Abstract
OBJECTIVE
To quantify the association of treated diabetes with cancer incidence and cancer mortality as well as cancer case fatality and all-cause mortality in adults who subsequently develop cancer and to calculate attributable fractions due to diabetes on various cancer outcomes.
RESEARCH DESIGN AND METHODS
Prospective data on 599 diabetic and 17,681 nondiabetic adults from the CLUE II (Give Us a Clue to Cancer and Heart Disease) cohort in Washington County, Maryland, were analyzed. Diabetes was defined by self-reported use of diabetes medications at baseline. Cancer incidence was ascertained using county and state cancer registries. Mortality data were obtained from death certificates.
RESULTS
From 1989 to 2006, 116 diabetic and 2,365 nondiabetic adults developed cancer, corresponding to age-adjusted incidence of 13.25 and 10.58 per 1,000 person-years, respectively. Adjusting for age, sex, education, BMI, smoking, hypertension treatment, and high cholesterol treatment using Cox proportional hazards regression, diabetes was associated with a higher risk of incident cancer (hazard ratio 1.22 [95% CI 0.98–1.53]) and cancer mortality (1.36 [1.02–1.81]). In individuals who developed cancer, adults with diabetes had a higher risk of cancer case fatality (1.34 [1.002–1.79]) and all-cause mortality (1.61 [1.29–2.01]). For colorectal, breast, and prostate cancers, the attributable fractions resulting from diabetes were larger for cancer fatality and mortality than cancer incidence.
CONCLUSIONS
In this prospective cohort, diabetes appears to exert a greater influence downstream on the risk of mortality in people with cancer than on upstream risk of incident cancer.
Although previous cohort studies have examined the influence of diabetes on particular aspects of cancer outcomes (1–9), none have sought to quantify the impact of diabetes across the full continuum of cancer control, from cancer development to survival after a cancer diagnosis. We, therefore, analyzed prospective data from CLUE II (Give Us a Clue to Cancer and Heart Disease), a community-based, cohort study in Washington County, Maryland, to test the hypothesis that preexisting, treated diabetes would predict 1) cancer incidence and cancer mortality in people at risk for cancer and 2) cancer case fatality and all-cause mortality in adults who subsequently develop cancer. Because data on cancer incidence and mortality were available in the same population, we further calculated the attributable fractions (AFs) to isolate effects of diabetes on cancer incidence from its effects on cancer case fatality and death from all causes. We hypothesized that diabetes would exert a stronger influence on downstream risk of mortality in people with cancer than upstream on the development of incident cancer.
RESEARCH DESIGN AND METHODS
In 1989, a local campaign against cancer and heart disease named CLUE II was conducted in Washington County, Maryland. Mobile trailers were stationed in a wide variety of locations in the county in an effort to give all segments of the community an opportunity to participate. At baseline, written informed consent was obtained, and a short questionnaire on demographics and medical history were administered to all participants. A total of 32,894 individuals, nearly one-third of the adult population of Washington County at that time, took part in this study. To reduce the likelihood of a case subject not being identified through the cancer registries, the analysis cohort was limited to 25,076 CLUE II participants who lived in Washington County at baseline. We further excluded participants aged <30 years (n = 5,470) or with history of invasive cancer at baseline (n = 1,360; individuals could be excluded for more than one reason). The final study sample consisted of 18,280 adults aged ≥30 years without a history of cancer at baseline.
Diabetes status
Individuals were classified as having diabetes if they reported taking antidiabetes medications in the last 48 h on the baseline questionnaire.
Cancer incidence and mortality assessment
Cancer cases in the CLUE II cohort have been identified through linkage of the cohort participants with the Washington County Cancer Registry and, since 1992, with the Maryland Cancer Registry. For all-cause mortality, we identified deaths among cohort members via searches of the National Death Index, Maryland death certificates, reviews of obituaries of the local newspaper, and reports by next of kin. Death certificates were reviewed to determine underlying cause of death.
Other baseline variables
Other information collected at baseline included demographic characteristics (e.g., race, marital status, and education); self-reported height, weight, and weight at age 21; brief medical history (e.g., treated hypertension and treated high cholesterol); use of medications in the past 48 h; and a modified Block Food Frequency Questionnaire. Clinical variables, including resting blood pressure and plasma total cholesterol, were measured for each participant. A follow-up questionnaire in 1996 ascertained whether participants had a first-degree family history of cancer.
Data analysis
Participants were grouped by diabetes status at baseline, and the χ2 test and t test were used to evaluate differences in proportions and means for the baseline characteristics between diabetic and nondiabetic participants. For cancer incidence, follow-up time for each participant was determined from the date of study entry to the date of cancer diagnosis, last date known to be free of a cancer diagnosis, death, or the end of follow-up (31 December 2006), whichever came first. For cancer mortality, person-years of follow-up were calculated from the date of study entry to either the date of cancer death, the date of death from other causes, or the end of follow-up, whichever came first. We subsequently excluded cancer incidence and cancer death in the first 2 years of follow-up to minimize the potential that participants may have had subclinical cancer prior to baseline. To determine cancer-specific death in participants with cancer, we calculated follow-up time from the date of cancer diagnosis to either the date of death from that cancer, the date of death from other causes, or the end of follow-up, whichever came first. In a similar manner, for death from all causes, in participants with cancer, person-years of follow-up were calculated from the date of cancer diagnosis to either the date of death from any cause or the end of follow-up, whichever came first.
The associations between treated diabetes and the cumulative risk of cancer incidence, cancer mortality, and survival after cancer diagnosis were first evaluated using the Kaplan-Meier method with log-rank tests to determine the significance of differences in cumulative risk using age as the time scale. Cox proportional hazards models were subsequently used to calculate hazard ratios (HRs) and 95% CIs for cancer incidence and cancer mortality, and in those diagnosed with cancer, death from cancer and death from all causes comparing baseline-treated diabetes to no diabetes after adjusting for age (continuous), age squared (continuous), sex, BMI (<25, 25–29, 30–35, and >35 kg/m2), smoking (never, former ≤20 cigarettes/day, former >20 cigarettes/day, current ≤20 cigarettes/day, and current >20 cigarettes/day), education (<12 vs. ≥12 years), hypertension treatment (yes/no), and high cholesterol treatment (yes/no). We confirmed the proportionality assumption by examining Schoenfeld residuals and used the Efron method to handle ties.
Additional analyses were performed to determine the association between diabetes and risk of advanced stage of major cancers, including colorectal (stage 3/4), breast (stage 3/4), and prostate cancer (stage 3/4 or Gleason score ≥7). For participants with valid responses (10) to a dietary questionnaire (n = 13,690; 75% of study population), we additionally adjusted for alcohol, fruit, vegetable, and red meat intakes and use of a multivitamin. To reduce possible detection bias from regular medical encounters in participants with treated diabetes, for the analysis of cancer incidence, a subgroup analysis was further conducted in adults who received treatment for hypertension and/or high cholesterol at baseline. To further reduce possible confounding as a result of the difference in the age distribution between diabetic and nondiabetic groups on mortality, we conducted a separate subgroup analysis limited to participants aged ≥60 years at baseline.
AFs (11) of cancer incidence, cancer case fatality, and all-cause mortality due to diabetes among individuals with preexisting, treated diabetes were estimated based on adjusted HRs to give crude, relative measures of the percentages of events that are expected to be the result of diabetes, should there be a causal relationship {AF = [(HR − 1)/HR] × 100}.
Tests of significance were two-tailed, with an α-level of 0.05. We performed analyses using SAS version 9.2 (Cary, NC) and Stata/SE version 10.0 (College Station, TX).
RESULTS
Baseline characteristics
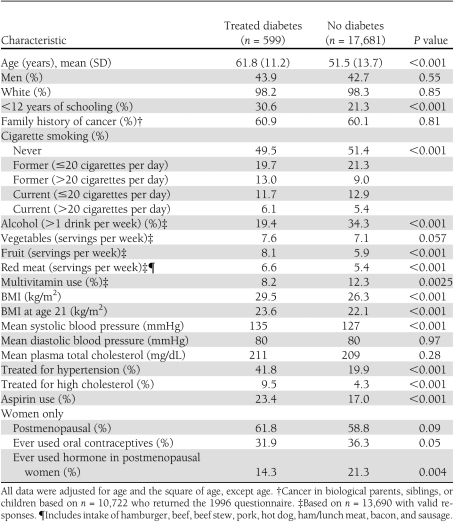
Baseline characteristics are shown in Table 1 by diabetes status. Of 18,280 participants, 599 (3.3%) had been treated for diabetes, including 354 taking oral medication, 197 receiving insulin, and 48 taking unspecified diabetes medications. In age-adjusted comparisons, compared with their nondiabetic counterparts, adults with diabetes were less educated, were more likely to be smokers, reported higher intake of red meat and fruit but lower alcohol consumption, were less likely to take multivitamins, had higher BMI at age 21 and at baseline, had higher systolic blood pressure, were more likely to have been treated for hypertension or high cholesterol, and were more likely to use aspirin (all P < 0.05). Women with diabetes were less likely to have used oral contraceptives or hormone replacement therapy.
Table 1.
Age-adjusted baseline characteristics by treated diabetes status in 18,280 adults, CLUE II, Washington County, Maryland, 1989–2006
Cancer incidence
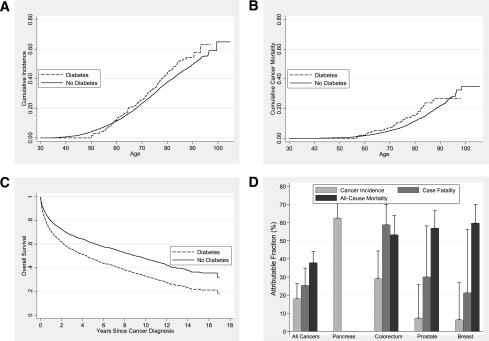
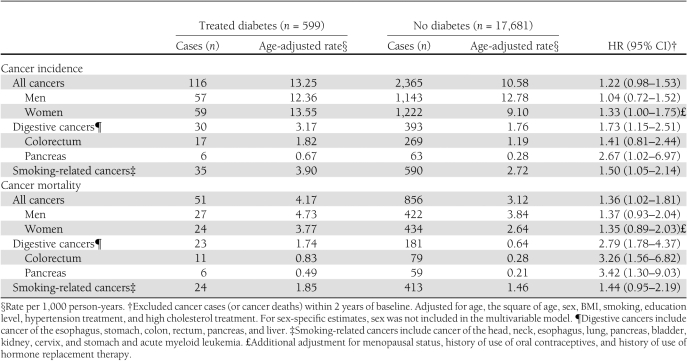
During 17 years of follow-up, 116 diabetic and 2,365 nondiabetic adults developed cancer, corresponding to age-adjusted incidence rates of 13.25 and 10.58 per 1,000 person-years, respectively. The cumulative cancer incidence for adults with diabetes was elevated constantly after age 60 (Fig. 1A). Compared with their nondiabetic counterparts, adults with diabetes were slightly more likely to develop cancer overall (multivariable-adjusted HR 1.22 [95% CI 0.98–1.53]), especially cancers of the pancreas and digestive system and cancers related to smoking (Table 2). Diabetes was not statistically significantly associated with other cancers: the HRs for colorectal, lung, kidney, breast, and prostate were 1.41 (0.81–2.44), 1.26 (0.73–2.18), 2.06 (0.79–5.37), 1.07 (0.61–1.87), and 1.08 (0.66–1.77), respectively.
Figure 1.
A: Cumulative cancer incidence in 18,280 adults by treated diabetes status, log-rank P = 0.012. B: Cumulative cancer mortality in 18,280 adults by treated diabetes status, log-rank P = 0.015. C: Age-adjusted overall survival after cancer diagnosis in 2,481 participants diagnosed with cancer by treated diabetes status, log-rank P < 0.0001. D: AFs and SEs of cancer incidence, cancer case fatality, and total mortality due to diabetes in participants with preexisting diabetes.
Table 2.
Association between treated diabetes and cancer incidence and cancer mortality in 18,280 adults, CLUE II, Washington County, Maryland, 1989–2006
No statistically significant associations were observed between diabetes and risks of advanced stage of colorectal or prostate cancer. There were no advanced breast cancer cases in participants with diabetes. In the subset of participants with valid responses for the food frequency questionnaire, the results were similar to the overall cohort. In another subset analysis including participants receiving treatments for hypertension and/or high cholesterol, the fully adjusted HR of overall cancer incidence in the treated diabetic group was somewhat stronger than but not different from the estimate in the total study population (HR 1.37 [95% CI 1.04–1.80]).
Cancer mortality
A total of 51 diabetic and 856 nondiabetic participants died of cancer during follow-up, corresponding to age-adjusted cancer mortality rates of 4.17 and 3.12 per 1,000 person-years, respectively. Similar to cancer incidence, diabetes was associated with an increased risk of cancer mortality after age 60 (Fig. 1B). The multivariable-adjusted HR of all-cancer mortality was 1.36 (95% CI 1.02–1.81) comparing adults with and without diabetes (Table 2). Diabetes-related cancer mortality risk was significantly increased for cancers of the digestive system, especially pancreatic cancer and colorectal cancer (Table 2). There were no significant associations observed in other site-specific analyses (data not shown).
Cancer case fatality and all-cause mortality after a cancer diagnosis
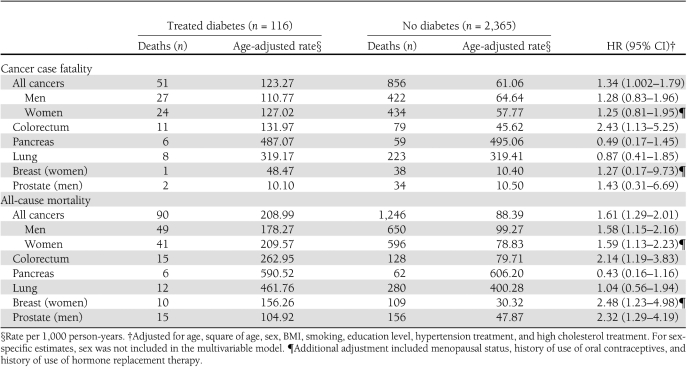
During follow-up, 2,481 adults developed cancer. The mean age of cancer diagnosis was 71.7 ± 8.8 and 67.9 ± 11.4 years in adults with and without treated diabetes, respectively (P < 0.0001). Cancer patients with diabetes experienced a rapid decline in survival within the first 3 years after cancer diagnosis (Fig. 1C). Overall, 69% of participants with cancer died of cancer and 13% died of circulatory disease. Cancer case fatality was higher in individuals with diabetes compared with those without diabetes (Table 3). Among participants with colorectal cancer, individuals with diabetes had a higher risk of death from colorectal cancer than those without diabetes. Diabetes also conferred a higher risk of death from all causes in adults who developed a wide array of cancers, including cancers of the colorectum, breast, and prostate. Pooling across cancers, diabetes was associated with a nearly 60% excess risk of all-cause mortality. In the subset of participants with valid responses for the food frequency questionnaire, the results were similar to the overall cohort after further adjustment for alcohol consumption (data not shown).
Table 3.
Association between treated diabetes and cancer fatality and all-cause mortality in 2,481 adults diagnosed with cancer, CLUE II, Washington County, Maryland, 1989–2006
AFs resulting from diabetes
To compare the impact of diabetes across the full range of cancer outcomes, we calculated a series of AFs (Fig. 1D). The AF can be interpreted as the percentage of events in people with preexisting diabetes attributable to diabetes itself, if the relationships are causal. In the CLUE II cohort, the fraction of events attributable to diabetes rose in a graded fashion from incident cancer, to cancer case fatality, to all-cause mortality in cancer patients. The largest AF for incident cancer risk was for pancreatic cancer (>60%). The largest AFs for all-cause mortality in cancer patients were for colon, prostate, and breast—all >50%. Colon cancer showed a moderate-to-strong influence of diabetes across its full natural history, from incidence to cancer case fatality and all-cause mortality.
CONCLUSIONS
This community-based, prospective study provides support for the following conclusions. First, adults with treated diabetes were more likely to develop cancer than their nondiabetic counterparts, especially pancreatic cancer. Second, diabetic adults were more likely to die of cancer than their nondiabetic counterparts. Third, diabetes was associated with greater cancer-specific case fatality for adults with cancer, particularly with colorectal cancer. Fourth, in patients with cancer, individuals with diabetes had higher all-cause mortality than those without diabetes. Fifth, in individuals with diabetes, the AFs due to diabetes were larger for cancer case fatality and total mortality than for cancer incidence, with the exception of pancreatic cancer. Thus, diabetes appears to exert a greater influence downstream on the risk of mortality in people with cancer than on upstream risk of incident cancer.
Our study is consistent with prior literature in several ways, including our overall relationship of diabetes with incident cancer (7) and our estimates for diabetes-related risk of incident pancreatic cancer (5), risk of mortality owing to cancer of the pancreas and colon (7,8), and risk of all-cause mortality in cancer patients (9). A unique strength of our study, however, is its quantification of the influence of diabetes on cancer outcomes across the full range of its clinical history, from risk of incident cancer to risk of cancer death, case fatality, and all-cause mortality in cancer patients. Previous studies did not adopt this more global perspective, instead focusing more specifically on individual relationships with particular cancers, separating the impact on incidence from the impact on mortality.
Type 2 diabetes (which accounts for >90% of prevalent cases of diabetes in the U.S.) shares common lifestyle risk factors with several cancer types. These factors include obesity (12) and physical inactivity (13), which increase the risk of insulin resistance and compensatory hyperinsulinemia. An important role of hyperinsulinemia in colorectal and pancreatic carcinogenesis is supported by in vitro studies, animal models, and several epidemiologic studies (14). Another mechanism may be that hyperglycemia itself promotes tumor growth (15), but results are not consistent among studies (16).
We found preexisting diabetes to be an independent risk factor for death from cancer in adults with any cancer and for colorectal cancer death among adults with colorectal cancer. Possible explanations for diabetes-related case fatality include tumor proliferation due to hyperinsulinemia and hyperglycemia (17), less aggressive cancer treatment resulting from the presence of diabetes-related comorbidities (18), poorer response to cancer treatment in adults with diabetes (19), and suboptimal cancer screening practices related to diabetes status (20).
Diabetes is a strong independent predictor of all-cause mortality in patients with a variety of cancer types. Diabetes-related cardiovascular disease no doubt plays a major role here. It is possible that cancer’s adverse effects on thrombosis and oxygenation, as well as the cardiovascular risks imposed by cancer surgery, will create adverse biological interactions (21). Besides, the urgent need to treat cancer might distract from optimal care for diabetes and related conditions (22). For these reasons, some experts favor all-cause mortality as an end point in cancer outcomes research that is not biased by attribution of cause of death and captures the full effect of possible interactions (23).
A main strength of our study is the availability of data on cancer incidence and mortality across multiple cancer types in the same population that allowed us to isolate the effects of diabetes on cancer incidence from its effects on cancer case fatality and death from all causes. Other strengths of our study include a community-based cohort, comprehensive ascertainments of cancer and mortality, data on potential confounders, and 17 years of follow-up that offered the opportunity to study long-term associations.
Nevertheless, several limitations deserve mention. First, the diagnosis of diabetes and fasting glucose data were not available in the study, and diabetes status was not updated during follow-up. We therefore relied exclusively on self-reported use of diabetes medications at baseline to identify cases of diabetes. Thus, the diabetic individuals in our study may be later in the natural history of their diabetes than in other studies. The consequent misclassification of adults with undiagnosed and untreated diabetes as nondiabetic likely biased our results toward the null. Second, as a result of the lack of fasting blood assessment, we were not able to isolate the effects on cancer from hyperglycemia, diabetes, or antidiabetes treatment (e.g., insulin). Third, it is possible that detection bias occurred if adults with diabetes had more frequent contact with their physicians and therefore were more likely to be detected with cancer; although we attempted to rule out this bias by conducting a subgroup analysis among participants who presumably accessed care because they had been treated for hypertension and/or high cholesterol. Fourth, we lacked optimal data to characterize adiposity: BMI was calculated based on self-reported weight and height (which tend to underestimate true BMI), and we lacked data on waist circumference or percent body fat. Residual confounding by adiposity is therefore a potential concern. Fifth, we had a limited number of cases to investigate less common cancer sites, such as endometrial, lymphoma, esophageal, and liver cancers. Sixth, given the significant difference between diabetic and nondiabetic individuals at baseline, even the multiple adjustments for covariates leave the possibility of residual confounding. Finally, our sample was almost entirely white; generalizability to nonwhite populations warrants further investigation.
Our study suggests that for many common cancers like colon, breast, and prostate, diabetes exerts a stronger adverse influence downstream, after cancer occurs, than upstream, in relation to incident cancer risk. Whether improvements in diabetes management might reduce the risk of mortality in cancer patients with preexisting cancer deserves further attention.
Acknowledgments
H.-C.Y. has received support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diabetes Research and Training Center (P60-DK-079637). N.-Y.W. has received support from the NIDDK Diabetes Research and Training Center (P60-DK-079637) and the National Center for Research Resources (UL1 RR 025005). F.L.B. has received support from the NIDDK Diabetes Research and Training Center (P60-DK-079637) and from the NIDDK (K24-DK-62222). Funding for the CLUE II cohort was provided by grants from the National Cancer Institute (U01-CA-086308) and the National Institute on Aging (U01-AG-18033). The authors acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped support the availability of the cancer registry data.
No potential conflicts of interest relevant to this article were reported.
H.-C.Y. researched data, contributed to discussion, wrote the manuscript, had full access to all data in the study, and takes responsibility for the integrity of data and the accuracy of the data analysis. E.A.P., N.-Y.W., K.V., K.J.H., and F.L.B. contributed to discussion and reviewed and edited the manuscript.
The authors appreciate the continued efforts of the staff members, particularly Sandra Clipp and Judith Hoffman-Bolton, of the Johns Hopkins George W. Comstock Center for Public Health Research and Prevention in the conduct of the CLUE studies.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–862 [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–1687 [DOI] [PubMed] [Google Scholar]
- 3.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007;50:1365–1374 [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369–380 [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao C, Page JH. Type 2 diabetes mellitus and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Am J Epidemiol 2008;168:471–480 [DOI] [PubMed] [Google Scholar]
- 7.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202 [DOI] [PubMed] [Google Scholar]
- 8.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004;159:1160–1167 [DOI] [PubMed] [Google Scholar]
- 9.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300:2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett WC. Nutritional Epidemiology. 2nd ed. New York, Oxford University Press, 1998 [Google Scholar]
- 11.Gordis L. Epidemiology. 4th ed. Philadelphia, W.B. Saunders Company, 2008 [Google Scholar]
- 12.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007;132:2208–2225 [DOI] [PubMed] [Google Scholar]
- 13.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol 2005;98:3–30 [DOI] [PubMed] [Google Scholar]
- 14.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346–1353 [DOI] [PubMed] [Google Scholar]
- 15.Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2003;12:412–418 [PubMed] [Google Scholar]
- 16.Rinaldi S, Rohrmann S, Jenab M, et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2008;17:3108–3115 [DOI] [PubMed] [Google Scholar]
- 17.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2005;2:48–53 [DOI] [PubMed] [Google Scholar]
- 18.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 2007;120:1986–1992 [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 2003;21:433–440 [DOI] [PubMed] [Google Scholar]
- 20.McBean AM, Yu X. The underuse of screening services among elderly women with diabetes. Diabetes Care 2007;30:1466–1472 [DOI] [PubMed] [Google Scholar]
- 21.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med 2007;262:157–172 [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst 2002;94:1044–1045 [DOI] [PubMed] [Google Scholar]
- 23.Welch HG, Black WC. Are deaths within 1 month of cancer-directed surgery attributed to cancer? J Natl Cancer Inst 2002;94:1066–1070 [DOI] [PubMed] [Google Scholar]