Abstract
OBJECTIVE
The study aimed to evaluate the outcome of transatrial–transpulmonary repair of tetralogy of Fallot in relation to a right-ventricular outflow tract (RVOT)-sparing surgery.
METHODS
Based on the surgical management of right-ventricular outflow tract obstruction (RVOTO) at repair of tetralogy of Fallot, 140 children were retrospectively divided into three groups: (1) pulmonary valve (PV)-sparing, (2) infundibulum-sparing and (3) extended trans-annular patch (TAP). Clinical and echocardiographic outcome was assessed with regards to three equally divided study time eras between January 1994 and June 2010.
RESULTS
Over a 15-year study period, median age decreased from 11 (2–101) to 5 (1–11) months (p < 0.001), whereas type of RVOT repair changed significantly between the first and the last era (group 1: 18–40%, group 2: 25–40% vs group 3: 57–20% (p = 0.002)). Mortality was 0%. Complications were mainly related to clinical restrictive RV physiology (27%) and arrhythmia (10%). This cardiac morbidity remained constant over the eras and was associated with younger age (p = 0.04), increased postoperative right ventricle/left ventricle (RV/LV) pressure ratio (p = 0.01) and type of RVOT repair at the cost of TAP (p = 0.03). Median follow-up of 8 years (1–16 years) showed an overall freedom from RVOT re-operation of 84% and 73%, respectively at 5 and 10 years. Most re-operations were for residual/recurrent RVOTO (12%) occurring more frequently in the latter era: 16% versus 7% in era 1 (p = 0.08). Late echocardiographic evaluation revealed a strong correlation between severity of pulmonary regurgitation and increased RV/LV size ratio, which was mainly determined by increased TAP length (p < 0.001) and duration of follow-up (p = 0.06).
CONCLUSION
In a 15-year’s experience with transatrial–transpulmonary correction of tetralogy of Fallot, a valve- and infundibulum-sparing approach has been advanced by lowering the age for elective repair. This change has been performed without compromising immediate clinical outcome, despite an increased early re-operation rate for residual obstruction. However, longer follow-up will disclose whether this approach is protective against progressive and late RV dysfunction.
Keywords: Tetralogy of Fallot, Right-ventricular outflow tract, Transatrial repair
INTRODUCTION
More than 50 years’ experience with surgical treatment of tetralogy of Fallot (ToF) has revealed that the physiological sequelae of the formerly performed transventricular repair with a large trans-annular patch are not benign in the long-term [1–3]. Over the past decades, two issues have been addressed to optimize the surgical outcome: the type of right-ventricular outflow tract (RVOT) correction and the timing for complete repair.
Regarding the first issue, the transatrial–transpulmonary approach has gained much popularity by minimizing transmural myocardial scarring of the right ventricle [4,5]. Even though a trans-annular enlargement is required in 60–70% of the patients, leading to pulmonary valve incompetence, preservation of the RVOT integrity is often pursued by limiting the infundibular incision, considered as a right-ventricle infundibulum-sparing procedure [6]. Some authors have also advocated a pulmonary-valve- or annulus-sparing strategy in selected cases of severe pulmonary annulus hypoplasia, eventually associated with a separate transventricular patch plasty for adequate relief of the infundibular stenosis [7,8].
The second debate concerns the optimal time of primary repair. Protagonists of early corrective heart surgery have introduced complete repair of ToF in the neonatal period to overcome the potentially morbid effects of cyanosis and advanced RV hypertrophy, as well as the complications related to prior shunt palliation [9,10]. Opponents of this early repair have used the argument of increased morbidity and even mortality in these neonates, to strengthen their preference for a two-stage approach in infants with symptomatic ToF. In accordance with the ideas claimed by Fraser et al. [11], we have been adopting an individualized strategy in the surgical therapy of children with ToF. This included the use of modified Blalock–Taussig shunt (BTS) in the symptomatic neonate, particularly with ductal-dependent pulmonary flow, and in presence of small, native pulmonary branches. Complete repair has consistently been performed by a transatrial–transpulmonary approach within the first year of life. However, during this 15-year experience, we have gradually introduced a more RVOT-sparing approach, while lowering the age of repair simultaneously. The purpose of this study is to report on the early and late effects of both changes on clinical outcome, and to assess specifically the usefulness of an infundibulum-sparing approach in comparison with more extensive trans-annular patch plasty.
PATIENTS AND METHODS
Patients
This study has been approved by the Ethical Committee of the University Hospital of Gent (registration number B67020109388) and informed consent was waived for its retrospective design. The medical records of all patients, who underwent complete repair of ToF between January 1994 and June 2010, were reviewed, and consisted of 140 patients (84 males and 56 females). Patients with absent pulmonary valve syndrome and pulmonary atresia requiring insertion of a valved conduit for primary correction were excluded.
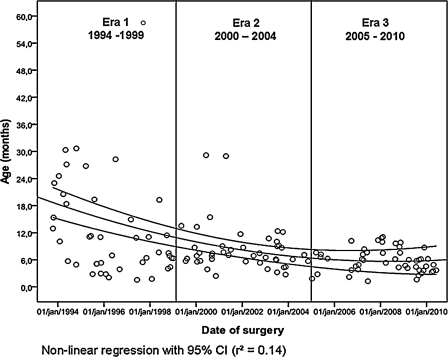
During the study period, the age at the time of complete repair progressively decreased, as depicted in Fig. 1. Based on this graphical analysis, three equally divided time periods were differentiated as era 1 for the period 1994–1999, era 2 for the period 2000–2004, and era 3 for the period 2005–2010.
Figure 1:
Evolution of age at repair over study period.
Surgical policy
During the whole study period, the operative management of children with ToF remained constant. Using standard cardiopulmonary bypass with moderate systemic hypothermia and cold crystalloid cardioplegia, complete repair entailed consistently transatrial closure of the ventricular septal defect with a 0.4-mm polytetrafluoroethylene (PTFE) patch (Gore-Tex, WL Gore & Associates, Flagstaff, AZ, USA) through the tricuspid valve, and excision/transsection of obstructive parietal and septal bundles in the infundibulum. Intra-atrial communications were closed routinely. Via a longitudinal arteriotomy of the pulmonary trunk, the size of the pulmonary branches was measured with Hegar dilators. After commissurotomy of the pulmonary valve, the adequacy of the pulmonary annulus size was compared with the minimal acceptable pulmonary size from Rowlatt’s tables [12]. If the pulmonary annulus was found appropriate within the lower limits of the expected normalized z-value and relief of the infundibular stenosis seemed satisfactory after further transpulmonary resection, the arteriotomy was closed with an autologous pericardial patch. When the pulmonary annulus was deemed too small, the pulmonary arteriotomy was extended across the annulus, by preference through the most anterior commissure, into the infundibulum. Based on this approach, we defined three types of RVOT repair: (group 1) a pulmonary valve-sparing procedure with preservation of the pulmonary valve annulus after commissurotomy, (group 2) an infundibulum-sparing procedure with a trans-annular incision limited to the first 5 mm of the RVOT, and (group 3) an extended trans-annular patch plasty, where the RVOT incision was extended beyond the first 5 mm, to cross the level of the hypertrophic infundibular septum.
At the end of the procedure, peak RV/LV pressure ratio determination and transesophageal echocardiography were performed in all patients. The RVOT repair was judged adequate when RV/LVP ratio was <0.8 and echocardiography excluded residual anatomic obstruction. When RV/LV pressure ratio was initially higher than 0.8, the decision regarding the appropriateness of RVOT relief was made by echocardiography to differentiate between anatomic versus dynamic obstruction, after intra-operative optimization of hemodynamics and withdrawal of inotropics.
The approximate length of the trans-annular incision, the Hegar size defining the final z-value of the RVOT, and the intra-operative RV/LV pressure ratio were noted in the operative record. In addition, the length of trans-annular incision was indexed to body surface area of each patient, to allow proper comparison of TAP length in relation to age at repair.
Postoperative follow-up
The end points of early outcome were mortality, ventilation support time, duration of intensive care and hospital stay, and morbidity defined by complications affecting one of the former end points. In particular, cardiac complications were related to RV dysfunction with clinical signs of restrictive RV physiology and temporarily increased need for inotropic support, and/or arrhythmia, mainly defined as junctional ectopic tachycardia.
Median follow-up was 7.7 years (range 6 months–16 years), and complete clinical data were available for at least 1 year since the closing date of this study in 85% of the patients. Follow-up focused on clinical evolution and need for late re-operation and/or re-intervention for RVOT-related issues. Clinical data were obtained from routine medical records, QRS duration from the last electrocardiogram and parameters as residual RVOT gradient, and grade of pulmonary valve regurgitation and RV/LV size ratio by measuring the maximal diameter of both ventricles on four-chamber view on the latest transthoracic echocardiography.
Statistical analysis
Continuous data are expressed as mean value ± standard deviation or median value and range. Categorical data are expressed as frequencies. Comparison of continuous data between different time periods or types of RVOT procedure has been done by one way-analysis of variance (ANOVA) with Tukey correction for multiple comparisons, in case of normally distributed data with equal variance. Kruskal–Wallis analysis followed by Mann–Whitney analysis for post hoc subgroup differences was used for non-normally distributed data. Between-group differences of categorical data were compared by chi-square or Fisher’s exact test.
Risk factor analysis of categorical outcome was performed by multivariate logistic regression in a backward likelihood-ratio model. A multivariate stepwise linear regression model was used to identify risk factors for increased RV/LV size ratio at the last echocardiography, after exclusion of patients who underwent late pulmonary valve implantation. Additional data exploration with the classification tree method has been carried out to determine a significant cut-off value for continuous variables reaching statistically significant influence in a multivariate model.
Freedom from time-related events was calculated with the Kaplan–Meier method using the log-rank test for univariate analysis. Multivariate Cox regression analysis was used to define independent predictors for late re-operation. Statistical analysis was performed with Statistical Package for Social Sciences (SPSS) 18 – Predictive Analysis Software (PASW) software version (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and operative results
Demographic data of the study population are depicted in Table 1. In the first era, patients were significantly older, having a median age of 11 months at the time of complete correction (p < 0.001). Although there was no significant age difference between era 2 and era 3, Fig. 1 shows a greater variability of age in era 2, whereas the children in era 3 were consistently operated on between 3 and 6 months of age. This evolution involved a higher proportion of children in era 1, that was operated on in a symptomatic status (66% in era 1 vs 40% in era 2 vs 28% in era 3, p = 0.001), which was reflected by the lower arterial saturation (p = 0.03). However, the percentage of patients treated previously by a shunt, as well as the frequency of associated genetic disorders, such as microdeletion 22q11 (n = 16) and trisomy 21 (n = 8) were equal over eras.
Table 1:
Demographic patient data over study eras
| Overall | Era 1 1994–1999 |
Era 2 2000–2004 |
Era 3 2005–2010 |
p-value | |
|---|---|---|---|---|---|
| Number patients | 140 | 44 | 43 | 53 | 0.48 |
| Median age (m) | 6.6 | 11a | 7 | 5 | <0.001 |
| Weight (kg) | 7.2 ± 2.3 | 8.1 ± 3.2a | 7.3 ± 1.9 | 6.5 ± 1.4 | 0.003 |
| Gender (m/f) | 84/56 | 22/22 | 28/15 | 24/19 | 0.26 |
| Genetic disorder | 24% | 18% | 28% | 25% | 0.55 |
| Previous shunt | 19% | 14% | 26% | 17% | 0.33 |
| Symptoms | 44% | 66% b | 40% | 28% | 0.001 |
| Median SaO2 | 87% | 84% b | 86% | 88% | 0.03 |
| Preop PV z-value | –2.32 ± 1.43 | –2.40 ± 1.40 | –2.08 ± 1.60 | –2.47 ± 1.30 | 0.41 |
a p < 0.05 between era 1 and eras 2–3.
b p < 0.05 between era 1 and era 3.
Within the study period, the morphologic features of ToF remained unchanged. Associated malformations were a persistent left superior caval vein (n = 8), a right-sided aortic arch (n = 18), atrial septal defect or patent foramen ovale (n = 67), aberrant coronary anatomy (n = 3), and atrioventricular septal defect (n = 6). RVOT characteristics showed no significant differences between the eras, as shown by the preoperative z-value of the pulmonary annulus. The mean size of the pulmonary branches was, respectively, 6.8 ± 1.1 mm for the right pulmonary artery and 6.7 ± 1.2 mm for the left pulmonary artery.
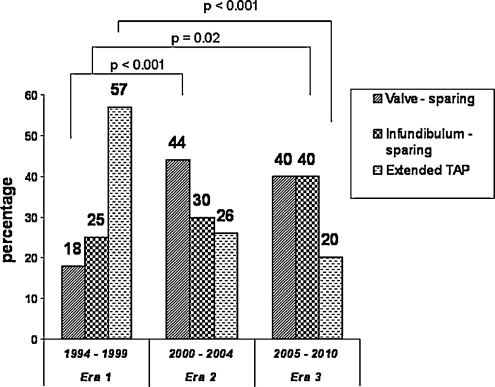
The evolution of the surgical approach of the right-ventricular outflow tract obstruction (RVOTO) reflects a significant higher proportion of extended trans-annular patch plasty in era 1, moving progressively to a more frequent valve- or infundibulum-sparing approach in eras 2 and 3 (p = 0.002) (Fig. 2). At the same time, the length of the trans-annular incision, indexed to the patient’s body surface area, decreased significantly from era 1 to eras 2 and 3: 19.9 ± 12.9 mm cm−2 in era 1 versus 11.5 ± 13.5 mm cm−2 in era 2 and 11.9 ± 12.8 mm cm−2 in era 3 (p = 0.005). In 21 (15%) patients, a pericardial patch plasty of a pulmonary artery stenosis was performed. The immediate operative result over the different time periods indicated a significant difference of the postoperative RVOT z-value between era 1 and era 3 (0.40 ± 0.81 in era 1, 0.22 ± 0.83 in era 2, and −0.11 ± 0.72 in era 3) (p = 0.007), whereas the intra-operative peak RV/LVP ratio remained equal (0.76 ± 0.17 in era 1, 0.74 ± 0.17 in era 2, and 0.69 ± 0.18 in era 3) (p = 0.16). In six (4%) patients, adjacent surgical revision by extending the trans-annular patch was required due to unacceptable residual RVOTO and suprasystemic RV pressures.
Figure 2:
Evolution of type of RVOT repair over study period.
When comparing the three RVOT treatment categories, some important differences in anatomic and physiologic characteristics were observed (Table 2). Patients in whom the pulmonary valve was preserved had a better preoperative arterial saturation, a larger native pulmonary annulus, and a more favorable RV/LV pressure ratio at the end of the procedure. However, patients undergoing an infundibulum-sparing repair or a more extended trans-annular incision had similar pre- and perioperative characteristics, unless there was a higher proportion of prior shunt use in the latter group (34% in group 3 vs 10% in group 2, p = 0.004).
Table 2:
Demographic and operative data between types of RVOT repair
| Group 1 Valve-sparing | Group 2 Infundibulum-sparing | Group 3 TAP | p-value | |
|---|---|---|---|---|
| Number patients | 48 | 45 | 47 | 0.66 |
| Median age (m) | 7 | 6 | 7.5 | 0.21 |
| Median SaO2 | 91%b | 87% | 85% | 0.03 |
| Previous shunt | 10% | 11% | 34%c | 0.004 |
| Preop PV z-value | −0.86 ± 1.15a | −2.84 ± 0.75 | −3.26 ± 0.95 | <0.001 |
| TAP length/BSA (mm cm”2) | − | 13.2 ± 3.6 | 30.2 ± 8.1 d | <0.001 |
| Postop PV z-value | 0.34 ± 0.94 | 0.0 ± 0.61 | 0.12 ± 0.84 | 0.13 |
| Postop RV/LVP | 0.65 ± 0.17a | 0.74 ± 0.16 | 0.79 ± 0.16 | <0.001 |
a p < 0.05 between group 1 and 2–3.
b p < 0.05 between group 1 and 3.
c p < 0.05 between group 3 and 1–2.
d p < 0.05 between group 2 and 3.
Early outcome
There was no operative mortality. The median ventilation time was 6 h (range 3–336 h). Median intensive care unit (ICU) and hospital stay were, respectively, 2 (range 1–21 days) and 9 days (range 7–42 days).
Two-thirds of the patients (68%) had an uneventful postoperative course. Major morbidity was mainly related to temporary RV dysfunction (27%) and arrhythmia (10%), such as junctional ectopic tachycardia. Other complications were pulmonary problems (pneumonia and chylothorax) (n = 8), renal failure requiring peritoneal dialysis (n = 5), and seizures (n = 4). Four patients needed early re-operation during the same hospital period, within 2–15 days after repair, because of residual RVOTO and subsequent RV dysfunction.
Table 3 shows an overview of the early results with regard to the different eras and the type of RVOT repair. There were no significant differences between the three eras, and regarding the type of RVOT procedure, only the patients undergoing a pulmonary valve-sparing surgery had less postoperative cardiac events. Multivariate analysis revealed three independent predictors for increased cardiac-related morbidity: higher RV/LV pressure ratio at the end of repair (odds ratio (OR) 29.7, 95% confidence interval (CI) 2.3–384.5, p = 0.01), type of RVOT repair at the cost of an extended trans-annular patch (OR 3.1, 95% CI 1.1–9.1, p = 0.03), and lower age at the time of correction (OR 0.93, 95% CI 0.86–0.99, p = 0.04), indicating a cut-off value at the age of 3 months.
Table 3:
Early outcome over study eras and between types of RVOT repair
| Era 1 1994-1999 | Era 2 2000-2004 | Era 3 2005-2010 | p-value | |
|---|---|---|---|---|
| All complications | 27% | 37% | 32% | 0.61 |
| Cardiac complications | 23% | 33% | 26% | 0.58 |
| Median ventilation time (h) | 8 | 5 | 5 | 0.62 |
| Median ICU stay (days) | 3 | 2 | 2 | 0.48 |
| Early 30-day re-operation | 0% | 2% | 6% | 0.26 |
| Group 1 PV-sparing | Group 2 Infund-sparing | Group 3 TAP | p-value | |
| All complications | 23% | 31% | 43% | 0.12 |
| Cardiac complications | 15%a | 29% | 38% | 0.03 |
| Median ventilation time (h) | 4 | 7 | 8 | 0.63 |
| Median ICU stay (days) | 2 | 2 | 3 | 0.20 |
| Early 30-day re-operation | 0% | 2% | 6% | 0.17 |
a p < 0.05 between group 1 and 3.
Late outcome and re-operations
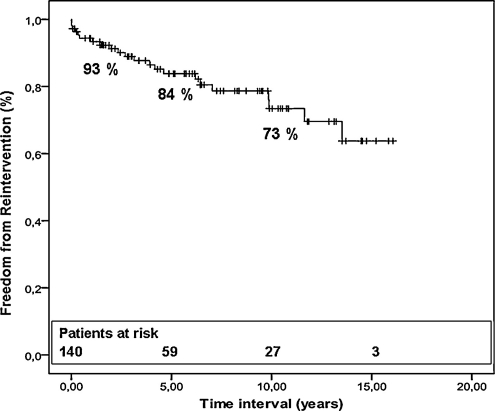
Within the mean follow-up of 7.5 ± 4.7 years (range 6 months–16 years), there was one late death, occurring during a re-operation for recurrent RVOTO 4 months after the primary repair. All patients in follow-up were doing well, with only three patients necessitating medication for cardiac reasons. Freedom from re-operation and/or re-intervention was 93 ± 2% at 1 year, 84 ± 3% at 5 years, and 73 ± 4% at 10 years (Fig. 3). Recurrent RVOTO (n = 16, 12%) was the major cause for re-intervention, treated by pulmonary artery stenting (n = 1), infundibular patch plasty (n = 2), extension of the trans-annular patch (n = 2), and resection of residual infundibular muscle (n = 5). In six patients with recurrent RVOTO, a pulmonary valve homograft was implanted together with resection of obstructing bundles, to treat simultaneously pulmonary regurgitation. Valvulation using a pulmonary homograft (n = 8) or a bovine jugular vein conduit (n = 1) was performed in nine (7%) patients for progressive RV dilation due to isolated pulmonary insufficiency. Finally, one patient required a pacemaker for atrioventricular block.
Figure 3:
Actuarial freedom curve from all re-operations.
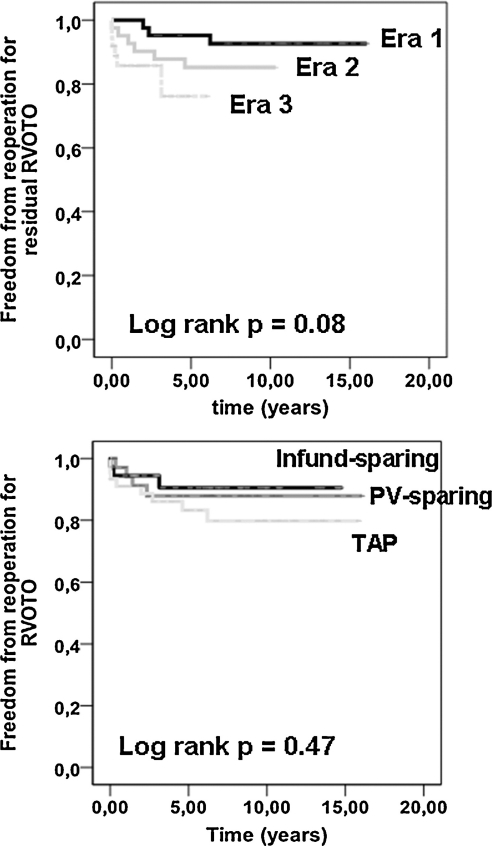
Univariate log-rank analysis for late re-intervention for recurrent RVOTO or pure pulmonary insufficiency (PI) was not influenced by the type of RVOT repair (p-value 0.47 and 0.34, respectively). However, there was a trend for a higher rate of late re-operation for recurrent RVOTO during the latter era of the study period (p = 0.08) (Fig. 4). Multivariate Cox-regression analysis revealed the following independent risk factors for any re-operation: type of RVOT repair, determined by extended trans-annular patch plasty (hazard ratio (HR) 12.3, 95% CI 2.6–58.7, p = 0.004) and era of surgery (era 3 vs era 1, HR 37.4, 95% CI 1.5–913.8, p = 0.005). However, concerning specifically re-operation for recurrent RVOTO, only increased RV/LV pressure ratio at the end of repair (HR 111.7, 95% CI 6.1–2059.9, p = 0.002) was predictive, indicated by a significant cut-off value of 0.84 (p = 0.006). The factor ‘era of surgery’ achieved statistical significance only regarding the comparison of era 3 versus era 1 (HR 5.4, 95% CI 1.3–22.8, p = 0.02). Considering the late need for PV implantation, type of repair (only group 3 vs group 1) (HR 39.7, 95% CI 3.1–501.1, p = 0.004) and increased RV/LV size ratio at echocardiography (HR 2.1, 95% CI 1.2–4.6, p = 0.03) were relevant risk factors.
Figure 4:
Actuarial freedom from re-operation for RVOTO recurrence. (a) Comparison between study eras. (b) Comparison between types of RVOT repair.
Last echocardiographic assessment
Complementary investigation during follow-up was routinely based on 12-lead electrocardiogram and transthoracic echocardiography. Table 4 shows the commonly available measurements in most patients by both examinations. Univariate analysis demonstrated a significant higher RV/LV diameter ratio (p < 0.001) in group 3, whereas patients of group 1 had significantly less pulmonary regurgitation (p < 0.001). Linear regression analysis revealed one major independent predictor for late RV dilation, as indicated by increased RV/LV size ratio: length of trans-annular incision (unstandardized β coefficient 0.16, 95% CI 0.009–0.897, p < 0.001). Duration of follow-up reached near-statistical significance (unstandardized β coefficient 0.008, 95% CI 0.000–0.17, p = 0.06).
Table 4:
Late echocardiographic results and QRS duration between types of RVOT repair
| All | Group 1 PV-sparing |
Group 2 Infund-sparing |
Group 3 TAP |
p-value | |
|---|---|---|---|---|---|
| Late RV/LV size ratio | 0.96 ± 0.20 | 0.88 ± 0.18 | 0.90 ± 0.15 | 1.15 ± 0.16b | <0.001 |
| Late PI grade | 2.4 ± 0.9 | 1.9 ± 0.9a | 2.5 ± 0.7 | 3.0 ± 0.9 | <0.001 |
| Late RVOTO (mmHg) | 15 ± 14 | 14 ± 14 | 16 ± 13 | 17 ± 15 | 0.62 |
| Late QRS (ms) | 120 ± 19 | 115 ± 21 | 122 ± 18 | 123 ± 17 | 0.26 |
ap < 0.05 between group 1 and 3.
bp < 0.05 between group 3 and 1–2.
DISCUSSION
This study actually reviews a single-center experience with the surgical treatment of ToF over the past 15 years. This included a management individualized to the patient by using a two-stage approach in symptomatic neonates with duct-dependent pulmonary flow, with first palliation by a modified BTS, followed by complete repair in infancy. A one-stage approach was adopted beyond the neonatal age, independent of their clinical status, and preferably within the first year of life. Complete correction has consistently been achieved by a transatrial–transpulmonary technique. According to Fraser et al. [11], our strategy has focused primarily on the type of RVOT repair, preserving by preference the ventricle and then the valve. During the study period, we have gradually lowered the age at repair, which facilitated simultaneously an RVOT-sparing approach.
Implications on early outcome
Although the morphology of the RVOTO in tetralogy patients has not changed substantially over time, this conceptual change allowed to decrease significantly the extent of the trans-annular incision, especially in patients with pulmonary annulus hypoplasia, and favored the use of an infundibulum-sparing repair instead of the more extensive trans-annular patch relief.
Advancing the timing of surgery has been an important issue to improve the surgical outcome of ToF. Neonatal repair has been favored to avoid the physiological consequences of hypoxemia and RV hypertrophy, as well as the problems associated to prior shunting. However, even in experienced institutions, this approach is associated with increased morbidity, leading to significantly longer ventilation times and intensive care stay, and eventual mortality [9,10]. However, it remains uncertain if such early repair is really advantageous to the RV in the long term, considering the high proportion of trans-annular patches and the decreased benefit of restrictive RV physiology. In a study of 227 patients reviewing a local change of practice lowering the age for primary repair, Van Arsdell et al. found that the optimal age for repair, in terms of physiological tolerance, is probably between 3 and 11 months of age [13]. Our results have shown that decreasing the age at repair – at least to the age of 3 months – while adapting a RVOT-sparing policy, did not affect the early clinical outcome. Postoperative morbidity was mainly related to transient RV function impairment and arrhythmia, which were independently supported by age younger than 3 months, and use of an extended trans-annular patch, but, especially an increased RV/LV pressure ratio at the end of repair. Both the last two factors are the direct effect of the interaction between repair type and the severity of RV hypertrophy, which in a sense is also age-related. The group of Great Ormond Street and Brompton Hospital has first demonstrated that impaired diastolic RV function was determined by the type of repair, in particular by trans-annular patch plasty [14]. However, in a later study, younger age at repair appeared to prevent the development of early restrictive RV physiology, as 80% of their patients aged <6 months, underwent a TAP repair. Hence, TAP repair was not an independent predictor for later restriction [15]. Subsequently, one can assume that the degree of RV hypertrophy is the main substrate for a restrictive RV pattern, certainly when the ventricle is challenged by the volume load due to variable pulmonary valve insufficiency. Based on the knowledge that a restrictive RV physiology has a beneficial long-term effect on RV function [14,16], this RVOT-sparing surgery seems justified in the attempt to maintain the functional advantage of RV hypertrophy against late RV dilation by pursuing some residual restrictive effective opening – as depicted by a lower pulmonary z-value in the last versus the first era.
Presently, we are proposing elective complete correction between 3 and 6 months of age, irrespective of symptomatic status or even previous palliation. We believe this age range enhances the opportunity to relieve the hypoplastic RVOTO with minimal trans-annular incision and less infundibular resection to deal with the balance between the postoperative adverse, but long-term protective effect of a restrictive physiology on RV function.
A side effect of the adoption of a RVOT-sparing policy in our series is a higher re-operation rate for residual or recurrent RVOTO. At 3 years, the freedom from re-operation was respectively 95 ± 3% in era 1, 88 ± 5% in era 2, and 86 ± 6% in era 3. Previous reports have already pointed to the higher residual outflow gradients after transatrial correction [4,17]. Based on a disturbing increased incidence of recurrent RVOTO, Alexiou et al. even reverted to a transventricular repair in tetralogy patients with severe infundibular stenosis [18]. According to Kaushal et al. [19], we have been using the addition of transesophageal echocardiography to differentiate between a fixed anatomical obstruction and a dynamic obstruction, when systemic or even suprasystemic RV pressures were recorded. On-site revision for inadequate RVOTO relief was 12% in their commonly older population, and noticeably higher than the 4% in our cohort. As a peak RV/LV pressure ratio above 0.84 was the strongest predictor of re-operation for recurrent RVOTO in our experience, we should question whether we had wrongly appreciated the first surgical result. Therefore, in the pursuit of the optimal equilibrium between adequate relief of pressure load and the potential inconvenience of pulmonary insufficiency-related volume load, the benefit of a RVOT-sparing procedure has to be weighed against the risk of incomplete RVOT relief and subsequent re-operation, by respecting more rigorously the recommended threshold of 0.8 for intra-operative RV/LV pressure ratio.
Considerations on late outcome
As the evidence is growing that the deleterious long-term complications of ToF repair are the result of the interaction between surgical-induced RVOT dysfunction and the mode of RV compliance, our surgical strategy is aimed to minimize the insult to the RV. Undeniably, the infants in whom a valve-sparing technique was carried out, belong to the better part of the tetralogy spectrum, with intrinsically an adequate-sized pulmonary valve and less RV hypertrophy. The most interesting comparison in this series concerns the patients with the narrowest outflow tract, treated by an infundibulum-sparing approach versus a more extensive trans-annular patch. Basic measurements on routine echocardiography at last follow-up indicated a close relationship between RV dilation and the extent of trans-annular patch repair. Moreover, the time span for which the RV is subjected to the PI-related volume load is an additional factor for increased RV dilation. These results are not surprising, as the use of TAP has currently a negative impact on both late clinical outcome and RV function. In a study of 59 patients, who were evaluated 14 years after contemporary transatrial–transpulmonary repair, van den Berg et al. found impaired functional capacity and moderate RV dysfunction in relation to the use of TAP [20].
However, the question remains whether an infundibulum-sparing repair will ultimately delay this physiological post-PI dilation process, as actually the follow-up of these RVOT-sparing procedures is too short to be conclusive. The exact role of the infundibulum on RV function is controversial. In a study concerning systolic RV function in young individuals without heart disease, Geva et al. demonstrated that the infundibulum contributed to only 13% of the total RV stroke volume [21]. Otherwise, d’Udekem et al. found that patching the subvalvular outflow tract affected both PV and RV function equally to trans-annular patch repair, and subsequently pointed out the peculiar function of this anatomic substrate as a kind of contractile support of the pulmonary valve [22,23]. We believe that the main advantage of the infundibulum-sparing technique is related to (1) the proper absorptive support to important pulmonary regurgitation and (2) the controlled restriction that helps to sustain the long-term benefit of RV hypertrophy.
Study limitations
There are some obvious shortcomings of this study, primarily because of its retrospective design. It covers a considerable time span, during which two of the utmost important variables of surgical outcome changed, that is, age at repair and type of RVOT reconstruction. Therefore, it is not easy to evaluate the clear effect of each change as the result of the confounding interference between both variables on the final results. Moreover, the number of patients in each era and repair group is small, perhaps limiting the strength of some interpretations.
In addition, the differentiation into three groups of RVOT repair is subjected to bias. ToF is known by a wide spectrum of RVOTO morphology. Children treated by a pulmonary-valve-sparing procedure have definitely a more friendly RVOT anatomy, resulting in better functional outcome and prognosis. Although it was retrospectively impossible to delineate the features of the infundibular stenosis, such as the infundibular length and the degree of infundibular septal hypertrophy and malalignment, we feel confident that the morphological RVOT differences between groups 2 and 3 are negligible. The conclusions on the comparison between both repair groups are thereby valid, but need further follow-up to prove consistency.
Finally, the echocardiographic analyses are only based on measurements of some rude parameters, as afforded by routine ambulatory echocardiographies performed beyond study purposes. More in-depth elaboration of parameters of systolic as well as diastolic RV performance, using echocardiography and/or magnetic resonance, will probably better clarify the exact contribution of each type of repair.
In conclusion, a valve- and infundibulum-sparing reconstruction of the RVOT has been advanced reciprocally through and together with lowering the age at repair during transatrial–transpulmonary correction of ToF. This change has been achieved without compromising the immediate clinical outcome, but at the cost of an increased early re-operation rate for residual and/or recurrent obstruction. As the length of the trans-annular relief of the RVOT is, with time, the main determinant of RV dilation, further long-term follow-up is needed to disclose whether such infundibulum-sparing approach is more protective against progressive RV dysfunction.
Conflict of interest: none declared.
REFERENCES
- 1.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Eng J Med. 1993;329:593–9. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Kirklin JW, Blackstone EH, Milano A, Pacifico AD. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann Thorac Surg. 1989;48:783–91. doi: 10.1016/0003-4975(89)90671-1. [DOI] [PubMed] [Google Scholar]
- 3.Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot: QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231–7. doi: 10.1161/01.cir.92.2.231. [DOI] [PubMed] [Google Scholar]
- 4.Karl TR, Sano S, Pornviliwan S, Mee RB. Tetralogy of Fallot: favorable outcome of nonneonatal transatrial, transpulmonary repair. Ann Thorac Surg. 1992;54:903–7. doi: 10.1016/0003-4975(92)90646-l. [DOI] [PubMed] [Google Scholar]
- 5.Stellin G, Milanesi O, Rubino M, Michielon G, Bianco R, Moreolo GS, Boneva R, Sorbara C, Casarotto D. Repair of tetralogy of Fallot in the first six months of life: transatrial versus transventricular approach. Ann Thorac Surg. 1995;60:S588–91. doi: 10.1016/0003-4975(95)00849-7. [DOI] [PubMed] [Google Scholar]
- 6.Morales DL, Zafar F, Fraser CD. Tetralogy of Fallot repair: the right ventricle infundibulum sparing strategy. Semin Thorac Cardiovasc Pediatr Card Surg Ann. 2009;12:54–8. doi: 10.1053/j.pcsu.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005;80:1431–9. doi: 10.1016/j.athoracsur.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Boni L, Garcia E, Galletti L, Pérez A, Herrera D, Ramos V, Marianeschi SM, Comas JV. Current strategies in tetralogy of Fallot: pulmonary valve sparing and evolution of right ventricle/left ventricle pressure ratios. Eur J Cardiothorac Surg. 2009;35:885–90. doi: 10.1016/j.ejcts.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Parry AJ, McElhinney DB, Kung GC, Mohan Reddy V, Brock MM, Hanley FL. Elective primary repair of acyanotic tetralogy of Fallot in early infancy: overall outcome and impact on the pulmonary valve. J Am Coll Cardiol. 2000;36:2279–83. doi: 10.1016/s0735-1097(00)00989-x. [DOI] [PubMed] [Google Scholar]
- 10.Hennein HA, Mosca RS, Urcelay G, Crowley DC, Bove EL. Intermediate results after complete repair of tetralogy of Fallot in neonates. J Thorac Cardiovasc Surg. 1995;109:332–44. doi: 10.1016/S0022-5223(95)70395-0. [DOI] [PubMed] [Google Scholar]
- 11.Fraser CD, McKenzie ED, Cooley DA. Tetralogy of Fallot: surgical management individualized to the patient. Ann Thorac Surg. 2001;71:1556–63. doi: 10.1016/s0003-4975(01)02475-4. [DOI] [PubMed] [Google Scholar]
- 12.Rowlatt JF, Rimoldi HJA, Lev M. The quantitative anatomy of the normal child’s heart. Pediatr Clin North Am. 1963;10:499. [Google Scholar]
- 13.Van Arsdell GS, Maharaj GS, Tom J, Rao VK, Coles JG, Freedom RM, Williams WG, McCrindle BW. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000;102(Suppl. III):III-123–2. doi: 10.1161/01.cir.102.suppl_3.iii-123. [DOI] [PubMed] [Google Scholar]
- 14.Norgard G, Gatzoulis MA, Moraes F, Lincoln C, Shore DF, Shinebourne EA, Redington AN. Relationship between type of outflow tract repair and postoperative right ventricular diastolic physiology in tetralogy of Fallot: implications for long-term outcome. Circulation. 1996;94:3276–80. doi: 10.1161/01.cir.94.12.3276. [DOI] [PubMed] [Google Scholar]
- 15.Munkhammar P, Cullen S, Jogi P, De Leval M, Elliot M, Norgard G. Early age at repair prevents restrictive right ventricular physiology after surgery for tetralogy of Fallot. J Am Coll Cardiol. 1998;32:1083–7. doi: 10.1016/s0735-1097(98)00351-9. [DOI] [PubMed] [Google Scholar]
- 16.Gatzoulis MA, Clark AL, Cullen S, Newman CGH, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot: restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–81. doi: 10.1161/01.cir.91.6.1775. [DOI] [PubMed] [Google Scholar]
- 17.Mee RB. Transatrial transpulmonar repair of tetralogy of Fallot. Ann Card Surg. 1998;10:141–7. [Google Scholar]
- 18.Alexiou C, Chen Q, Galogavrou M, Gnanapragasam J, Salmon AP, Keeton BR, Haw MP, Monro JL. Repair of tetralogy of Fallot in infancy with a transventricular or a transatrial approach. Eur J Cardiothorac Surg. 2002;22:174–83. doi: 10.1016/s1010-7940(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal SK, Radhakrishanan S, Singh Dagar K, Iyer PU, Girotra S, Shrivastava S, Iyer KS. Significant intraoperative right ventricular outflow gradients after repair of tetralogy of Fallot: to revise or not to revise? Ann Thorac Surg. 1999;68:1705–13. doi: 10.1016/s0003-4975(99)01069-3. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg J, Hop WC, Strengers JLM, de Jongste JC, van Osch-Gevers L, Meijboom FJ, Pattynama PM, Bogers AJJC, Helbing WA. Clinical condition at mid-to-late follow-up after transatrial-transpulmonary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2007;133:470–7. doi: 10.1016/j.jtcvs.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Geva T, Powell AJ, Crawford EC, Chung T, Colan SD. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation. 1998;98:339–45. doi: 10.1161/01.cir.98.4.339. [DOI] [PubMed] [Google Scholar]
- 22.d’Udekem d’Acoz Y, Pasquet A, Lebreux L, Ovaert C, Mascart F, Robert A, Rubay JE. Does right ventricular outflow tract damage play a role in the genesis of late right ventricular dilatation after tetralogy of Fallot repair? Ann Thorac Surg. 2003;76:555–61. doi: 10.1016/s0003-4975(03)00434-x. [DOI] [PubMed] [Google Scholar]
- 23.d’Udekem Y, Ovaert C, Grandjean F, Gerin V, Cailteux M, Shango-Lody P, Vliers A, Sluysmans T, Robert A, Rubay JE. Tetralogy of Fallot: transannular and right ventricular patching equally affect late functional status. Circulation. 2000;102(Suppl. III):III-116–2. doi: 10.1161/01.cir.102.suppl_3.iii-116. [DOI] [PubMed] [Google Scholar]