Abstract
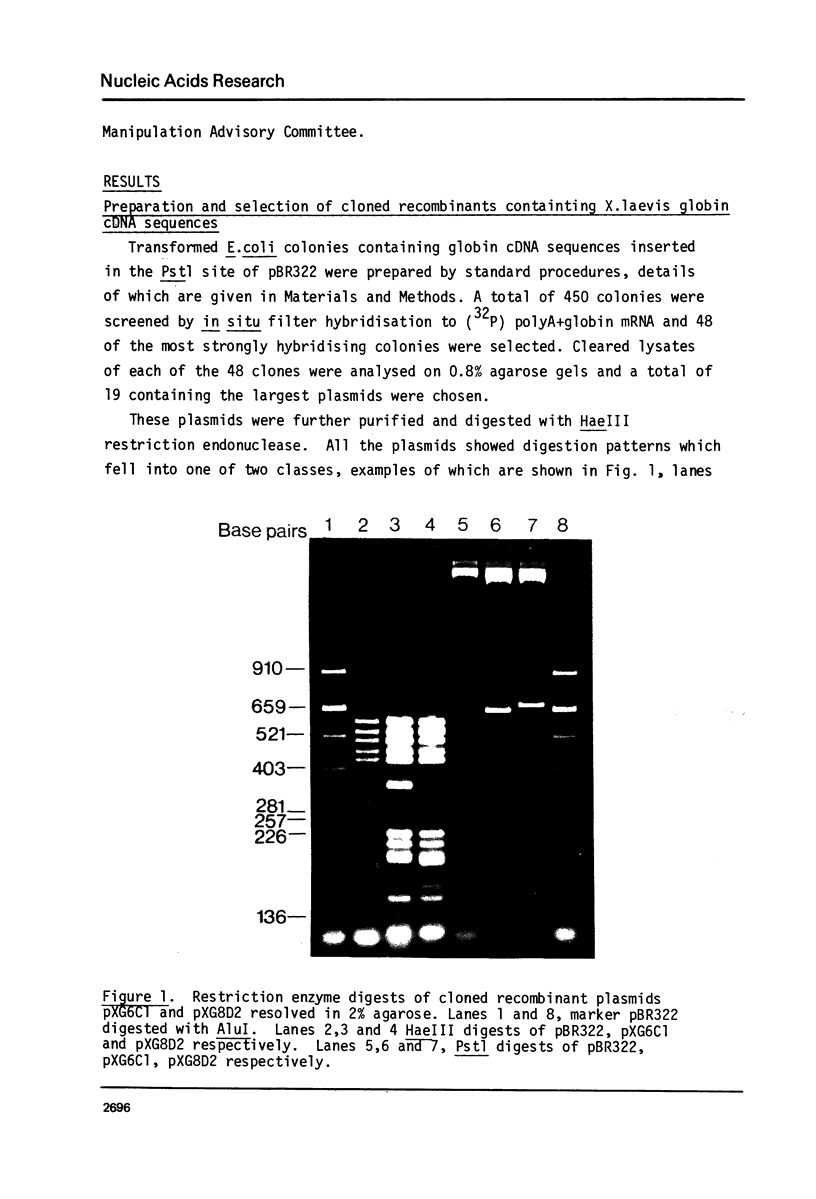
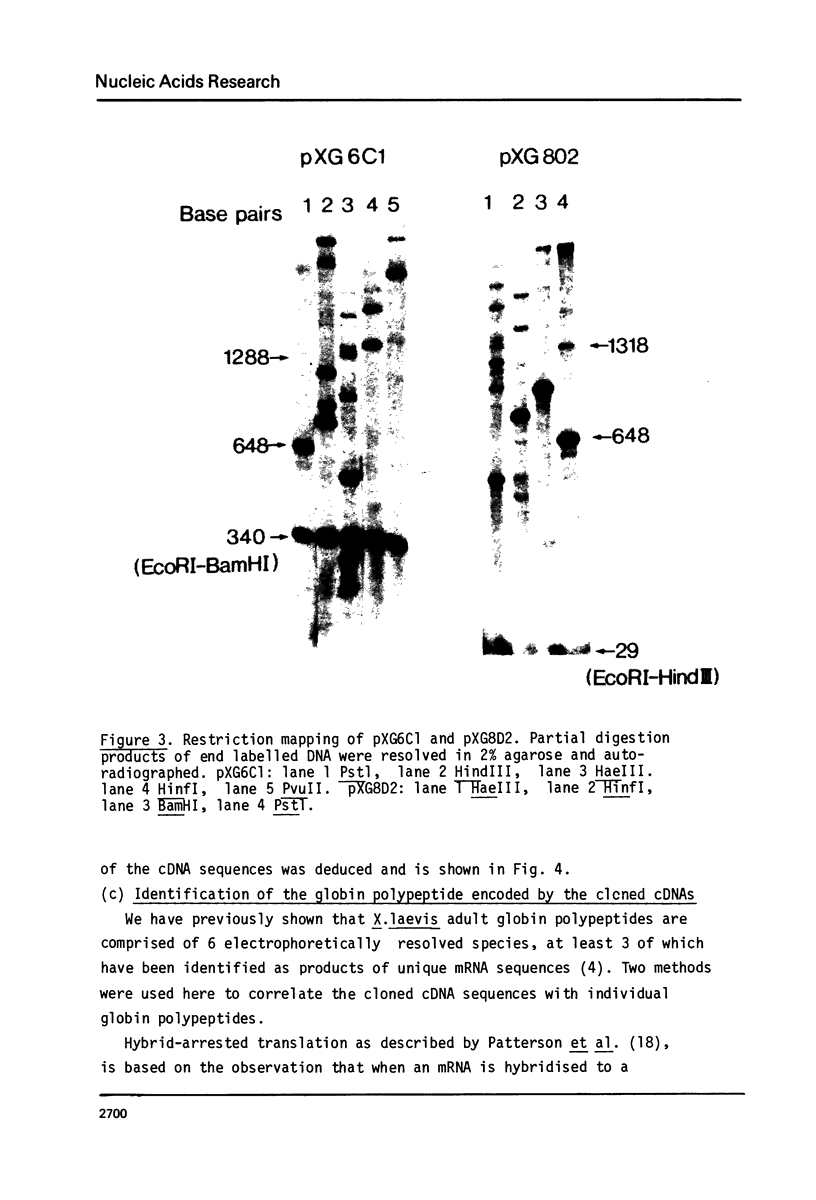
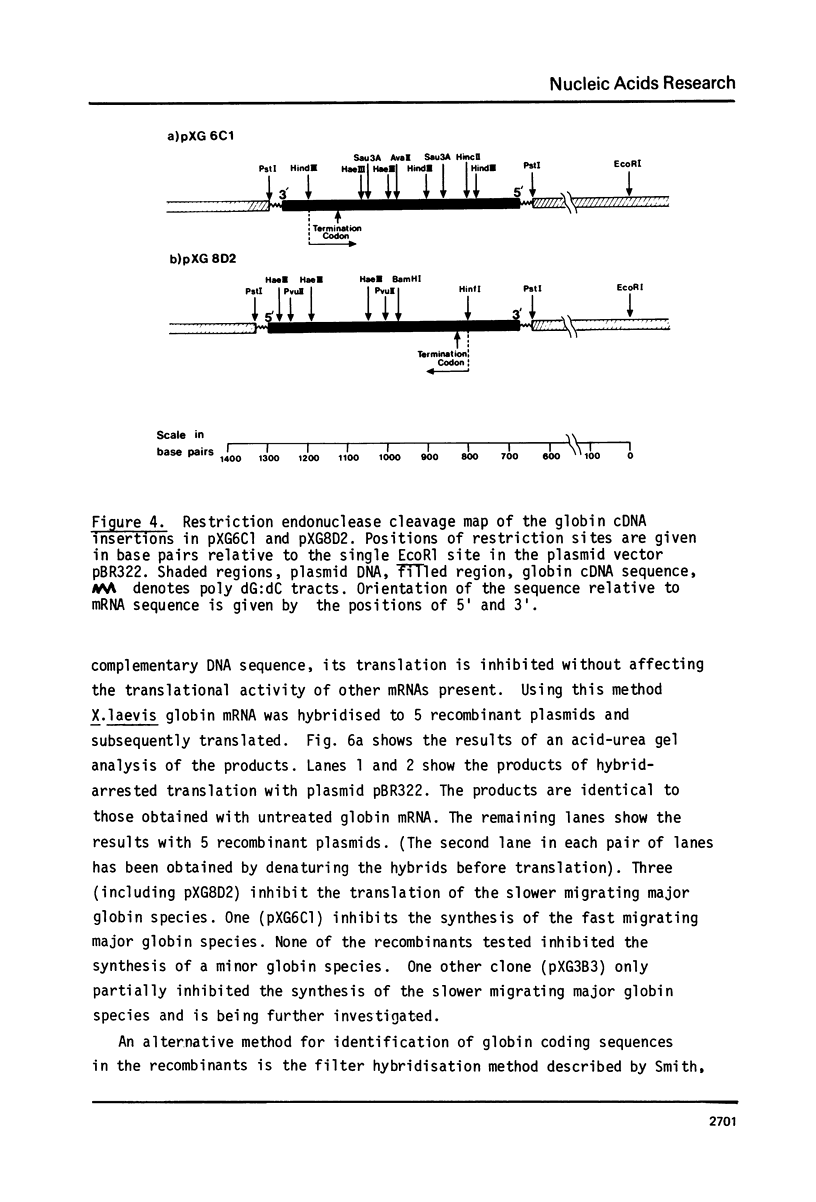
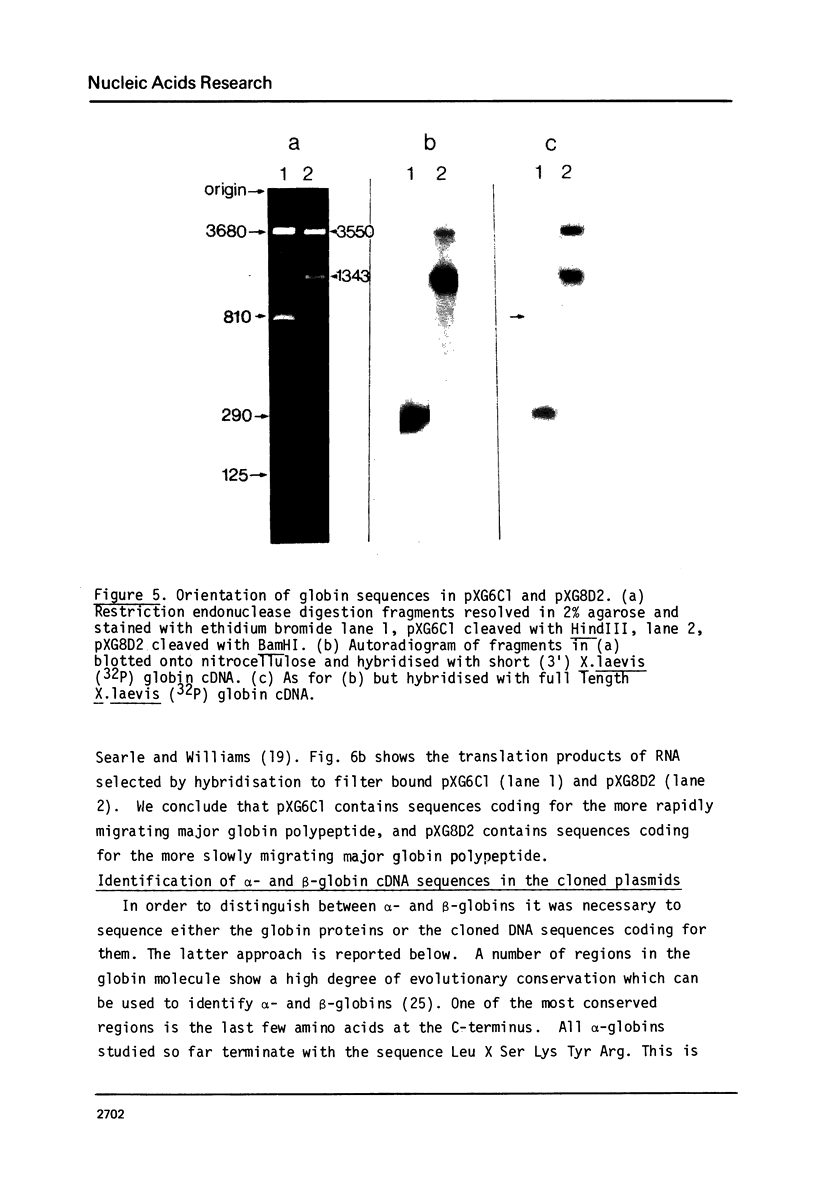
This report describes the synthesis and cloning of almost complete DNA copies of the mRNAs encoding the major alpha-globin and major beta-globin of X. laevis. Double-stranded globin cDNA was inserted into the PstI site of the plasmid pBR322 and two cloned recombinants (designated pXG6C1 and pXG8D2) were selected. These were shown to contain almost complete copies of X. laevis globin mRNA. Restriction enzyme maps were determined for each cDNA sequence using the established method of partial digestion of end labelled DNA. However, this procedure was modified such that isolation of individual DNA fragments was no longer required. Each plasmid was shown, by both hybrid arrested translation and filter selection of complementary RNA, to contain a sequence coding for one or other of the two major globin polypeptides. Sufficient DNA sequence information has been determined from each cDNA clone to demonstrate that pXG8D2 contains a beta-globin sequence and pXG6C1 contains an alpha-globin sequence.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battaglia P., Melli M. Isolation of globin messenger RNA of Xenopus laevis. Dev Biol. 1977 Oct 15;60(2):337–350. doi: 10.1016/0012-1606(77)90132-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Kay R. M., Williams J. G. Analysis of Xenopus laevis globins during development and erythroid cell maturation and the construction of recombinant plasmids containing sequences derived from adult globin mRNA. Dev Biol. 1979 Oct;72(2):350–363. doi: 10.1016/0012-1606(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Humphries P., Old R., Coggins L. W., McShane T., Watson C., Paul J. Recombinant plasmids containing Xenopus laevis globin structural genes derived from complementary DNA. Nucleic Acids Res. 1978 Mar;5(3):905–924. doi: 10.1093/nar/5.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean N., Jurd R. D. The control of haemoglobin synthesis. Biol Rev Camb Philos Soc. 1972 Aug;47(3):393–437. doi: 10.1111/j.1469-185x.1972.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., McReynolds L. A., O'Malley B. W. The ovalbumin gene. In vitro enzymatic synthesis and characterization. J Biol Chem. 1976 Dec 10;251(23):7355–7362. [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas N., Maclean N. The erythroid cells of anaemic Xenopus laevis. I. Studies on cellular morphology and protein and nucleic acid synthesis during differentiation. J Cell Sci. 1975 Dec;19(3):509–520. doi: 10.1242/jcs.19.3.509. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M. Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol. 1979 Mar 25;129(1):19–35. doi: 10.1016/0022-2836(79)90056-1. [DOI] [PubMed] [Google Scholar]