Abstract
OBJECTIVE
This study aims to develop a logistic regression model and a simple score system for the prediction of significant coronary artery disease (CAD) in patients undergoing operations for rheumatic mitral valve disease.
METHODS
A total of 1241 rheumatic patients (mean age 57 ± 6 years), who underwent routine coronary angiography (CAG) before mitral valve operations between 1998 and 2009, was analyzed. To identify low-risk (≤5%) patients, a bootstrap refined logistic regression model on the basis of clinical risk factors was developed, from which an additive model was derived. Receiver operating characteristic (ROC) curves were used to compare discrimination, and precision was quantified by the Hosmer–Lemeshow statistic. Significant coronary atherosclerosis was defined as 50% or more luminal narrowing in one or more major epicardial vessels by means of CAG.
RESULTS
One hundred twenty-seven (10.2%) patients had significant coronary atherosclerosis. Independent predictors of significant CAD include age, male sex, hypertension, angina, smoking, and hypercholesterolemia. Five hundred and fifty patients were designated as low risk according to our logistic regression and additive models. Of these patients, only 6 (1.1%) had single-vessel disease, and none had multivessel disease. Our models proved more efficient than established regression models.
CONCLUSIONS
Our logistic regression model could estimate the risk of significant CAD in rheumatic patients undergoing mitral valve operations, while the additive simple score system could reliably identify the low-risk patients in whom routine preoperative angiography might be safely avoided.
Keywords: Rheumatic heart valve disease, Mitral valve disease, CAD, Risk factor, Logistic regression model
INTRODUCTION
It is of great importance to detect the presence of coexistent coronary artery disease (CAD) before operations on heart valves because of the impact of untreated CAD on perioperative and long-term postoperative survival [1,2]. Accurate identification by coronary angiography (CAG) for every patient is not wise due to the low positive rate, the possible complications, and the cost. To predict the coronary artery stenosis in patients undergoing operation for mitral valve regurgitation, some logistic regression models have been established [3,4]. However, the patients from which such models were derived suffered mostly from degenerative valve disease. In China and many other developing countries, rheumatic heart disease accounts for a majority of mitral valvular operations. In these patients, the risk of coexistent CAD is much lower [5–7] and therefore the models might not be applicable for prediction. In this article, we sought to develop a new model to predict the risk of CAD in patients undergoing operations for rheumatic mitral valve disease.
METHODS
The study was performed with protocols approved by the Ethics Committee in Research of Changhai Hospital.
Patient selection
We retrospectively identified 1454 consecutive patients of 50 years or older who underwent cardiac operation for rheumatic mitral valve disease in Changhai Hospital between 1998 and 2009. Of them, 187 patients did not undergo CAG at the discretion of the attending staff. Another 26 patients with previous myocardial infarction were excluded because the diagnosis of CAD was undoubted and CAG was clearly needed. Therefore, 1241 patients were included in our study. Routine CAG was performed before the cardiac surgery. Significant CAD was defined as at least 50% diameter narrowing of a major coronary artery.
Risk factor selection
Risk factors for CAD were defined as follows: age, family history (first-degree relative with a myocardial infarction), smoking, diabetes, hypertension (systolic pressure over 140 mmHg or diastolic pressure over 90 mmHg), and hypercholesterolemia (serum cholesterol of 5.8 mmol l−1 or more). In addition, obesity (body mass index (BMI) of 28 or more), the presence of ischemic changes on electrocardiogram (resting ST or T wave abnormalities), coexistent aortic valve lesion, abnormal left ventricular function (an ejection fraction of 50% or less), and New York Heart Association class III or IV were also designated as variables for analyses. Etiology was determined by echocardiographic and histopathologic examinations.
Statistical analysis
Logistic regression model development
All the designated variables were entered into univariable analyses. The criterion for variable retention was significance of 0.2. Predictors of significant CAD in the univariable analyses were entered into a forward stepwise multivariable logistic regression model. Only independent predictors (P < 0.05) were included in the model:
The multivariable logistic model was then refined by using the bootstrap technique [8], which utilized 2000 random resamplings to assess the stability of odds ratio estimates with sampling variation.
Additive model
To simplify the bedside calculation of clinical risk, an additive scoring system was developed according to the bootstrap logistic regression model. We chose a probability of significant CAD of greater than 0.05 as a cut-off, which corresponded to a bootstrap logistic regression model score of −3.2.
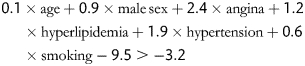 |
Age was rescaled as a variable with intervals of 5 years when above 50 years (interval age = (age − 50)/5). The equation was modified as follows:
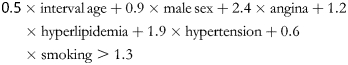 |
By multiplying each coefficient by a factor of 2 and rounding off the values to the nearest whole number, a simple additive model was established.
Comparing the various models
Receiver operating characteristic (ROC) curves were used to compare discrimination of the previous regression models [3,4] with ours for the prediction of significant CAD on our sample population. Precision was quantified by the Hosmer–Lemeshow goodness-of-fit statistic.
Continuous variables are expressed as mean values ± SD. Categoric variables are expressed as frequencies or percentage. All statistical analyses were performed with PASW statistics 18 software.
RESULTS
The analyses were based on angiographic data obtained from 1241 patients. Clinical characteristics of the study population are summarized in Table 1. One hundred twenty-seven (10.2%) patients in the study population had significant CAD.
Table 1:
Clinical characteristics of the study population
| Variable | Study population (n = 1241) |
|---|---|
| Age (years) | 57 ± 6 |
| Male (%) | 623 (50.2) |
| Obesity (%) | 280 (22.6) |
| Hypertension (%) | 168 (13.5) |
| Hypercholesterolemia (96) | 92 (7.4) |
| Diabetes mellitus (%) | 70 (5.6) |
| Smoking (%) | 167 (13.5) |
| Family history of coronary artery disease (%) | 29 (2.3) |
| Abnormal LV function (%) | 314 (25.3) |
| NYHA class III or IV (%) | 1039 (83.7) |
| Angina (%) | 72 (5.8) |
| Ischemic changes on electrocardiogram (56) | 268 (21.6) |
| Concurrent aortic valve replacement | 571 (46.0) |
| Significant coronary artery disease (%) | 127 (10.2) |
| Concurrent coronary artery bypass graft | 96 (7.7) |
Logistic regression modeling
The univariable risk factors for the prediction of coexistent coronary disease are presented in Table 2. Abnormal LV function, NYHA class III or IV, family history, ischemic changes on ECG, and coexistent aortic valve lesion did not reach the significance cut-off of 0.2 for variable retention. Male sex, age, hyperlipidemia, hypertension, and smoking were predictors in the logistic multivariable analysis, and further validated as predictive in the bootstrap refined model as follows: model score (LnOR)
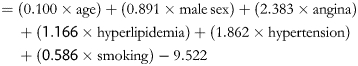 |
Table 2:
Univariable associations with significant coronary artery disease
| Variable | Coefficient | SE | OR | P value |
|---|---|---|---|---|
| Agea | 0.120 | 0.015 | 1.128 | <0.01 |
| Male | 0.773 | 0.206 | 2.167 | <0.01 |
| Obesity | 0.355 | 0.210 | 1.426 | 0.1 |
| Hypertension | 1.996 | 0.205 | 7.358 | <0.01 |
| Hypercholesterolemia | 1.209 | 0.262 | 3.349 | <0.01 |
| Diabetes mellitus | 0.947 | 0.308 | 2.579 | <0.01 |
| Smoking | 1.088 | 0.218 | 2.969 | <0.01 |
| Family history of coronary artery disease | 0.012 | 0.617 | 1.012 | 1.0 |
| Abnormal left ventricle function | −0.006 | 0.216 | 0.994 | 1.0 |
| NYHA class III or IV | −0.278 | 0.238 | 0.758 | 0.2 |
| Angina | 2.202 | 0.260 | 9.044 | <0.01 |
| Ischemic changes on electrocardiogram | 0.030 | 0.226 | 1.030 | 0.9 |
| Coexistent aortic valve lesion | 0.063 | 0.188 | 1.065 | 0.7 |
a For every additional 5 years.
The odds ratios with confidence intervals from the original regression model and the bootstrap model were shown in Table 3. The strong similarity confirmed the stability of the model.
Table 3:
Odds ratios and confidence intervals from the original regression model and the bootstrap model
| Variable | Original model OR (95% CI) | Bootstrap model OR (95% CI) |
|---|---|---|
| Agea | 1.104 (1.068–1.141) | 1.105 (1.069–1.142) |
| Male sex | 2.461 (1.529–3.962) | 2.431 (1.516–3.921) |
| Angina | 10.617 (5.551–20.309) | 10.838 (5.686–20.658) |
| Hypercholesterolemia | 3.143 (1.682–5.874) | 3.210 (1.727–5.967) |
| Hypertension | 1.59 (1.24–2.04) | 6.438 (4.056–10.218) |
| Smoking | 1.765 (1.017–3.062) | 1.797 (1.040–3.106) |
a For every additional 5 years of age.OR: odds ratio; CI: confidence interval
Additive model
The additive scores were listed in Table 4. A model score of more than 3 corresponding to a risk of significant CAD of greater than 0.05 would be an indication for angiography. Hypertension and angina yielded a score of 4 and 7, respectively. Because any score more than 3 would be an indication for angiography, both the variables were reassigned a value of 3 to simplify the values in the model.
Table 4:
An additive scoring system for the prediction of significant coronary artery disease
| Variable | Score |
|---|---|
| Age | 1 point for each 5 years over the age of 50 years |
| Smoking | 1 point |
| Male | 2 points |
| Hyperlipidemia | 2 points |
| Hypertension | 3 points |
| Angina | 3 points |
A score equal or more than 3 points is an indication for coronary angiography.
Comparison of discrimination of the various models
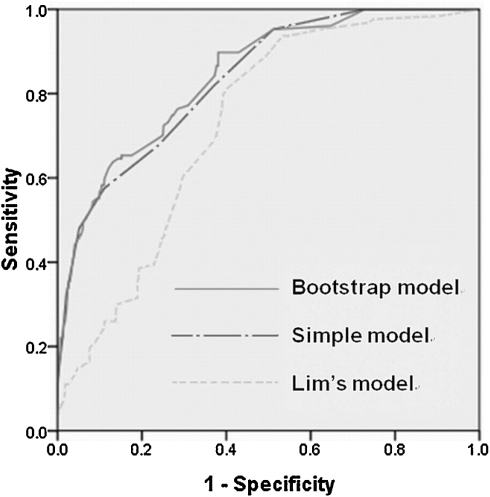
The area under the curve (AUC) of our logistic regression model was 0.843, and similar discriminating ability was achieved by the simple additive model (AUC of 0.835). The Hosmer–Lemeshow statistic revealed good fit with values of 6.2 (P = 0.6) and 7.3 (P = 0.5), respectively. Lim’s model yielded acceptable discrimination but no goodness of fit on our patient population, with an AUC of 0.727 (see Fig. 1) and a Hosmer–Lemeshow score of 17.016 (P < 0.05).
Figure 1:
Comparison of curves from the various models. The AUC of the bootstrap model, our simple model and Lim’s model were 0.843 (95% CI: 0.809–0.877), 0.835 (95% CI: 0.800–0.870) and 0.727 (95% CI: 0.689–0.765), respectively.
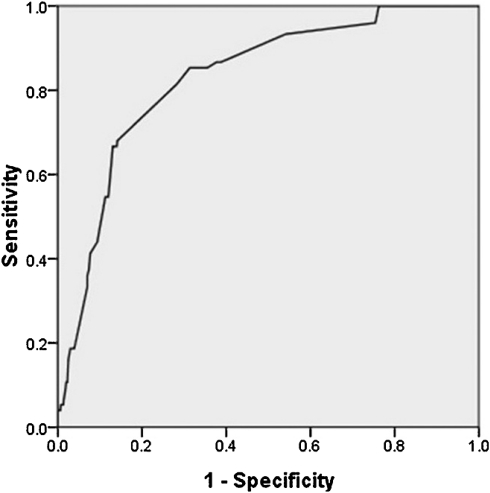
Lin’s model was tested on part of our study population, including 917 patients without angina or ischemic changes on ECG, according the premise of using their model. It produced an AUC of 0.829 (see Fig. 2). The Hosmer–Lemeshow test revealed no good fit with a value of 25.667 (P < 0.005).
Figure 2:
Receiver operating characteristic curve of Lin’ model on 917 patients without angina or ischemic changes on electrocardiogram. The AUC was 0.829 (95% CI: 0.784–0.875).
DISCUSSION
It has been recommended that patients before valve operation are selected for CAG based on risk factor identification in the American Heart Association and the American College of Cardiology (AHA/ACC) guidelines [9]. High sensitivity can be achieved with unacceptably low specificity, the possible complications, and the cost. The guidelines also advise CAG should be performed before valve surgery in men aged >35 years, pre-menopausal women aged >35 years with cardiovascular risk factors, and post-menopausal women. Appling these criteria to our study population, we would find almost every patient to be of high risk because of their age. To eliminate the cost and morbidity of unnecessary studies, Lin et al. developed a logistic regression model for the prediction of possible coronary artery stenosis in presurgical patients with mitral degeneration [3]. Lim et al. also described a similar model, which was derived from patients who had mitral repair [4]. However, patients with degenerative valve disease were in the majority (100% and 83%) in the sample population of both studies. The prevalence of CAD in the two models was 19.3% and 36%, respectively. In patients with rheumatic heart valve disease, the incidence of CAD was low and thus the strength of prediction and respective relationships between predictors derived in previous analyses might be lost.
In this study, both the logistic regression model and the simple additive model derived from our rheumatic patients showed better discriminative ability than Lim’s model. With a probability of 0.05 as a cut-off, both of them identified 550 low-risk patients, including six (1.1%) with single-vessel disease and no concurrent coronary artery bypass graft (CABG). As for Lin’s model, the ROC area showed that it produced strong discriminating ability when applied to part of our population without angina or ischemic ECG changes. It identified 210 low-risk patients, including four (1.8%) with CAD, none of whom met an indication of CABG. However, in the whole cohort, Lim’s strategies would submit 1041 patients to CAG and detected CAD in 123 of them, whereas our models submitted only 691 and found 121 with CAD. In comparison, our models acted more efficiently, reducing 33.6% of CAG with an increase of 1.57% in the incidence of miss-diagnosis.
Angina is reckoned as a less specific indicator of CAD in patients with valvular heart disease than in the general population, because multiple causes, such as LV chamber enlargement, increased wall stress or wall thickening with subendocardial ischemia [10], and Right Ventricular (RV) hypertrophy [11], may account for it. In our study, however, angina remained predictive, whereas some commonly recognized risk factors, including diabetes mellitus, family history, and obesity, were not predictive. In our study, a low prevalence of significant CAD of 10.2% in the rheumatic patients was present, as observed by other investigators [5–7]. The reason cannot be the rheumatic-fever-preventing intramuscular antibiotics which could have a protective antiinflammatory effect on the genesis of coronary atherosclerosis because the patients in our study seldom received such prophylaxis. Kruczan et al. inferred that the low prevalence could be due to the demographic and clinical characteristics [7]. In other words, the rheumatic patients were younger, containing more female sex, and, thus, with fewer comorbidities in comparison with those having degenerative valve disease. However, the mean age of our sample population was 57, and in the patients of male sex, the prevalence was only 13.6%. The reason of the low prevalence remains unknown.
It is a very important issue whether we should perform additional CABG at the time of valve surgery for patients with significant CAD. According to the AHA guidelines, concomitant revascularization is recommended in patients with mitral valve disease due to diseases other than ischemia. However, there are no data to indicate the wisdom of this general strategy [9]. Some randomized trials in nonvalve populations have found that only patients with multivessel CAD in the presence of proximal left anterior descending artery disease, left ventricular dysfunction, or left main trunk disease derived survival benefit from surgical revascularization [12–14]. Furthermore, coronary bypass of non-flow-limiting lesions may lead to proximal native vessel atherosclerosis [15]. In our population, 127 patients had significant CAD and 96 of them received concomitant CABG. The other 31 patients (including six low-risky ones) only had valve operations and there were no perioperative coronary events among them.
Computed tomography angiography (CTA) is another option to identify low-risk patients. It has proved that the negative predictive value of CTA was promising [16–20] whether the prevalence of CAD in the study population was high or low. In patients undergoing valve surgery, several studies have received encouraging results in ruling out the presence of significant CAD by CTA [21–25]. However, the authors used 16- or 64-slice computed tomography and thus the effectiveness of CTA would be marred by atrial fibrillation, a quite common complication in patients with rheumatic valve disease. Application of a 320-slice or dual-source computed tomography (CT) scanner might overcome the barrier, and clinical trials in this regard are needed. In developing countries where such devices are not always available, regression models for prediction of CAD might come in handy.
Limitations
This was a retrospective study in a single center, requiring verifications in more populations.
In summary, for the prediction of significant CAD in patients undergoing operations for rheumatic mitral valve disease, we established a logistic regression model for an accurate risk estimation and a simple additive score system to identify low-risk patients at the bedside with simplicity and accuracy.
Funding
This work was supported by Medical Research Fund of the Ministry of Health of China (200802096).
Conflict of interest: none declared.
REFERENCES
- 1.Czer LS, Gray RJ, DeRobertis MA, Bateman TM, Stewart ME, Chaux A, Matloff JM. Mitral valve replacement: impact of coronary artery disease and determinants of prognosis after revascularization. Circulation. 1984;70:I198–207. [PubMed] [Google Scholar]
- 2.Kay PH, Nunley DL, Grunkemeier GL, Pinson CW, Starr A. Late results of combined mitral valve replacement and coronary bypass surgery. J Am Coll Cardiol. 1985;5:29–33. doi: 10.1016/s0735-1097(85)80081-4. [DOI] [PubMed] [Google Scholar]
- 3.Lin SS, Lauer MS, Asher CR, Cosgrove DM, Blackstone E, Thomas JD, Garcia MJ. Prediction of coronary artery disease in patients undergoing operations for mitral valve degeneration. J Thorac Cardiovasc Surg. 2001;121:894–901. doi: 10.1067/mtc.2001.112463. [DOI] [PubMed] [Google Scholar]
- 4.Lim E, Ali ZA, Barlow CW, Jackson CH, Hosseinpour AR, Halstead JC, Barlow JB, Wells FC. A simple model to predict coronary disease in patients undergoing operation for mitral regurgitation. Ann Thorac Surg. 2003;75:1820–5. doi: 10.1016/s0003-4975(03)00171-1. [DOI] [PubMed] [Google Scholar]
- 5.Jose VJ, Gupta SN, Joseph G, Chandy ST, George OK, Pati PK, John B, George P. Prevalence of coronary artery disease in patients with rheumatic heart disease in the current era. Indian Heart J. 2004;56:129–31. [PubMed] [Google Scholar]
- 6.Guray Y, Guray U, Yilmaz MB, Mecit B, Kisacik H, Korkmaz S. Prevalence of angiographically significant coronary artery disease in patients with rheumatic mitral stenosis. Acta Cardiol. 2004;59:305–9. doi: 10.2143/AC.59.3.2005186. [DOI] [PubMed] [Google Scholar]
- 7.Kruczan DD, Silva NA, Pereira Bde B, Romão VA, Correa Filho WB, Morales FE. Coronary artery disease in patients with rheumatic and non-rheumatic valvular heart disease treated at a public hospital in Rio de Janeiro. Arq Bras Cardiol. 2008;90:197–203. doi: 10.1590/s0066-782x2008000300010. [DOI] [PubMed] [Google Scholar]
- 8.Effron BE, Tibshirani RJ. New York: Chapman and Hall; 1993. An introduction to the bootstrap. [Google Scholar]
- 9.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FP, Delforge MR, Carré AG. Coronary sinus blood flow at rest and during isometric exercise in patients with aortic valve disease. Mechanism of angina pectoris in presence of normal coronary arteries. Am J Cardiol. 1981;47:199–205. doi: 10.1016/0002-9149(81)90384-2. [DOI] [PubMed] [Google Scholar]
- 11.Ross RS. Right ventricular hypertension as a cause of precordial pain. Am Heart J. 1961;61:134–5. doi: 10.1016/0002-8703(61)90527-0. [DOI] [PubMed] [Google Scholar]
- 12.The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med. 1984;311:1333–9. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 13.Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–71. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 14.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332–7. doi: 10.1056/NEJM198808113190603. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove DM, Loop FD, Saunders CL, Lytle BW, Kramer JR. Should coronary arteries with less than fifty percent stenosis be bypassed? J Thorac Cardiovasc Surg. 1981;82:520–30. [PubMed] [Google Scholar]
- 16.Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, Wildermuth S. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–7. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 17.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, Wintersperger B, Reiser M, Becker CR, Steinbeck G, Boekstegers P. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 19.Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, Serruys PW, Krestin GP, de Feyter PJ. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 20.Meijboom WB, van Mieghem CA, Mollet NR, Pugliese F, Weustink AC, van Pelt N, Cademartiri F, Nieman K, Boersma E, de Jaegere P, Krestin GP, de Feyter PJ. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol. 2007;50:1469–75. doi: 10.1016/j.jacc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Gilard M, Cornily JC, Pennec PY, Joret C, Le Gal G, Mansourati J, Blanc JJ, Boschat J. Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol. 2006;47:2020–4. doi: 10.1016/j.jacc.2005.11.085. [DOI] [PubMed] [Google Scholar]
- 22.Manghat NE, Morgan-Hughes GJ, Broadley AJ, Undy MB, Wright D, Marshall AJ, Roobottom CA. 16-detector row computed tomographic coronary angiography in patients undergoing evaluation for aortic valve replacement: comparison with catheter angiography. Clin Radiol. 2006;61:749–57. doi: 10.1016/j.crad.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Meijboom WB, Mollet NR, Van Mieghem CA, Kluin J, Weustink AC, Pugliese F, Vourvouri E, Cademartiri F, Bogers AJ, Krestin GP, de Feyter PJ. Preoperative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48:1658–65. doi: 10.1016/j.jacc.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Reant P, Brunot S, Lafitte S, Serri K, Leroux L, Corneloup O, Iriart X, Coste P, Dos Santos P, Roudaut R, Laurent F. Predictive value of noninvasive coronary angiography with multidetector computed tomography to detect significant coronary stenosis before valve surgery. Am J Cardiol. 2006;97:1506–10. doi: 10.1016/j.amjcard.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 25.Scheffel H, Leschka S, Plass A, Vachenauer R, Gaemperli O, Garzoli E, Genoni M, Marincek B, Kaufmann P, Alkadhi H. Accuracy of 64-slice computed tomography for the preoperative detection of coronary artery disease in patients with chronic aortic regurgitation. Am J Cardiol. 2007;100:701–6. doi: 10.1016/j.amjcard.2007.03.087. [DOI] [PubMed] [Google Scholar]