. Briefly, 300 mg of crude mitochondria was suspended in 300 μl Buffer 2 at 37 °C. After equilibration for at least 2 min, the substrates malate/pyruvate (10 mM each) were added followed by 0.2 mM ADP. State 2 respiration was measured for 30 s after malatate/pyruvate, and 30 s after addition of ADP state 3 respiration was determined (30 s). The rates of state 2 and state 3 respirations were computed from slopes of oxygen uptake vs time (dO2/dt). The respiratory control ratio (RCR) resulted from the ratio of state 3/state 2 respiration. The activity of respiratory chain complexes I–IV was determined according to Rustin et al. modified by Zini et al.
Complexes I–V: Mitochondria were suspended in 300 μl Buffer 2. Mitochondrial protein concentration was adjusted to 1 μg protein/μl. After equilibration, pyruvate followed by malate was added. State 2 respiration was determined. After addition of ADP, state 3 respiration was measured.
Complexes II–V: As much as 300 μg of mitochondria was suspended in 300 μl Buffer 2. After equilibration, complex I was blocked by rotenone (0.2 mM). Mitochondria were energized by succinate (1 M) and state 2 respiration was checked. State 3 respiration was quantified after addition of adenosine diphosphate (0.2 mM).
Complexes III–V: According to complexes II–V, mitochondria were suspended in Buffer 2. After addition of rotenone and succinate, succinate dehydrogenase (complex II) was blocked by malonate (1 M). Respiration was determined for 30 s (slope I). After adding glycerol-3-phosphate (G3P), respiration was measured again (slope II).
Complexes II–IV: In 300 μl Buffer 2 supplemented by 0.2 mM rotenone and 1 M succinate, mitochondrial state 2 oxygen consumption and state 3 respiration after supplementation of 0.2 mM ADP were checked, respectively. Complex V was blocked adding 1 mM oligomycin and respiration was recorded for 30 s (slope 1). Uncoupling of mitochondrial respiration was achieved by 1 M carbonyl cyanide m-chlorophenylhydrazone (CCCP), and oxygen consumption was monitored for 30 s (slope 2). The ratio of slopes was calculated.
Complexes III, IV: Mitochondrial oxygen consumption in Buffer 2 containing 0.2 mM rotenone, 1 mM oligomycin, and 1 mM succinate was determined for 30 s. After addition of 1 mM CCCP, oxygen consumption was recorded for 30 s. 1 M Malonate was added and slope was recorded for 30 s. After addition of 1 M G3P, oxygen take-up was measured.
Complex IV: Mitochondrial oxygen consumption in Buffer 2 containing 0.2 mM rotenone, 1 mM oligomycin, and 1 mM succinate was determined for 30 s; CCCP was added and oxygen uptake was measured. After addition of 0.1 mM antimycin a, 0.5 M ascorbate and 0.1 M TMPD oxygen consumption were recorded for 1 min.
Mitochondrial viability testing by Ca2+-induced swelling of energized mitochondria
Ca2+-induced swelling of energized mitochondria was determined according to Halestrap and Davidson [17] with slight modifications. Mitochondria of 300 μg were suspended in 200 μl Buffer 3. Complex I was blocked by 3 mM rotenone. Mitochondria were energized by 6 mM succinate. Swelling in the presence of 25 mM Ca2+ was determined by measuring the decrease of optical absorption at 520 nm using an Ultrospect 3000 spectrophotometer (GE Healthcare Munich, Germany).
Analysis of mitochondrial membrane potential (ΔΨm) by flow cytometry analysis
In the presence of rotenone and succinate, 100 μg of mitochondria was suspended in 100 μl Buffer 5 and energized by 10 mM succinate after inhibition of complex I by 2 μM rotenone. Mitochondria were stained by 2 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC1, Enzo Life Sciences GmbH Lörrach, Germany). ΔΨm stability was assessed after CCCP-induced (0.5 μM) mitochondrial uncoupling. Flow cytometry (FACS) was performed using a BD FACScan Flow-Cytometer (Becton Dickenson, Heidelberg, Germany) as described by Lecoeur et al. [18]. Briefly, settings of FACScan were applied as followed: mitochondrial size was measured by the forward scatter (FWS) adjusted to an E-00 setting with logarithmic amplification of 5.41. Mitochondrial granulation was detected by the sideward scatter (SSC) adjusted to linear amplification at a voltage of 581 mV and a gain of 4.27. JC-1 green fluorescence reflects JC-1 monomers and was detected using the FL-1 channel. Formation of J-aggregates led to orange fluorescence, measured in the FL-2 channel. Fluorescence was recorded using the logarithmic amplifier mode. FL-1 was adjusted to a voltage of 907 and 622 mV for FL-2, respectively. Spectral compensation was performed as follows: FL1 – 18.5% FL2 and FL2 – 25.4% FL1. As many as 25 000 counts of the main mitochondrial region, gated on FSW/SSC parameters, were recorded. Data were analyzed using the WinMDI software (http://facs.scripps.edu/software.html). The ratio of J-aggregate+-mitochondria and JC1+-mitochondria was used to assess ΔΨm. Measurements were performed in native mitochondria, immediately after JC 1 staining, 10 min after JC 1, immediately after uncoupling with CCCP, and 1, 2,and 3 min after CCCP.
Quantification of cytochrome C (Cyt C) content from subcellular fractions
After tissue disruption using a potter pistil, the supernatant was collected. Total protein content was determined using the BCA assay. Cyt C content was analyzed using the R&D system’s rat/mouse Cyt C immunoassay (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany) according to the manufacturer’s recommendations. Cyt C content was expressed as ng Cyt C/μg total protein content.
Mitochondrial membrane integrity analysis by citrate synthase assay
Latent citrate synthase (CS) activity was evaluated according to Bugger et al. [5] and Chemnitius et al. [19]. In brief, latent CS (CSlatent) is calculated from total CS (CStotal) and free CS (CSfree). The CS ratio (CSR) represents the ratio of latent and free CS activity. The CSR resembles structural integrity of the mitochondrial preparation. Citrate synthase activity was determined isolated mitochondria (60 μg) in Buffer 4 at 25 °C. Maximal enzyme activity was measured spectrophotometrically at λ = 412 nm after addition of 50 μl 10 mM oxaloacetate in 0.1 M Tris–HCl at pH 8.5. CStotal was determined after pre-incubation of mitochondria with 2.5% Triton X-100 and CSfree after preincubation with H2O, respectively. Enzyme kinetics was analyzed by computing slopes from resulting curves.
Testing for activity of MMP-9 using gelatin-zymography
Snap frozen Lung tissue was homogenized in Buffer 6 using a Tissue-lyser (Quiagen, Hilden, Germany). The suspension was centrifuged 10 000 RCF for 10 min at 4 °C. The pellet was discarded. The supernatant’s protein content was determined using the BCA-test. As much as 40 μg of protein suspended in SDS-sample Buffer (2:1 volume ratio) was loaded on zymography gel. Electrophoresis was performed for 2.5 h at constant 125 mV. Gels were repeatedly washed in aqua bidest and re-natured by immersing for 4 × 30 min in renaturing buffer (Invitrogen Darmstadt, Germany). Gels were developed overnight at 37 °C using Invitrogen LC2671 developing buffer (Invitrogen, Darmstadt, Germany). After washing, gels were stained using Invitrogen LC6060 Simply Blue Safe stain (Invitrogen, Darmstadt, Germany). After washing, gels were digitized. Analysis was performed using the ImageJ open source software suite (http://rsbweb.nih.gov/ij/index.html). Data are expressed semi-quantitatively.
Assessment of neutrophil granulocyte sequestration by tissue MPO assay
Relative neutrophil sequestration into lung tissue was assessed by MPO assay. Frozen lung tissue was homogenized in 1.5 ml of 0.02 M potassium phosphate buffer (pH 7.4). The suspension was centrifuged at 10 000 × g for 15 min. The supernatant was discarded and the pellet was washed 3 times in potassium phosphate buffer followed by centrifugation at 10 000 × g for 15 min. Incubation at 60 °C for 2 h was carried out. The pellet was re-suspended in 1 ml of 0.5% hexaolecyltrimethyl ammonium bromide (HTAB) in 50 mM potassium phosphate solution (pH 6.0) and homogenized. Tissue was disrupted by sonication and three freeze–thaw cycles (liquid nitrogen bath/37 °C water bath). The suspension was centrifuged at 10 000 × g for 15 min. Protein content of the supernatant was assessed using the BCA-test (Thermo Fischer Scientific, Germany). Aliquots (70 μl) of supernatant were added to 630 μl of tetramethylbenzidine substrate system (Sigma-Aldrich GmbH Munich, Germany) at pH 6.0. The change in absorption at 655 nm at 25 °C over 3 min was recorded. Assays were performed as repeated measures and results are expressed as means in mU MPO/μg protein.
TWC measured using tissue wet to dry weight ratio
Lung water content was determined from fresh tissue. Specimens harvested were weighed before drying overnight at 60 °C (mwet). Dry weight (mdry) was measured and tissue water content (TWC) was calculated: TWC = 1 − (mwet/mwet + mdry). Values are expressed in percentages.
Statistical analysis
Results are expressed as means ± standard deviations (SD) or 95% confidence intervals (CI95%), unless otherwise stated. A non-parametric Kruskal–Wallis test combined with a Dunn’s multiple comparisons test was applied to test for differences between groups. Analysis of continuous data was performed using a repeated measures ANOVA combined with the Bonferroni’s multiple comparison test. A P-value <.05 was considered significant. All tests were computed using GraphPad Prism for Mac OS X Version 5.0c.
RESULTS
Mitochondrial respiratory chain dysfunction at complex II was ameliorated by GSH preconditioning
Ischemia followed by reperfusion impairs state 2 and state 3 respiration of pulmonary mitochondria (Table 1). GSH preconditioning improved function of complex cascades II–V and II–IV (P < .05, Kruskal–Wallis test). Regarding the calculated ratio of complexes II–IV, values determined from IR30/60 and GSH-IR30/60 differed significantly (P < .05, Dunn’s multiple comparison test).
Table 1:
Polarograhic determination of respiratory complex activities: during IR activities revealed a significant decline in O2 consumption compared to controls. GSH preconditioning protected complex cascades II-V and II-IV. Differences between groups reached statistical significance (P < .05). Complex II-IV activities of IR30/60 differed significantly from GSH-IR30/60. Data are expressed as means ± SD
| Control | Sham30/60 | IR30/60 | GSH-IR30/60 | |||
|---|---|---|---|---|---|---|
| I-V | State 2-respiration | 2.6 ± 0.5 | 2.6 ± 0.8 | 1.6 ± 0.4 | 1.5 ± 0.6 | P < .01 |
| State 3-respiration | 3.1 ± 0.9 | 2.7 ± 0.9 | 1.6 ± 0.3 | 1.6 ± 0.6 | P < .01 | |
| RCR | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.2 | n.s. | |
| II—V | State 2-respiration | 3.2 ± 0.4 | 3.3 ± 0.7 | 1.4 ± 0.4 | 2.1 ± 0.3 | P < .001 |
| State 3-respiration | 4.2 ± 1.2 | 4.2 ± 1.3 | 1.5 ± 0.7 | 2.4 ± 0.6 | P < .001 | |
| III—V | State 3-respiration | 6.5 ± 1.5 | 6.2 ± 1.3 | 3.9 ± 0.9 | 3.9 ± 1.4 | P < .01 |
| ll-IV | Slope 1 | 3.6 ± 0.8 | 3.0 ± 1.1 | 1.1 ± 0.3 | 3.6 ± 1.1 | P < .001 |
| Slope II | 5.8 ± 1.0 | 5.8 ± 1.3 | 3.2 ± 0.9 | 4.5± 1.0 | P < .001 | |
| Ratio | 1.7 ± 0.5 | 1.9 ± 0.2 | 2.5 ± 0.6 | 1.3 ± 0.15 | P > .0001 | |
| III—IV | Slope 1 | 1.3 ± 0.2 | 1.5 ± 0.6 | 1.0 ± 0.2 | 1.1 ± 0.4 | n.s. |
| Slope II | 2.3 ± 0.7 | 2.6 ± 0.2 | 1.8 ± 0.1 | 2.4 ± 0.7 | P = .058 | |
| Ratio | 1.8 ± 0.2 | 1.8 ± 0.7 | 1.7 ± 0.3 | 2.2 ± 0.4 | n.s. | |
| IV | Slope | 37.4 ± 6.5 | 33.1 ± 6.0 | 29.2 ± 4.4 | 27.3 ± 6.8 | n.s. |
Mitochondrial viability determined by Ca2+-induced swelling was improved by GSH preconditioning during IR
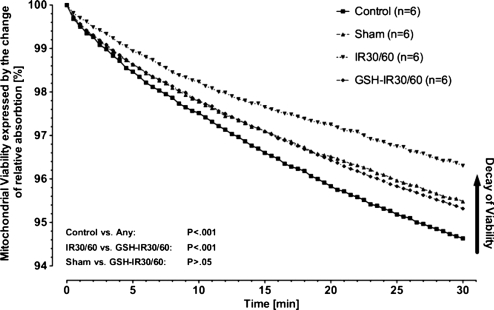
Ca2+-induced swelling reflects mitochondrial viability. Differences between groups were highly significant (P < .0001, ANOVA). Mitochondria of controls achieved high declines in light absorption indicating good viability (Fig. 1). A significant decay in viability between controls and sham was evident (P < .001, Bonferroni’s multiple comparison test). Compared to controls or sham, IR30/60-mitochondria in both conditions demonstrated substantially impaired swelling (P < .001, Bonferroni’s multiple comparison test). Compared to IR30/60, GSH preconditioning (GSH-IR30/60) significantly preserved mitochondrial viability during IR (P < .001, Bonferroni’s multiple comparison test).
Figure 1:
Mitochondrial viability by Ca2+-induced mitochondrial swelling: differences between study groups were highly significant (P < .001). Compared to controls mitochondrial viability was significantly impaired in IR30/60 (P < .01). Compared to IR30/60 GSH preconditioning (GSH-IR30/60) significantly protected mitochondrial viability during IR (P < .01). Data are expressed as means.
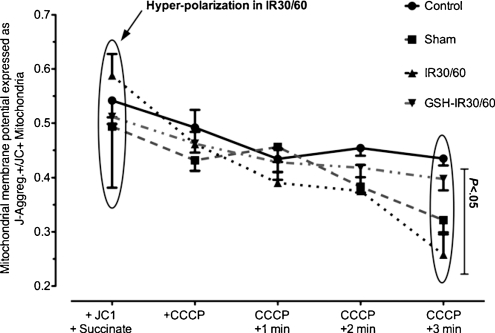
During IR, mitochondrial membrane potential (ΔΨm) of energized mitochondria revealed hyper-polarization and increased susceptibility to uncoupling by CCCP. GSH-preconditioning normalized ΔΨm during IR
Energized mitochondria of controls (Fig. 2) demonstrated a moderate ratio of J-aggregate+ to JC1+ mitochondria (0.54 ± 0.09). The ratio increased during IR in IR30/60 (0.61 ± 0.14) reflecting mitochondrial hyper-polarization. GSH preconditioning (GSH-IR30/60) normalized ΔΨm of energized mitochondria during IR (0.51 ± 0.04). The ΔΨm of energized mitochondria (+JC1/+succinate; Fig. 2) inter-group value did not differ significantly (P > .05, Kruskal–Wallis test). ΔΨm was jeopardized by the addition of CCCP. The decay of ΔΨm against time (0–3 min after CCCP) differed insignificantly between groups but showed a clear trend toward significance (P = .07, ANOVA). Decline in ΔΨm of IR30/60 was higher when compared with controls but GSH preconditioning inhibited ΔΨm decay. Inter group differences of ΔΨm kinetics were not significant (P > .05, Bonferroni’s multiple comparison test). Compared to controls (0.43 ± 0.03), ΔΨm decay was most pronounced in IR30/60 (0.26 ± 0.09) 3 min after CCCP. GSH preconditioning (GSH-IR30/60) normalized values (0.40 ± 0.06). ΔΨm 3 min after CCCP differed significantly between groups (P = .011, Kruskal–Wallis test). Compared to controls, IR30/60 showed a significant decay of ΔΨm 3 min after CCCP (CI95%: 0.39–0.48 vs 0.14–0.37, P < .05, Dunn’s multiple comparison test). Compared to IR30/60, values of GSH-IR30/60 did not differ significantly but showed a trend toward significance as expressed by 95% confidence intervals (CI95%: 0.14–0.37 vs 0.35–0.45, P > .05, Dunn’s multiple comparison test).
Figure 2:
FACS-analysis of ΔΨm in energized mitochondria and after uncoupling with CCCP: Values reflect the ratio of J-aggregate+-mitochondria and JC1+-mitochondria. Compared to controls membrane potential (ΔΨm) of succinate-energized mitochondria demonstrated hyper-polarization during IR (IR30/60). After uncoupling with CCCP ΔΨm declined faster in IR30/60-mitochondria when compared to controls. GSH preconditioning (GSH-IR30/60) prevented hyper-polarization and accelerated decline after CCCP comparing to IR30/60. Differences between groups reached statistical significance 3 min after CCCP addition (P < .05). Compared to controls values differed significantly in IR30/60 (P < .05). Differences between IR30/60 and GSH-IR30/60 did not differ significantly (P > .05). Data are expressed means ± SEM.
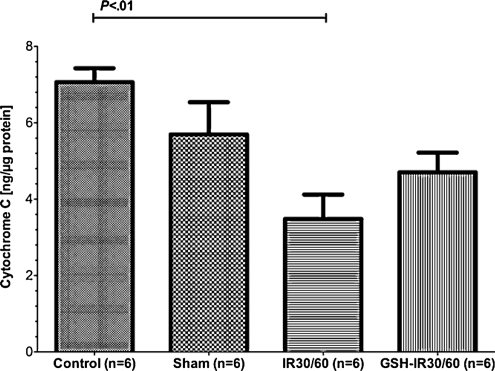
Mitochondrial Cyt C content determined from subcellular fractions was reduced during pulmonary IR and GSH-preconditioning ameliorated IR-related Cyt C loss
Cyt C was determined from supernatant obtained from mitochondria isolation. Regarding Cyt C content, inter group differences reached statistical significance (P = .003, Kruskal–Wallis test). When compared to controls, there was no significant change of Cyt C content in sham (CI95%: 6.22–7.91 vs 3.63–7.76, P > .05, Dunn’s multiple comparison test). Cyt C content was high in supernatant from control mitochondria (Fig. 3) and was significantly reduced in IR30/60 (P < .05, Dunn’s multiple comparison test). GSH preconditioning (GSH-IR30/60) insignificantly reduced loss of Cyt C determined from the subcellular fraction when compared to IR30/60 (CI95%: 3.49–5.92 vs 2.00–4.96, P > .05, Dunn’s multiple comparison test). Differences between sham and IR30/60 (CI95%: 3.63–7.76 vs 2.00–4.96, P > .05, Dunn’s multiple comparison test) as well as sham and GSH-IR30/60 (CI95%: 3.63–7.76 vs 3.49–5.92, P > .05, Dunn’s multiple comparison test) were statistically insignificant.
Figure 3:
Mitochondrial cytochrome C (Cyt C) content determined from sub-cellular fractions: regarding Cyt C, inter-group differences differed significantly (P = .003). Between controls and sham no statistical significant difference on Cyt C was detectable (P > .05). Cyt C content was high in controls and significantly declined during IR (P < .01). GSH preconditioning preserved tissue from Cyt C loss without reaching statistical significance when compared to controls or IR30/60 (P > .05). Data are expressed means ± SEM.
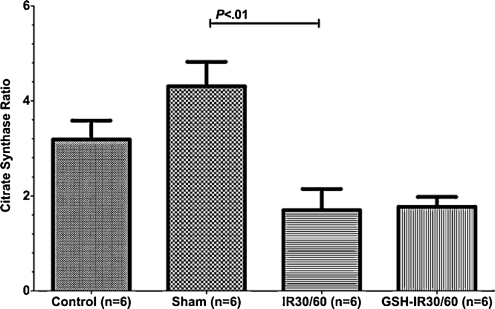
During warm pulmonary IR the CSR decreased and was not stabilized by GSH preconditioning
The CSR reflects mitochondrial membrane integrity (Fig. 4). Differences between groups differed significantly (P = .002, Kruskal–Wallis test). Between control and sham, differences of CSR were statistically insignificant (CI95%: 0.77–0.85 vs 0.81–0.89, P > .05, Dunn’s multiple comparison test) Mitochondria during IR (IR30/60) revealed a declined CSR comparing to sham (CI95%: 0.81–0.89 vs 0.67–0.83, P < .01, Dunn’s multiple comparison test). GSH preconditioning (GSH-IR30/60) did not improve CSR ratio when compared to IR30/60 group (CI95%: 0.68–0.74 vs 0.67–0.83, P > .05, Dunn’s multiple comparison test).
Figure 4:
Mitochondrial integrity reflected by the citrate synthase ratio (CSR): differences of CSR demonstrated statistical significance (P = .002). There was no statistical significant difference between control and sham (P > .05). The CSR declined during ischemia–reperfusion injury when compared to sham (P < .01). Compared to IR30/60 decay of CSR remained detectable in GSH-IR30/60 (P > .05). Data are expressed as means ± SEM.
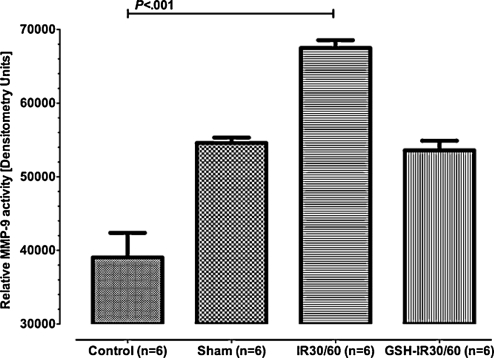
IR-related MMP-9 activation was limited by GSH-preconditioning
Semi-quantitative assessment of MMP9-activity was performed using gel zymography (Fig. 5). In control, values of MMP-9 activity were low, with significant MMP9-activation in tissue subjected to 30 min of ischemia and 60 min of reperfusion (IR30/60). GSH preconditioning ameliorated MMP9 activation. Inter group differences reached statistical significance (P = .0003, Kruskal–Wallis test), with significant differences between control and IR30/60 (P < .001, Dunn’s multiple comparison test). Differences between GSH-IR30/60 and control (CI95%: 50 233–56 950 vs 30 463–47 606, P > .05, Dunn’s multiple comparison test) or GSH-IR30/60 and IR30/60 did not reach statistical significance but differences of CI95% indicated a trend toward significance (CI95%: 50 233–56 950 vs 64 767–70 218, P < .05, Dunn’s multiple comparison test). MMP-9 activation differed insignificantly between controls and sham (CI95%: 30 463–47 606 vs 52 551–56 636, P > .05, Dunn’s multiple comparison test). Differences between sham and IR30/60 showed a trend toward statistical significance (CI95%: 52 551–56 636 vs 64 767–70 218, P > .05, Dunn’s multiple comparison test). MMP-9 activities after GSH preconditioning (GSH-IR30/60) equaled activities determined from sham (CI95%: 50 233–56 950 vs 52 551–56 636, P > .05, Dunn’s multiple comparison test).
Figure 5:
Activity of matrix metalloproteinase-9 determined by gelatin in vitro zymography. Values represent MMP-9 semi-quantitatively. Activities between groups differed significantly (P = .0003). Control and sham did not differ statistically significant (P > .05). Differences between control and IR30/60 reached a statistically significant level (P < .001). Differences between sham and IR30/60 showed a trend toward significance (P > .05). GSH preconditioning (GSH-IR30/60) effectively reduced MMP-9-activity during IR and showed to trend toward statistical significance when compared to IR30/60 (P > .05). Values of IR-IR30/60 almost equaled values of sham (P > .05). Data are expressed means ± SEM.
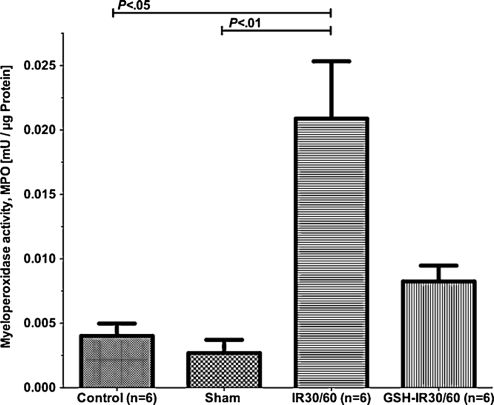
GSH preconditioning inhibited IR-induced sequestration of neutrophil granulocytes
Tissue MPO reflects sequestration of neutrophil granulocytes. Tissue samples of each group were examined (Fig. 6). Differences between groups reached statistical significance (P = .002, Kruskal–Wallis test). Between controls and sham, differences of MPO were statistically insignificant (CI95%: 0.001–0.006 vs 0.000–0.005, P > .05, Dunn’s multiple comparison test). When compared to sham, MPO was substantially elevated in IR30/60 (CI95%: 0.000–0.005 vs 0.011–0.031, P < .05, Dunn’s multiple comparison test). GSH-IR30/60 revealed statistical lower increase in MPO when compared to IR30/60, but differences in CI95% only indicated a trend toward significance (CI95%: 0.005–0.012 vs 0.011–0.031, P > .05, Dunn’s multiple comparison test).
Figure 6:
Myeloperoxidase assay (MPO) to assess sequestration of neutrophil granulocytes from lung tissue: MPO-activities of groups differed significantly (P < .0003). MPO activity remained low in controls and peaked in IR30/60 (P < .001). GSH-preconditioning (GSH-IR30/60) effectively suppressed MPO-activity during IR without reaching statistical significance when compared to controls or IR30/60 (P > .05). Data are expressed means ± SEM.
Development of pulmonary edema during IR was ameliorated after GSH preconditioning
TWC was determined from tissue specimens after termination of the experiment. Between groups, TWC differed significantly (P = .002, Kruskal–Wallis test). Regarding controls, TWC consisted of 82.0% (CI95%: 80.5–83.5%) and of 80.6% (CI95%: 78.5–82.5%) in shams, respectively. During IR (IR30/60), TWC was elevated up to 86.9% (CI95%: 85.9–87.8%). GSH preconditioning reduced increment of TWC to 85.0% (CI95%: 83.1–86.9%). When compared to sham, TWC differed significantly in IR30/60 (P < .01, Dunn’s multiple comparison test). Differences between IR30/60 and GSH-IR30/60 did not differ significantly (P > .05, Dunn’s multiple comparison test). When compared to sham, TWC was significantly increased in GSH-IR30/60 (P < .05, Dunn’s multiple comparison test).
DISCUSSION
This study elucidates the impact of GSH preconditioning on pulmonary mitochondrial integrity and function during warm IR of the lung in a rat model. We have demonstrated earlier that warm pulmonary IR impairs mitochondrial viability and function [2]. More recently, we have shown that reduced GSH as a supplement to LPD improves pulmonary graft function after prolonged cold ischemia [4]. Now, this study focuses on the impact of GSH preconditioning on pulmonary mitochondria and tissue damage in the setting of warm pulmonary IR.
Respiratory chain analysis demonstrates an amelioration of IR-related mitochondrial respiratory complex II dysfunction after GSH preconditioning. IR-related dysfunction of complexes I, III, IV and V remained unchanged despite preconditioning. Complex II (SQR) is known to be the major source of oxygen-derived radicals from the electron transport chain (ETC). To resist oxidation, complex II is conjugated to reducing thiols, especially to GSH. GSH depletion of complex II during oxidative stress has been described previously in the setting of Shiva et al. [20].
Our data suggest that GSH preconditioning preserves complex II function, but the underlying mechanism remains to be elucidated. Supposedly, GSH preconditioning inhibits free radical formation or it protects complex II glutathionylation due to an augmented availability of GSH. Future analysis of mitochondrial membrane peroxidation and determination of protein glutathionylation might provide more detailed information about the molecular pathways of GSH preconditioning regarding protection of mitochondrial integrity, complex function, and radical scavenging.
Beyond respiratory chain dysfunction, we detected IR-associated impaired mitochondrial viability combined with a loss of Cyt C. GSH preconditioning substantially improved mitochondrial viability and ameliorated loss of mitochondrial Cyt C during IR.
Reperfusion of ischemic organs rapidly normalized intracellular acidosis combined with an increase of intracellular Ca2+. Opening of the mitochondrial membrane transition pore (mMTP) is the consequence, followed by mitochondrial depolarization and Cyt C liberation. Cytoplasmic Cyt C activates the endogenous Caspase 9 pathway to induce apoptotic cell death [6].
We observed a decline in viable mitochondria combined with a loss of Cyt C in IR30/60, suggesting mitochondria-dependent induction of apoptosis in post-ischemic pulmonary tissue. GSH preconditioning may inhibit this pathway. This hypothesis is supported by previously published data, demonstrating that depletion of mitochondrial GSH directly induces mitochondrial transition [21]. Despite improvement of mitochondrial viability during IR after GSH preconditioning, it remains uncertain if GSH preconditioning is effective to inhibit induction of apoptosis.
ΔΨm is a pivotal factor, determining cellular survival during IR. It has been well documented that mitochondrial membrane potential hyper-polarization is associated with generation of ROS and cell death in the case of oxygen deprivation. It also reflects an intermediate state of apoptosis coming with mitochondrial liberation of Cyt C and disturbance of the ETC [7,22]. In addition, failure of ADP production in hyperpolarized mitochondria has been shown [23]. Our data reflect both aspects of ΔΨm alterations, hyper-polarization of energized mitochondria during IR, and an accelerated ΔΨm collapse after uncoupling with CCCP. GSH preconditioning (insignificantly) prevented hyper-polarization in state 2 respiration. After GSH preconditioning, the CCCP-induced decay of ΔΨm was ameliorated to untreated pulmonary tissue undergoing IR, suggesting GSH-dependent protection of pulmonary tissue during warm IR.
Regarding GSH preconditioning (IR30/60), our data demonstrate an insignificant but notable inhibition of Cyt C loss when compared to IR30/60, suggesting amelioration of mitochondria-induced apoptotic cell death in pulmonary tissue undergoing IR. However, our data only reveal a trend toward tissue protection, since statistical significance was not reached. Hence, analysis of the caspase cascade in following studies will be necessary to elucidate the impact of GSH on Cyt C-dependent induction of apoptosis during IR.
Mitochondrial viability, Cyt C quantification, and results of ΔΨm analysis suggest that GSH preconditioning protects mitochondria during pulmonary IR by normalizing ΔΨm. Results of the citrate synthase assay demonstrated partially contrary results. Although GSH preconditioning improved mitochondrial viability and ΔΨm kinetics, it did not prevent from altered CSR in mitochondria undergoing IR. CSR indicates an increased mitochondrial membrane permeability despite GSH preconditioning. This might result from sublethal mitochondrial injury not circumvented by GSH. However, data regarding the citrate synthase assay are only indirectly reflecting mitochondrial viability, unlike direct parameters such as ΔΨm and Cyt C.
Beyond mitochondrial damage, we quantified inflammation in pulmonary tissue (MPO), degradation (MMP-9), and edema formation (wet to dry weight ratio). Although none of these assays reached statistical significance, MMP-9 zymography and MPO demonstrated a statistical trend toward GSH-related protection of pulmonary tissue damage during IR. Regarding wet to dry weight ratio, we observed GSH-related amelioration of pulmonary tissue edema. However, these differences might have occurred by chance, since no statistic trend has been observed.
Data presented in this study might be influenced by the applied anesthetic regime. Volatile anesthetics, such as the used Isoflurane®, are known to ameliorate ischemia reperfusion responses of organs by inhibiting mitochondrial transition and ubiquitin-conjugated protein aggregation [24]. However, the effect of Isoflurane® on IR is controlled by using sham-operated animals. We used the autogenic warm ischemia model of the left lung. This experimental setup matches to warm pulmonary IR of lungs in the clinical setting of surgery with extracorporeal circulation. The influences of cellular interactions of MHC-disparate individuals influencing the pulmonary IR in lung transplantation are so far unknown. This study focused on the early reperfusion period and differences between warm and cold ischemia as a model for IR, which has not been addressed earlier [25]. Some end points reached statistical significance (3.1, 3.2), whereas others showed a trend toward significance (3.3, 3.6, 3.7). Regarding Cyt C and tissue wet to dry weight ratio, we detected GSH-related pulmonary tissue protection. However, these data might be coincidental, since no statistical trend was verifiable. However, presented data clearly indicate pulmonary tissue and mitochondria protection by GSH preconditioning.
CONCLUSION
We demonstrated GSH preconditioning as a potential regime to prevent mitochondrial injury in the setting of pulmonary IR. Our data suggest that (1) GSH preconditioning improves mitochondrial survival; (2) GSH preconditioning might prevent mitochondria from induction of apoptosis by inhibition of ΔΨm hyper-polarization or prevention of ΔΨm decay followed by an inhibition of Cyt C liberation; (3) it reduces damage of the respiratory chain complex II; and (4) inhibition of ΔΨm hyper-polarization or prevention of ΔΨm decay during IR may be the underlying mechanism.
However, additional work to elucidate molecular mechanisms in detail, especially induction of apoptosis, is necessary. We revealed some important information about the impact of GSH on mitochondrial integrity during warm pulmonary IR and demonstrated that mitochondrial injury is associated to tissue damage. These data contribute to the understanding of pulmonary IR and the impact of reduced GSH as a potential agent to prevent IR-related pulmonary failure.
ACKNOWLEDGEMENTS
The authors thank Ms. Renate Wahn and Ms. Heidi Linß for expert technical assistance and Dr Daniel Schäfer for helpful discussions.
APPENDIX A. CONFERENCE DISCUSSION
Dr S. Clark (Newcastle Upon Tyne, UK): Could you put this into context for those of us who are clinical rather than academi clung transplant surgeons, and say how important you feel it is for us to understand mitochondrial function in the context of is chemia—reperfusion injury.
Dr Sommer: I think it's essential, especially regarding mitochondrial viability and membrane hyperpolarization. Decay of mitochondrial viability initiates apoptosis via cytochrome c liberation and induction of caspase and from a scientific point of view, it has to be taken into account to avoid mitochondrial decay, to avoid delayed onset of ischemia—reperfusion injury days after transplantation.
Dr Clark: So if mitochondrial function is so crucial, do you have any plans to look at other agents apart from glutathione that are known to preserve mitochondrial structure and function? Hydrogen sulphide springs to mind, which has been used in in vivo experiments in cardiac reperfusion injury. I wondered if you had any thoughts about other directions that this type of work could go in.
Dr Sommer: Indeed. We are currently working with resveratrol, a polyphenol, and from a former study trying glycine, finding different patterns of mitochondrial protection. Forinstance, applying resveratrol for preconditioning; organ protection pretty much focuses on preserving respiratory chain function, whatever this means, but less on mitochondrial viability. So in my opinion, impairing respiratory chainfunction is bad, but I think protection of viability and inhibition of cytochrome c liberation is essential.
Dr D. Chambers (London, UK): I wondered whether you were able to correlate your mitochondrial changes with function. You showed function data in the pig, and now you have gone on to ther at to show mitochondrial data, but you need to show that your mitochondrial effects are actually correlated with functional improvement. Have you beenable to do that?
Dr Sommer: Yes and no. Assuming that our data from pig animal experiments are reliable, I pretty much focused on mitochondrial function and integrity in this experiment. It's clearly a flaw that I cannot correlate it with pulmonary function or hemodynamic data.
Dr Chambers: I would suggest that you try and do that. It may not have any effect at all.
Dr Sommer: I'm about to do that, yes, but we have not produced any data so far.
Funding
This work was supported by the Interdisciplinary Center for Clinical Research of the University Hospital Würzburg [A-132; 2010; http://www.izkf.uni-wuerzburg.de/].
Conflict of interest: none declared.
REFERENCES
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI. The registry of the international society for heart and lung transplantation: twenty-sixth official adult lung and heart-lung transplantation report-2009. J Heart Lung Transplant. 2009;28:1031–49. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.J Heart Lung Transplant. 2011;30:811–8. doi: 10.1016/j.healun.2011.02.001. Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, Schimmer C, Leyh RG. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. [DOI] [PubMed] [Google Scholar]
- 3.Sommer SP, Warnecke G, Hohlfeld JM, Gohrbandt B, Niedermeyer J, Kofidis T, Haverich A, Struber M. Pulmonary preservation with LPD and celsior solution in porcine lung transplantation after 24 h of cold ischemia. Eur J Cardiothorac Surg. 2004;26:151–7. doi: 10.1016/j.ejcts.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Sommer SP, Gohrbandt B, Fischer S, Hohlfeld JM, Warnecke G, Avsar M, Struber M. Glutathione improves the function of porcine pulmonary grafts stored for twenty-four hours in low-potassium dextran solution. J Thorac Cardiovasc Surg. 2005;130:864–9. doi: 10.1016/j.jtcvs.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Bugger H, Chemnitius J-M., Doenst T. Differential changes in respiratory capacity and ischemia tolerance of isolated mitochondria from atrophied and hypertrophied hearts. Metabolism. 2006;55:1097–1106. doi: 10.1016/j.metabol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Gogvadze V, Robertson JD, Zhivotovsky B, Orrenius S. Cytochrome c release occurs via Ca2+-dependent and Ca2+-independent mechanisms that are regulated by Bax. J Biol Chem. 2001;276:19066–71. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 7.Iijima T, Mishima T, Akagawa K, Iwao Y. Mitochondrial hyperpolarization after transient oxygen–glucose deprivation and subsequent apoptosis in cultured rat hippocampal neurons. Brain Res. 2003;993:140–5. doi: 10.1016/j.brainres.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Schild L, Reinheckel T, Wiswedel I, Augustin W. Short-term impairment of energy production in isolated rat liver mitochondria by hypoxia/reoxygenation: involvement of oxidative protein modification. Biochem J. 1997;328:205–10. doi: 10.1042/bj3280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renner A, Sagstetter MR, Gotz ME, Lange V, Bengel D, Harms H, Riederer P, Elert O. Heterotopic rat heart transplantation: severe loss of glutathione in 8-hour ischemic hearts. J Heart Lung Transplant. 2004;23:1093–102. doi: 10.1016/j.healun.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O’Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol. 2010;48:673–9. doi: 10.1016/j.yjmcc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thu VT, Kim HK, Ha SH, Yoo JY, Park WS, Kim N, Oh GT, Han J. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 12.Borutaite V. Mitochondria as decision-makers in cell death. Environ Mol Mutagen. 2010;51:406–16. doi: 10.1002/em.20564. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Armstrong JS. Role of calcium and cyclophilin D in the regulation of mitochondrial permeabilization induced by glutathione depletion. Biochem Biophys Res Commun. 2007;363:572–7. doi: 10.1016/j.bbrc.2007.08.196. [DOI] [PubMed] [Google Scholar]
- 14.Marina Prendes MG, Gonzalez MS, Torresin ME, Hermann R, Pascale NG, del Mar Jaitovich M, Savino EA, Varela A. Involvement of mitochondrial permeability transition, glutathione status, pentose phosphate pathway and oxidative damage in the protective effect of fasting on the ischaemic-reperfused rat heart. Clin Exp Pharmacol Physiol. 2009;36:637–42. doi: 10.1111/j.1440-1681.2008.05122.x. [DOI] [PubMed] [Google Scholar]
- 15.Messner KR, Imlay JA. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem. 2002;277:42563–71. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- 16.Zini R, Simon N, Morin C, Thiault L, Tillement JP. Tacrolimus decreases in vitro oxidative phosphorylation of mitochondria from rat forebrain. Life Sci. 1998;63:357–68. doi: 10.1016/s0024-3205(98)00284-7. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–60. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecoeur H, Langonne A, Baux L, Rebouillat D, Rustin P, Prevost MC, Brenner C, Edelman L, Jacotot E. Real-time flow cytometry analysis of permeability transition in isolated mitochondria. Exp Cell Res. 2004;294:106–17. doi: 10.1016/j.yexcr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Chemnitius JM, Häfner H, Kreuzer H, Zech R. Latent and free citrate synthase activity as enzymatic indicators for respiratory potential of isolated porcine heart mitochondria. J Appl Cardiol. 1988;3:301–10. [Google Scholar]
- 20.Shiva S, Crawford JH, Ramachandran A, Ceaser EK, Hillson T, Brookes PS, Patel RP, Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–66. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naoi M, Yi H, Maruyama W, Inaba K, Shamoto-Nagai M, Akao Y, Gerlach M, Riederer P. Glutathione redox status in mitochondria and cytoplasm differentially and sequentially activates apoptosis cascade in dopamine–melanin-treated SH-SY5Y cells. Neurosci Lett. 2009;465:118–22. doi: 10.1016/j.neulet.2009.08.082. [DOI] [PubMed] [Google Scholar]
- 22.Poppe M, Reimertz C, Dussmann H, Krohn AJ, Luetjens CM, Bockelmann D, Nieminen AL, Kogel D, Prehn JH. Dissipation of potassium and proton gradients inhibits mitochondrial hyperpolarization and cytochrome c release during neural apoptosis. J Neurosci. 2001;21:4551–63. doi: 10.1523/JNEUROSCI.21-13-04551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iijima T, Mishima T, Tohyama M, Akagawa K, Iwao Y. Mitochondrial membrane potential and intracellular ATP content after transient experimental ischemia in the cultured hippocampal neuron. Neurochem Int. 2003;43:263–9. doi: 10.1016/s0197-0186(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1152/ajpcell.00006.2010. Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T,Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+. Am J Physiol Cell Physiol. 2010 Aug;299(2):C506-15. Epub 2010 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warnecke G, Sommer SP, Gohrbandt B, Fischer S, Hohlfeld JM, Niedermeyer J, Haverich A, Struber M. Warm or cold ischemia in animal models of lung ischemia-reperfusion injury: is there a difference? Thorac Cardiovasc Surg. 2004;52:174–9. doi: 10.1055/s-2004-817977. [DOI] [PubMed] [Google Scholar]