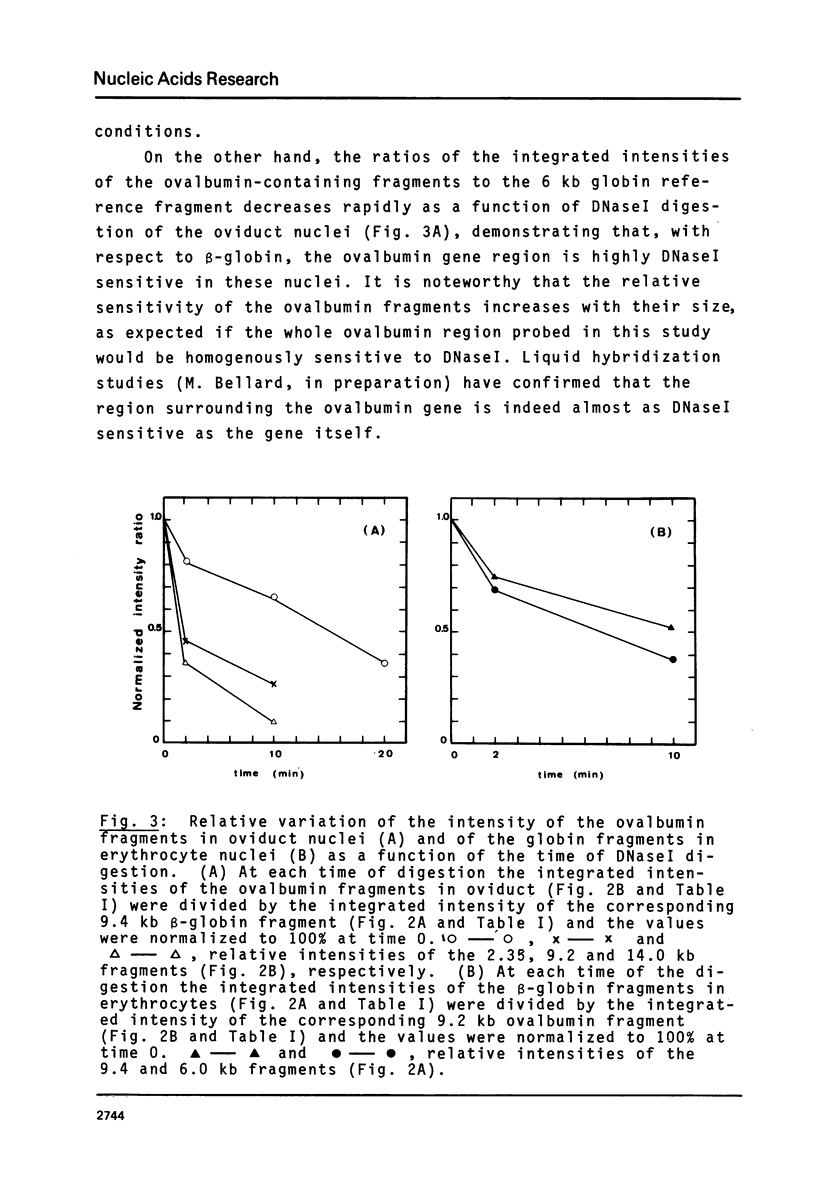
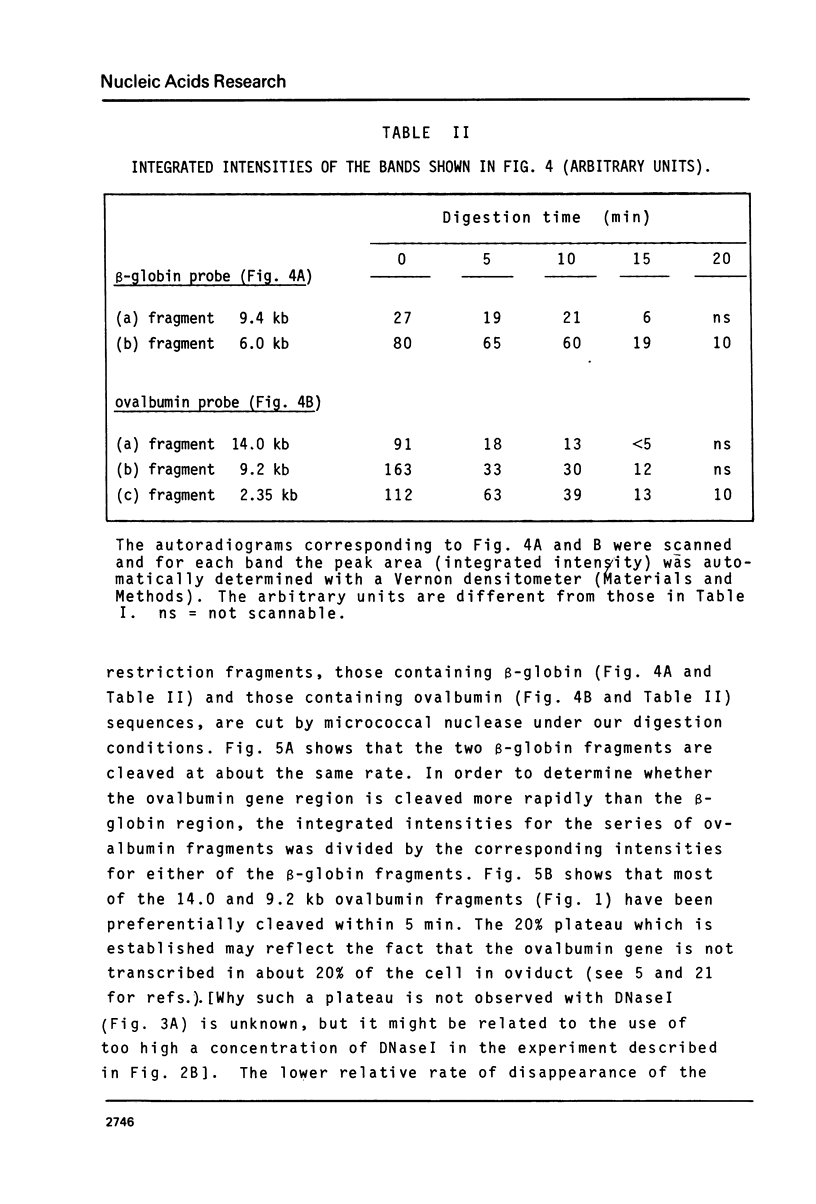
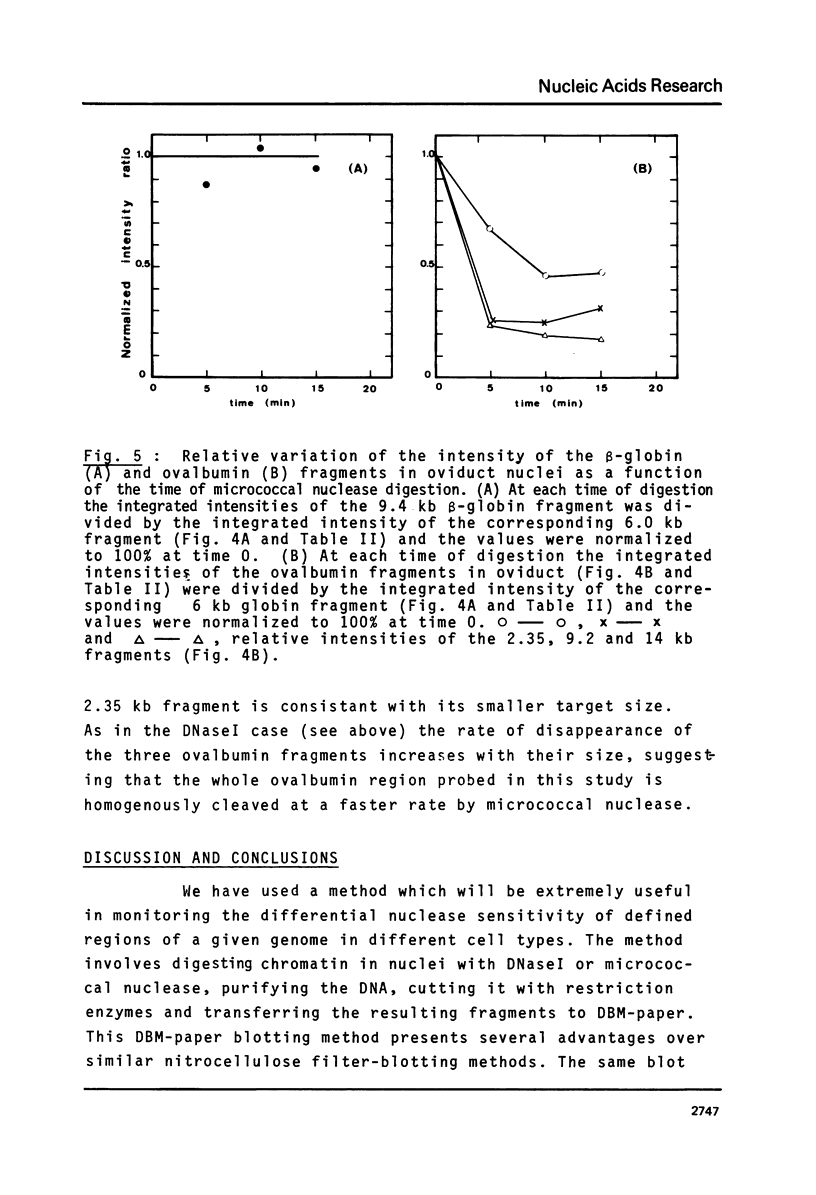
Abstract
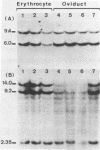
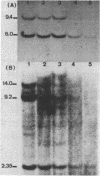
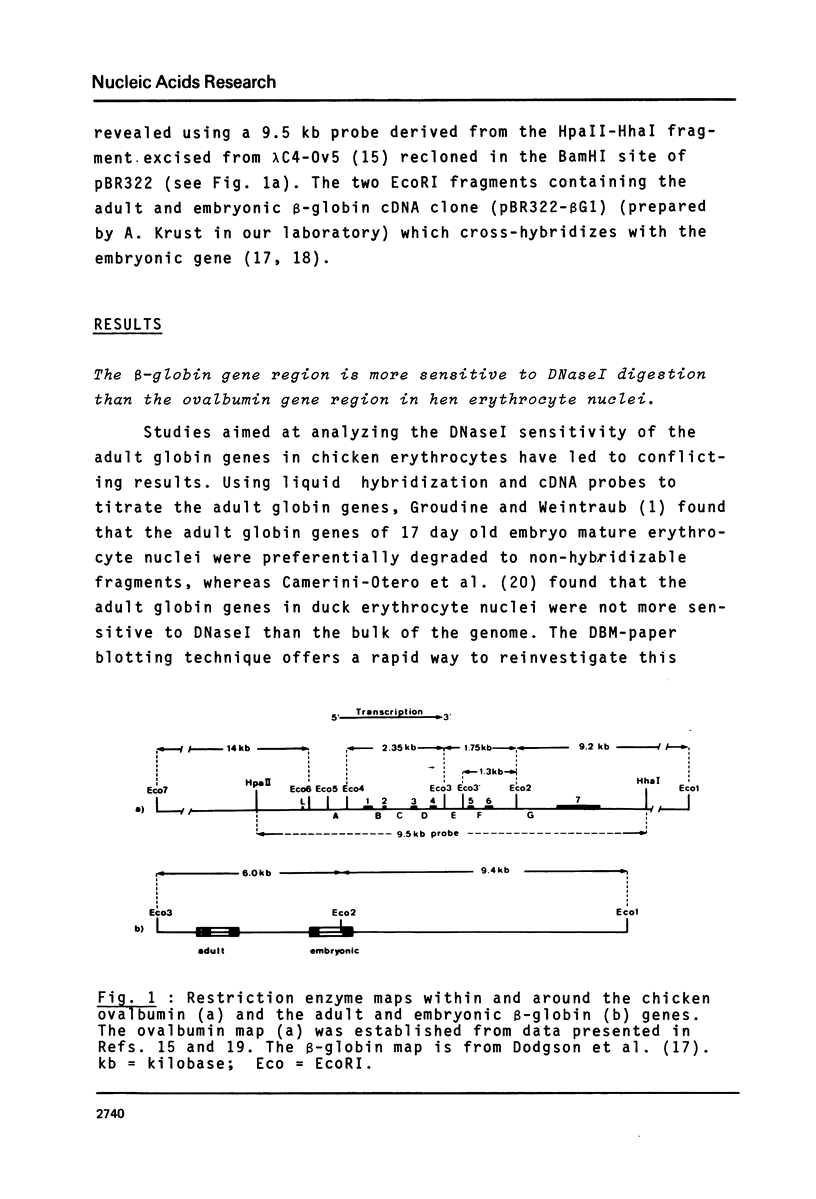
We have monitored the differential nuclease sensitivity of defined regions of the chicken genome in different cells using a method which combines restriction enzyme digestion and blotting to diazobenzyloxymethyl (DBM)-paper (see Ref. 11). By using different specific probes and by scanning the bands on the autoradiograms, it is possible to compare on the same blot the digestion patterns of similar-sized fragments from different regions of the genome corresponding to "active" and reference "inactive" genes. We have demonstrated the preferential sensitivity to DNaseI and micrococcal nuclease digestion of the ovalbumin gene region in hen oviduct chromatin. The beta-globin gene region (containing both an adult and an embryonic gene) is also preferentially digested by DNaseI in hen mature erythrocyte nuclei, but at a lower rate than the ovalbumin gene region in oviduct. These observations raise the possibility that there may be several types of preferential nuclease sensitivities, all characterized by increased rates of digestion but to different levels, the highest corresponding to the very actively transcribing genes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Conformation of ovalbumin and globin genes in chromatin during differential gene expression. J Biol Chem. 1979 Oct 25;254(20):10532–10539. [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the adult and embryonic chicken globin genes in chromosomal DNA. J Biol Chem. 1978 Nov 25;253(22):8239–8246. [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginder G. D., Wood W. I., Felsenfeld G. Isolation and characterization of recombinant clones containing the chicken adult beta-globin gene. J Biol Chem. 1979 Sep 10;254(17):8099–8102. [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]