Abstract
OBJECTIVE
To investigate the effect of treatment with the glucagon-like peptide 1 receptor agonist exenatide on weight loss and metabolic parameters in obese nondiabetic women.
RESEARCH DESIGN AND METHODS
Forty-one obese women (aged 48 ± 11 years and BMI 33.1 ± 4.1 kg/m2) participated in a 35-week randomized, double-blind, placebo-controlled, crossover study, including two 16-week treatment periods separated by a 3-week washout period. There was no lifestyle intervention. The primary outcome was change in body weight.
RESULTS
Subjects treated with exenatide lost an average of 2.49 ± 0.66 kg compared with a 0.43 ± 0.63 kg weight gain during placebo treatment. Weight loss with exenatide treatment was noted at 2 weeks. The degree of weight loss could be stratified. A total of 30% of subjects were high responders who lost ≥5% body weight (−7.96 ± 0.52%), 39% were moderate responders who lost <5% body weight (−2.43 ± 0.45%), and 31% were nonresponders who gained weight (1.93 ± 0.53%). Waist circumference also decreased significantly with exenatide treatment. Subjects experienced more nausea during exenatide treatment compared with placebo, but the severity decreased over time and did not correlate with weight loss.
CONCLUSIONS
Short-term exenatide treatment was associated with modest weight loss and decreased waist circumference in a cohort of obese nondiabetic women. A subset of individuals demonstrated robust weight loss that was detected very early in the course of treatment.
The recent withdrawal of sibutramine from the market leaves only two Food and Drug Administration–approved pharmacologic agents for the treatment of obesity, phentermine and orlistat (1). From this limited armamentarium, clinicians attempt medical management of a chronic disease that has substantial economic, physical, and emotional consequences. Although many studies have shown that weight loss can be achieved through highly supervised dietary interventions, weight regain following these interventions is almost universal (2–4). A recent meta-analysis showed that 25–88% (mean 54%) of weight loss achieved immediately after the intervention was maintained after 1 year of unsupervised follow-up (5). After 2 years, the percentage of maintained weight loss diminishes even more (6). In contrast, bariatric surgery is the most successful intervention for achieving long-term weight loss in morbid obesity (7). However, widespread use of a surgical intervention for obesity is problematic because many people who would benefit do not choose this approach, nor is it routinely available to individuals with BMI <39.9 kg/m2 and no other metabolic complications. Given the lack of long-term success with dieting, the invasive nature of bariatric surgery, and the limited pharmacologic options for the medical management of obesity, studies designed to investigate weight loss with agents approved for other primary indications have potentially high clinical value.
Glucagon-like peptide (GLP)-1 is an incretin hormone secreted by the enteroendocrine L cells of the small intestine in response to food intake. The major physiologic effects of GLP-1 include increased postprandial insulin secretion, decreased postprandial glucagon secretion, and delayed gastric emptying (8). Early studies of human GLP-1 showed that continuous peripheral infusion was associated with decreased appetite and increased satiety (9). Continuous infusion of GLP-1 also was shown to improve insulin sensitivity, glycemic control, and β-cell function in individuals with type 2 diabetes (10). Incretin mimetics evolved as therapeutic options for the treatment of type 2 diabetes primarily because of their effects on insulin and glucagon secretion, with weight loss as an additional benefit.
Weight loss ranging from 2 to 6 kg has been a consistent finding in studies designed to investigate the glycemic benefits of incretin mimetics in individuals with type 2 diabetes (11–14). There are very limited data on weight loss in nondiabetic individuals treated with these agents (15,16). To evaluate the effects of exenatide treatment in obese, nondiabetic women, we designed a randomized, placebo-controlled, crossover study. To minimize the placebo effect and study the effect of exenatide in a real-world setting, we did not include any lifestyle modifications, and we discouraged any change in diet or physical activity. To avoid confounding factors of body fat distribution, we enrolled only women.
RESEARCH DESIGN AND METHODS
We conducted a 35-week randomized, double-blind, placebo-controlled, crossover study, with two 16-week treatment periods separated by a 3-week washout period (Supplementary Fig. 1). All study visits were conducted in the Beth Israel Deaconess Medical Center General Clinical Research Center in Boston, Massachusetts. All participants gave written informed consent before participation. The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board and was conducted according to the principles expressed in the Declaration of Helsinki.
Between 2007 and 2010, we enrolled 41 women aged between 18 and 70 years with BMI between 28 and 40 kg/m2. Major exclusion criteria included having type 1 or type 2 diabetes, having uncontrolled hypertension or dyslipidemia, being treated with antiobesity medication within 1 year, having a history of bariatric surgery, and having previous treatment with exenatide. Subjects reported a stable weight within 6 months of the screening visit. A total of 24% of the study population had prediabetes at enrollment. Subjects injected 5 μg of either exenatide or identically dispensed placebo before breakfast and supper. After 2 weeks, subjects increased their doses to 10 μg exenatide or placebo twice daily. Study visits occurred every 2 weeks.
Primary outcome
The primary outcomes of body weight (kg) and BMI (kg/m2) were measured at all study visits, with subjects wearing light clothing and no shoes.
Secondary outcomes
Waist circumference (cm) was measured using a nonelastic measuring tape. Body composition was measured using bioelectrical impedance analysis (BIA; RJL System Quantum II Bioelectrical Body Composition Analyzer) after an overnight fast. Blood pressure and heart rate were measured using a Dynamap automated monitoring device. Lipid panels, leptin, and adiponectin were measured after an overnight fast at weeks 0, 10, and 16 during both treatment periods. Glycemic status was measured at the beginning and end of each treatment period with a standard 75-g, 2-h oral glucose tolerance test. Insulin was measured using the access chemiluminescent assay (Beckman Coulter, Fullerton, CA). Adiponectin was measured using an enzyme-linked immunosorbent assay technique (Alpco Diagnostics, Salem, NH), and leptin was measured using a radioimmunoassay technique (Linco Research, St. Charles, MN). Resting energy expenditure (REE; kcal) was measured after an overnight fast using the Viasys Indirect Calorimeter. Subjects wore a watch-sized sleep monitor and an accelerometer (Actigraph and Actical; Mini Mitter) on the nondominant wrist for 2 weeks during each treatment period. Subjects rated hunger, satiety, and nausea on a 10-cm visual analog scale every 2 weeks.
Statistical analysis
Our study was powered to assess the primary outcome of change in body weight during exenatide treatment. We calculated that we needed 42 subjects to detect a (mean ± SD) 2.6 ± 1.3 kg difference in body weight between exenatide and placebo treatment at 16 weeks with 80% power and an α value of 0.05. Using a 20–25% drop-out rate, we enrolled an additional 10 subjects. Differences in baseline characteristics between the two randomized groups of the study were assessed with t tests. Baseline demographic data for all study participants are presented as the mean ± SD. We used a least squares means procedure to compare outcomes between exenatide and placebo. We report the estimated mean or Δ along with the P value and 95% CIs. For repeated measures, we used linear mixed models to compare treatment differences over time. For subgroup analyses, we report the pairwise Tukey-Kramer adjusted P values. Subjects who completed at least 10 weeks of either treatment period were included in the outcome analysis for that treatment period by using the last-observation-carried-forward method. All statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
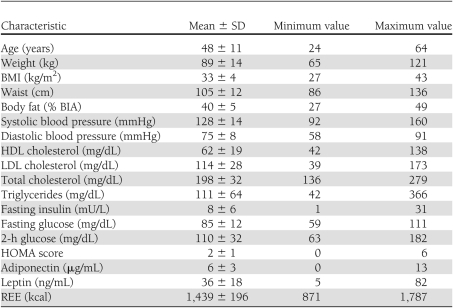
Baseline characteristics of the study population are provided in Table 1. There were no significant differences among subjects randomly assigned to exenatide or placebo for the first treatment period. Our study population as a whole had well-controlled cardiovascular risk factors (85% had systolic blood pressure <140 mmHg, 80% had total cholesterol levels <220 mg/dL, 93% had LDL <160 mg/dL, and 85% had triglycerides <150 mg/dL).
Table 1.
Baseline characteristics for subjects completing at least 10 weeks of treatment with either placebo or exenatide (n = 41)
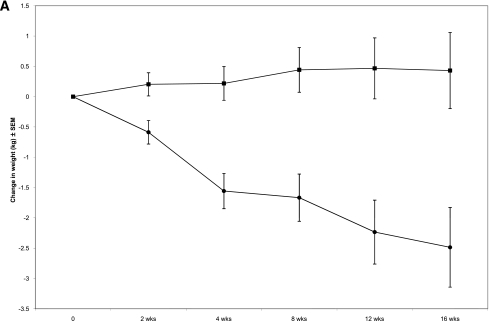
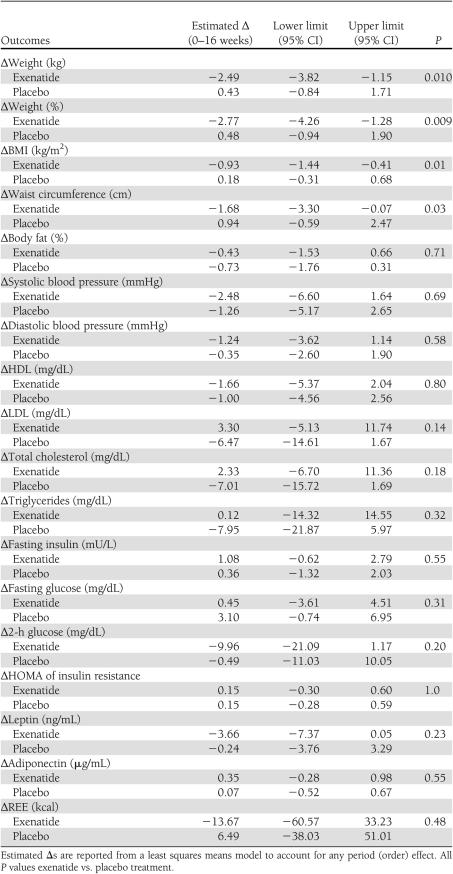
Sixteen weeks of exenatide treatment was associated with a significant decrease in body weight. Subjects lost 2.49 ± 0.66 kg during exenatide treatment compared with an increase of 0.43 kg ± 0.63 kg during placebo treatment (P < 0.01) (Fig. 1). This corresponded to a 2.7% decrease in body weight during exenatide treatment and a 0.2% increase in body weight during placebo treatment. A significant decrease in body weight was observed after 2 weeks of exenatide, and the difference persisted for the entire treatment period (Fig. 1A). Exenatide was associated with a small but statistically significant decrease in BMI, and waist circumference decreased by 1.68 cm (Table 2). There was no change in body composition using BIA.
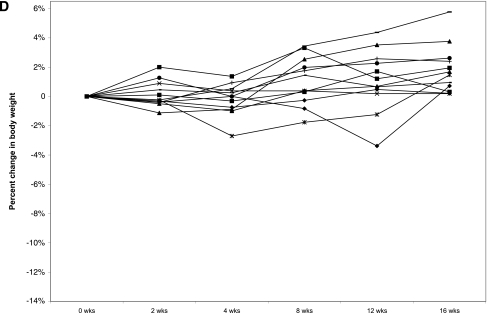
Figure 1.
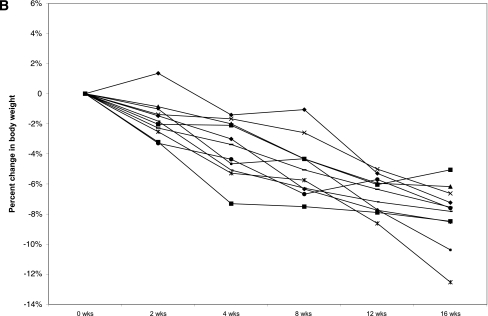
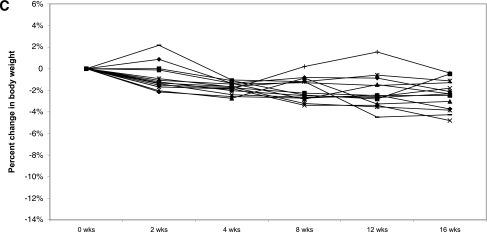
A: Change in body weight during exenatide and placebo treatment periods. Error bars represent ± SEM. P < 0.02 at 2 weeks. B: Individual weight profiles during exenatide treatment among high responders (n = 11). C: Individual weight profiles during exenatide treatment among moderate responders (n = 14). D: Individual weight profiles during exenatide treatment among nonresponders (n = 12).
Table 2.
Changes in metabolic parameters during exenatide and placebo treatment periods
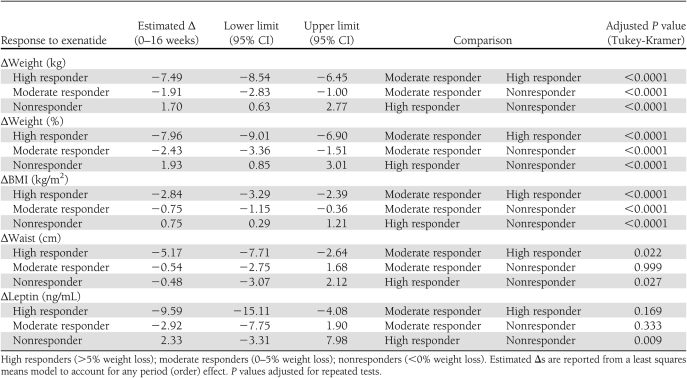
Of interest, retrospective analysis revealed that weight loss with exenatide treatment was variable (Table 3). Three levels of response could be identified. A total of 30% of subjects (n = 11) were high responders who lost >5% (range 5–12.5%) of their body weight. A total of 39% (n = 14) were moderate responders who lost <5% (range 0.4–4.8%) of their body weight, and 31% (n = 12) of subjects were nonresponders who achieved no weight loss or gained weight (range 0.19–5.8%) (Fig. 1B–D). Seven high responders were randomly assigned to exenatide first. Five regained weight when switched to placebo (2.62–8.17% regain), one continued to lose weight on placebo, and one was not included in the data analysis because she completed <10 weeks of placebo treatment. We did not identify any pretreatment metabolic parameters that predicted the degree of response to exenatide.
Table 3.
Changes in metabolic parameters during exenatide treatment among the three response groups
Exenatide was not associated with any significant change in metabolic parameters, including blood pressure, lipid profiles, insulin and adiponectin levels, and homeostasis model assessment (HOMA) scores. Two-hour glucose values trended lower during exenatide treatment compared with placebo, although there was wide variability (Table 2). We did not see any significant change in leptin levels when we compared exenatide with placebo (Table 2); however, we noted that leptin levels decreased significantly among high responders (39 ± 4.8 ng/mL to 28.8 ± 3.7 ng/mL) (Table 3), an effect not seen in nonresponders. There were no significant changes in adiponectin levels, insulin levels, or HOMA of insulin resistance scores when the data were analyzed by responder group (data not shown).
There was no significant change in REE, expressed as kilocalories per day or kilocalories per kilogram, for the study group as a whole or for any subgroup. High responders, who lost an average of 8% of their body weight, demonstrated a 0.6 kcal/kg increase in REE, whereas nonresponders demonstrated a −0.3 kcal/kg decrease in REE; neither change was significant. There was no significant change in quantitative or qualitative physical activity, nor was there a difference in sleep parameters, including sleep duration, sleep efficiency, or wake after sleep onset. There was, however, a trend toward decreased sleep onset latency during exenatide treatment compared with placebo (29.9 ± 17.6 min vs. 48.3 ± 42.9 min, P < 0.06).
Adverse events
Hypoglycemia was not observed in the fasting state or during oral glucose tolerance tests. The most common adverse event was nausea. A total of 56% of subjects experienced one or more episodes of nausea during exenatide treatment compared with 21% of subjects during placebo treatment. Two subjects dropped out during exenatide treatment and no subjects dropped out during placebo treatment because of nausea. All subjects who had a modified titration schedule during exenatide treatment reported nausea. Four subjects had a slower titration to the 10-μg dosage, and six subjects did not titrate above 7.5 μg. Two subjects had slower or incomplete titration on placebo.
Nausea visual analog scale scores increased during the exenatide treatment period compared with placebo. Scores were highest at week 4 and then decreased over the remainder of the treatment period. At week 16, nausea scores were not significantly different between exenatide and placebo (Supplementary Table 1). Satiety and hunger scores were not significantly different during exenatide and placebo treatment. Additional adverse events during exenatide treatment included irritation at the injection site (n = 1), constipation (n = 2), bloating (n = 1), uncomfortable satiety (n = 1), fatigue (n = 1), diarrhea (n = 1), heartburn (n = 1), belching (n = 1), menstrual irregularity (n = 1), and respiratory infection (n = 3). Three subjects with ongoing nausea or abdominal pain had lipase levels measured. No elevations were found, and in all cases symptoms resolved.
CONCLUSIONS
Incretin mimetics are quickly gaining popularity for the treatment of type 2 diabetes. Exenatide is associated with a reduction in hemoglobin A1C of 0.5–0.8% and liraglutide with a reduction of 1.1–1.5% (11–14,17). Of interest, a consistent finding in large clinical studies is that hemoglobin A1C levels declined but then plateaued while weight loss continued (18). Data on weight loss with exenatide in individuals without diabetes are limited. One group reported ~3.3 kg placebo-corrected weight loss over 24 weeks (15). Astrup et al. (16) showed that 1.2–3.0 mg liraglutide daily plus aggressive lifestyle intervention for 20 weeks was associated with dose-dependent, placebo-corrected weight loss, ranging from 2.0 to 4.4 kg among nondiabetic obese individuals. Neither study commented on individual responses to treatment.
Our cohort of women lost, on average, 2.5 kg after 16 weeks of exenatide treatment, and we observed significant weight loss after only 2 weeks. Importantly, we observed that 30% of our study population lost 8% of their body weight during exenatide treatment, whereas another 31% did not lose any weight or gained weight. The variable response to exenatide was apparent as early as 4 weeks. To our knowledge, this is the first study to report stratified weight loss in response to exenatide treatment in obese, nondiabetic individuals. Although we did not identify baseline characteristics that predicted the degree of response to exenatide treatment, our observation that significant weight loss was seen as early as 2 weeks and that high responders could be identified by 4 weeks has important clinical ramifications and demonstrates the value of a short clinical trial to assess the effectiveness in a given individual. A larger prospective study designed to investigate predictors of weight loss with exenatide is an important area for future investigation.
The mechanisms of weight loss with exenatide are not fully understood but may include changes in energy expenditure, changes in leptin sensitivity, or nausea resulting in decreased food intake. Significant weight loss is usually associated with a decrease in REE that favors weight regain (19). Leibel et al. (20) observed that a hypocaloric diet producing 10% weight loss resulted in a decrease in REE of ~130 kcal/day, and Pereira et al. (21) found that a low-fat diet producing 10% weight loss was associated with a decrease in REE of 176 kcal/day, compared with 96 kcal/day with a low–glycemic index diet producing the same degree of weight loss. Dietary or pharmacologic interventions that blunt the decrease in REE with weight loss are in great clinical demand. We identified a potential effect of exenatide on REE because those who had the most robust weight loss with exenatide did not have the expected decrease in this parameter. Rodent and human studies support a relationship between GLP-1 and energy expenditure, possibly by activating the sympathetic nervous system. In rats, both peripheral and central administration of GLP-1 receptor agonists increased blood pressure and heart rate by activating autonomic regulatory sites in the rat brain (22). In lean, healthy males, peripheral GLP-1 administration during a hyperglycemic clamp was associated with increased REE, and the GLP-1 analog liraglutide has been associated with increased heart rate (23,24).
Another mechanism through which exenatide may produce weight loss is by increasing leptin sensitivity. Obesity is considered to be a state of leptin resistance, and weight loss is thought to improve leptin sensitivity. There currently is no technique for measuring leptin sensitivity in humans, and the decrease in leptin levels seen with weight loss generally is used as indirect evidence of increased leptin sensitivity. Although there was no significant change in leptin levels during exenatide treatment when we analyzed our data in aggregate, we noted that leptin levels decreased significantly among exenatide high responders compared with nonresponders. However, this effect may be attributed to weight loss, per se, rather than exenatide treatment. A comparator group with calorie-restricted weight loss would help answer this question in future studies. Nausea, the most common adverse effect of exenatide, also may contribute to weight loss. Although exenatide was associated with more nausea than placebo, the severity of nausea decreased over time and nausea scores were not higher among exenatide high responders.
Our study included a small cohort of generally healthy obese women, which limits the generalizability of our findings. The overall drop-out rate was 35%, with 17% dropping out prior to being randomly assigned and 18% dropping out after being randomly assigned. There was no significant difference in baseline weight or BMI among dropouts versus completers. A crossover study design introduces the possibility of both an order effect and a carryover effect. Our statistical analysis was adjusted for possible treatment order effects. Given the short half-life of exenatide and normal renal function among study participants, a 3-week washout period should have been sufficient to exclude a carryover effect. Our study was powered for the primary outcome of weight loss during exenatide versus placebo treatment. Retrospective analysis revealed that weight loss among our cohort could be grouped as ≥5% body weight, <5% body weight, or weight gain. A larger, prospective study will be required to examine the metabolic parameters that may predict the degree of response to exenatide treatment.
The trend in obesity literature typically has been to report the average response of an entire study population to a particular dietary or pharmacologic intervention. However, the analysis and reporting of weight loss data in aggregate can obscure individual responses to treatment. Our study is the first study to describe weight loss variability with a GLP-1 receptor agonist and demonstrates that a subset of women have a robust response to exenatide treatment that can be identified very early in the course of therapy. Future studies examining the duration and magnitude of weight loss among individuals who have a robust response to exenatide treatment, as well as characteristics that predict response, may guide us toward a more personalized approach to pharmacotherapy for the treatment of obesity.
Acknowledgments
This study was supported by grant M01-RR-01032 from the Beth Israel Deaconess Medical Center General Clinical Research Center and by the Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1-RR-025758 and financial contributions from Harvard University and its affiliated academic health care centers).
Support for this study was also provided by Eli Lilly and Company and Amylin Pharmaceuticals, who are involved in the development of exenatide. No other potential conflicts of interest relevant to this article were reported.
J.D. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. C.G. contributed to discussion and reviewed and edited the manuscript. G.S.G. and M.C. researched data and reviewed and edited the manuscript. E.K.M., E.W., and J.M. contributed to discussion and reviewed and edited the manuscript. E.M.-F. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript.
Parts of this study were presented in abstract form at the Obesity Society 28th Annual Scientific Meeting, San Diego, California, 8–12 October 2010.
The authors thank the study subjects and the Beth Israel Deaconess Clinical Research Center nursing and administrative staff. The authors also thank Monika Haack, Beth Israel Deaconess Medical Center, for assistance with the measurements of sleep parameters and data analysis.
Footnotes
Clinical trial reg. no. NCT00456885, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0931/-/DC1.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
References
- 1.Glant M, Raz I. Present and future: pharmacologic treatment of obesity. J Obes 2011;2011:636181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 2010;153:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shai I, Schwarzfuchs D, Henkin Y, et al. ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–241 [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barte JCM, ter Bogt NCW, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 2010;11:899–906 [DOI] [PubMed] [Google Scholar]
- 6.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74:579–584 [DOI] [PubMed] [Google Scholar]
- 7.O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol 2010;25:1358–1365 [DOI] [PubMed] [Google Scholar]
- 8.Tong J, Sandoval DA. Is the GLP-1 system a viable therapeutic target for weight reduction? Rev Endocr Metab Disord 2011;12:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 2001;86:4382–4389 [DOI] [PubMed] [Google Scholar]
- 10.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 13.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Rosenstock J, Sesti G, et al. ; LEAD 6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care 2010;33:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astrup A, Rössner S, Van Gaal L, et al. ; NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 17.Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010;33:1759–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436–447 [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes 2010;34Suppl. 1:S47–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;322:621–628 [DOI] [PubMed] [Google Scholar]
- 21.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292:2482–2490 [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 2003;23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shalev A, Holst JJ, Keller U. Effects of glucagon-like peptide 1 (7-36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Invest 1997;27:10–16 [DOI] [PubMed] [Google Scholar]
- 24.Okerson T, Chilton RJ. The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther. 19 December 2010 [Epub ahead of print] [DOI] [PMC free article] [PubMed]