Abstract
OBJECTIVE
To assess associations of gestational diabetes, existing diabetes, and glycosuria with adiposity and cardiometabolic risk factors in offspring at adolescence.
RESEARCH DESIGN AND METHODS
Multivariable regression analyses were conducted in a prospective pregnancy cohort (n = 2,563–4,198 for different outcomes). Obstetric data were abstracted from clinical records. Offspring outcomes were assessed at mean age 15.5 years. Compared with those lost to follow-up, participants included in the analysis were of higher socioeconomic position. Outcomes included BMI, waist circumference, fat mass determined by dual-energy X-ray absorptiometry scan, systolic and diastolic blood pressure (sBP and dBP, respectively), fasting glucose, insulin, lipids, and C-reactive protein (CRP).
RESULTS
Maternal existing diabetes, gestational diabetes, and glycosuria were associated with higher offspring BMI and fat mass (z scores); however, this effect was attenuated in the confounder-adjusted model, and the CIs included the null value. Existing diabetes and gestational diabetes were associated with higher offspring fasting glucose levels (0.24 mmol/L [95% CI 0.03–0.45] and 0.20 mmol/L [0.02–0.39], respectively). Glycosuria was associated with higher fasting insulin (adjusted ratio of geometric means 1.12 [1.01–1.25]), but there were no clear associations of existing or gestational diabetes with offspring fasting insulin. There was little evidence of an association of maternal diabetes or glycosuria with offspring dBP, sBP, lipids, or CRP.
CONCLUSIONS
Maternal pregnancy glycosuria, gestational diabetes, and existing diabetes show some associations with higher offspring fasting glucose and insulin assessed in adolescence but are not clearly associated with a wider range of cardiometabolic risk factors.
Detailed analyses from Pima Indian populations have demonstrated that fetal exposure to maternal diabetes in utero increases the risk of obesity and type 2 diabetes for offspring in later life (1). Fewer studies examine these associations in non-Pima populations, in whom the prevalence of obesity and diabetes is much lower. A recent systematic review and meta-analysis identifies nine such studies and concludes that maternal diabetes is associated with increased offspring BMI (2). The authors highlighted that only three of the nine studies had adjusted for maternal prepregnancy or early pregnancy BMI and that pooling results from these studies after adjustment for maternal BMI suggested that this was a major confounder for the association with offspring BMI. However, there was evidence of marked heterogeneity between results from these three studies, potentially reflecting the different underlying types of diabetes examined and the prepubertal ages examined (2). Furthermore, the review does not include a recently published very large sibling study in Swedish men that similar to studies in the Pima, supports an intrauterine mechanism linking maternal diabetes in pregnancy with offspring BMI that is not confounded by maternal early pregnancy BMI (3). In that study, mean BMI at age 18 years of younger brothers born after their mother was diagnosed with diabetes in pregnancy was greater than their older brothers’ mean BMI who were born before this diagnosis (3). It is notable that to date, few studies have examined associations with other offspring cardiometabolic risk factors and whether associations are mediated by the associations with offspring adiposity.
The aim of this study was to assess the associations between gestational diabetes, existing diabetes, glycosuria, adiposity, and a range of cardiometabolic risk factors (including measures of glucose and insulin metabolism) in offspring at age 15.5 years and to examine whether any associations are mediated by excess offspring adiposity. This article adds to a previous published study from the same cohort in which we found that gestational diabetes and glycosuria were associated with greater offspring BMI, waist circumference, and fat mass assessed when the offspring were aged 9–11 years but in which we were unable to assess associations with other cardiometabolic risk factors because of lack of relevant data at that age (4).
RESEARCH DESIGN AND METHODS
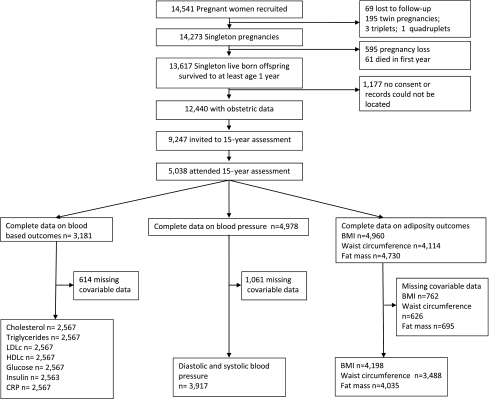
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective, population-based birth cohort study. In total, 14,541 pregnant women with expected dates of delivery between 1 April 1991 and 31 December 1992 were recruited (5), and there were 13,617 singleton live births who survived to 1 year. Data on existing and gestational diabetes status were available for 12,440 mother-offspring pairs; of these, 9,247 offspring (those who were still alive and engaged with the study) were invited to the follow-up assessment when they were mean age 15.5 years and 5,038 attended. Figure 1 shows the number of participants available for each analysis.
Figure 1.
Participation flow diagram through the study and the numbers included in each of the main analyses.
Ethics approval was obtained from the ALSPAC Law and Ethics Committee and the U.K. National Health Service Local Research Ethics Committee. Written informed consent was obtained from parents/guardians providing consent for offspring up to age 16 years, with offspring providing consent thereafter.
Assessment of maternal diabetes and glycosuria
Information on existing maternal diabetes and past history of gestational diabetes were collected by questionnaire from women at the time of recruitment. A standard protocol was used by research midwives to obtain information on gestational diabetes and glycosuria (recorded as none, trace, +, ++, and +++ or more) for the index pregnancy from the woman’s antenatal and postnatal medical records. The practice in the U.K. at the time was for all women to be offered urine tests for glycosuria at each antenatal clinic visit. Universal screening of women with a random or fasting blood glucose level or with an oral glucose tolerance test was not undertaken, and diagnostic tests for gestational diabetes will have been undertaken only in women with established risk factors (obesity, family history of diabetes and previous history of gestational diabetes or macrosomic birth, and south Asian ethnicity) or glycosuria. Glycosuria in our study was defined as a record of at least ++ (equal to 13.9 mmol/L or 250 mg/100 mL) on at least two occasions at any time during the pregnancy in the absence of existing diabetes and gestational diabetes. Because some level of glycosuria is common in pregnancy as a result of increased glomerular filtration rate (6), 250 mg/100 mL is the threshold that has generally been used for indicating the need for further testing to diagnose gestational diabetes (7). Women were categorized into one of four mutually exclusive categories of no existing diabetes or glycosuria (healthy), existing diabetes, gestational diabetes, and glycosuria.
Assessment of offspring adiposity and cardiometabolic risk factors
Offspring’s weight and height were measured in light clothing and without shoes and used to calculate BMI. Weight was measured to the nearest 0.1 kg using Tanita scales. Height was measured to the nearest 0.1 cm using a Harpenden stadiometer. Waist circumference was measured to the nearest 1 mm at the midpoint between the lower ribs and the pelvic bone using a flexible tape. A narrow fan beam densitometer (Lunar Prodigy; GE Healthcare Lunar Ltd., Cambridge, U.K.) was used to perform a whole-body dual-energy X-ray absorptiometry scan in which bone content and lean and fat mass were measured. Age- and sex-standardized z scores were calculated for BMI, waist circumference, and fat mass.
Overweight or obesity was defined using BMI and criteria defined by the International Obesity Task Force (8). Central obesity was defined as waist circumference greater than the 90th centile (9) of U.K. age- and sex-specific centile curves (10).
Blood pressure was measured with a Dinamap 9302 Vital Signs Monitor (Morton Medical, London, U.K.) with the correct cuff size. Two readings of systolic and diastolic blood pressure (sBP and dBP, respectively) were recorded with the participant at rest and their arm supported, and the mean of the two measures was used.
Fasting venous blood samples were taken, and samples were immediately spun and frozen at −80°C. Measurements were assayed 3–9 months after samples were taken, with no previous freeze-thaw cycles. Plasma lipid concentrations (total cholesterol, triglycerides, and HDL cholesterol [HDLc]) were measured by modification of the standard Lipid Research Clinical Protocol by using enzymatic reagents for lipid determination. Non–HDLc was calculated as total cholesterol minus HDLc. LDL cholesterol (LDLc) was calculated from directly measured lipid levels using Friedewald formula. Insulin was measured with an ELISA (Mercodia, Uppsala, Sweden) that does not cross-react with proinsulin. The recent consensus statement on insulin resistance in children suggests that homeostasis model assessment of insulin resistance is so highly correlated with fasting insulin (r = 0.98 in our sample) that it does not provide any added value to using fasting levels (11). Our findings were identical whether we used fasting insulin or homeostasis model assessment of insulin resistance, and we therefore present only those for fasting insulin (12). Plasma glucose was measured with an automated assay. C-reactive protein (CRP) was measured by automated particle-enhanced immunoturbidimetric assay (Roche, Welwyn Garden City, U.K.).
Assessment of covariables
Mode of delivery (caesarean section), gestational age, birth weight, and offspring’s sex were extracted from medical records. Parity, parental occupation, and maternal prepregnancy weight and height were obtained from questionnaires completed by the mothers at recruitment in early pregnancy. Information on smoking during pregnancy was obtained from questionnaires completed by mothers throughout pregnancy and was categorized as never smoked during pregnancy or smoked during first trimester only/throughout pregnancy. Highest parental occupation was used to allocate children to family social class groups (classes I [professional/management] to V [unskilled manual workers], using the 1991 British Office of Population and Census Statistics classification). Maternal-reported height and prepregnancy weight were used to calculate maternal prepregnancy BMI; reported prepregnancy weight correlated highly (Pearson correlation coefficient = 0.95) with measured weight at the first antenatal clinic.
Statistical analysis
All analysis was conducted using Stata version 11.0 MP2 (Stata Inc., College Station, TX). Triglycerides, insulin, and CRP were positively (right) skewed, but with a natural log transformation, they were approximately normally distributed. For these variables, we use geometric means or medians to describe them and natural logged values in multivariable models. In multivariable models with these outcomes, the resulting coefficients were exponentiated (back transformed) so that the results are then interpreted as the ratio of geometric means of the outcome comparing each exposure to the category of healthy women. Differences in distributions of participant characteristics (offspring outcomes and maternal/family covariables) in relation to maternal diabetes or glycosuria status were tested using χ2 tests for categorical variables and t tests or F tests for continuous variables. These analyses tested for the null hypothesis of no difference between the four exposure groups (i.e., with 3 df) so that there was no assumption of a linear trend across the exposure categories of healthy, existing diabetes, gestational diabetes, and glycosuria.
A series of multivariable linear regression models were conducted to examine the association of maternal diabetes or glycosuria with offspring outcomes and to explore the impact of adjustment for potential confounding and mediating characteristics on these associations. For each outcome, we included in the multivariable analyses only participants who had complete data on all variables used in any model. Therefore, numbers vary between each outcome but are the same across models for each outcome. In the basic model (model 1), we controlled for offspring age at outcome assessment and sex, and in the models with fat mass as the outcome, offspring’s height and height-squared at the time of this assessment. In model 2, we adjusted for possible confounding by maternal age, parity, social class, and smoking in pregnancy. We then adjusted for possible mediation by birth weight, gestational age, and mode of delivery (model 3). In analyses with cardiometabolic risk factors, we also examined whether associations were mediated by the association of maternal diabetes or glycosuria with offspring adiposity (model 4). In these models (model 4), we present results after adjustment for directly assessed fat mass, but adjusting instead for waist circumference or BMI produced identical results. In sensitivity analyses, we examined whether adjustment for the number of urine tests completed altered the associations of glycosuria with outcomes.
Associations did not differ for males and females, and there was no statistical evidence of interaction with sex (all P values for interaction ≥0.1). Therefore, all results are presented with males and females combined.
Dealing with missing data
Of the 5,038 eligible participants who attended the 15.5-year follow-up assessment, the numbers with each outcome varied (Fig. 1), and there were missing data on covariables. The numbers included in the main multivariable analyses are those with complete data on exposure, outcome, and all covariables included in any model for each outcome (this varies between 2,563 and 4,198). To examine whether missing data on covariables might have introduced selection bias, we also examined the basic age- and sex-adjusted associations in those with maximal data for each outcome and compared these results with those in the subgroups with complete data.
RESULTS
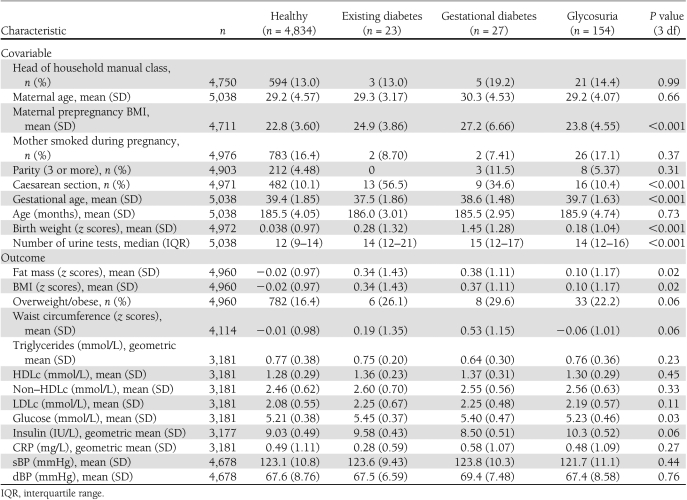
Of the 12,440 women who had a singleton live birth and valid obstetric data, the 5,038 whose offspring attended the follow-up assessment had similar prevalence of diabetes/glycosuria as the 7,402 who did not attend the follow-up clinic; however, those women whose offspring did not attend were younger and were more likely to be from manual social classes, to smoke during pregnancy, and to have had ≥3 previous pregnancies, and their offspring had lower birth weights (Supplementary Table 1). Table 1 shows the distributions of offspring outcomes and other maternal and family characteristics by maternal diabetes or glycosuria status. Maternal prepregnancy BMI varied by maternal diabetes or glycosuria status, with mothers who developed gestational diabetes having the highest prepregnancy BMI and mothers who were categorized as healthy (no existing diabetes, gestational diabetes, or glycosuria) having the lowest prepregnancy BMI. Proportion of caesarean sections also varied across the four groups, with the existing diabetes and gestational diabetes groups having the highest proportion of caesarean sections. Birth weight (age- and sex-standardized z scores) and fat mass also varied by maternal diabetes or glycosuria status, and the highest birth weight was for mothers with gestational diabetes.
Table 1.
Participants’ characteristics by maternal diabetes status in pregnancy at age 15-year follow-up (N = 5,038)
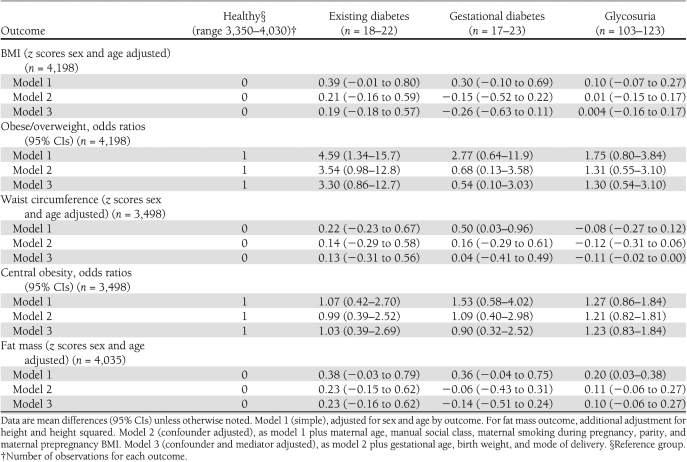
Table 2 shows the multivariable associations between maternal diabetes or glycosuria status and offspring adiposity at mean age 15.5 years. Maternal existing diabetes, gestational diabetes, and glycosuria were associated with higher BMI and fat mass (z scores) in the simple model unadjusted for confounders and mediators (model 1); however, this effect was attenuated and the CIs included the null value for the fully adjusted model. Gestational diabetes was associated with higher mean waist circumference z scores in the simple unadjusted model, but this relation was attenuated in the fully adjusted model. For overweight/obesity status and central obesity, the CIs for the estimates were wide and included the null value.
Table 2.
Multivariable models for the association of maternal diabetes/glycosuria status and offspring anthropometry at age 15 years
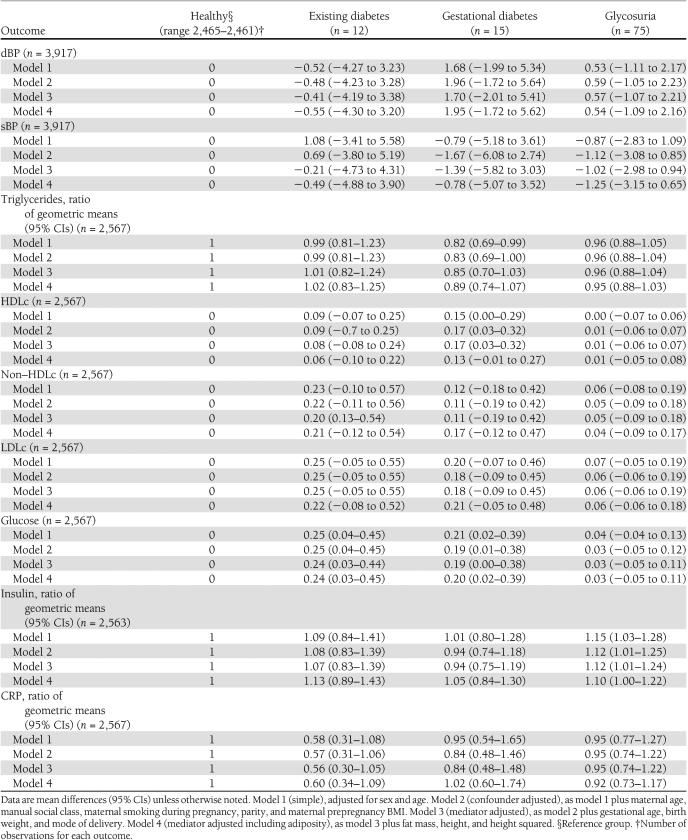
Table 3 shows the multivariable associations of maternal diabetes or glycosuria with offspring cardiometabolic risk factors. Existing diabetes and gestational diabetes were associated with higher offspring glucose levels, and adjustment for potential confounding factors and mediation by birth size or later adiposity did not notably alter this association. Maternal glycosuria was not associated with offspring glucose levels. Maternal glycosuria, but not existing or gestational diabetes, was associated with higher fasting insulin levels in offspring, and this association was not markedly altered by adjustment for potential confounding or mediating characteristics. Maternal diabetes or glycosuria were not associated with other offspring cardiometabolic risk factors (sBP, dBP, HDLc, non–HDLc, LDLc, triglycerides, or CRP). Additional adjustment for the number of urine tests did not change any associations presented in Tables 2 and 3.
Table 3.
Multivariable models for the association of maternal diabetes/glycosuria status and offspring cardiometabolic risk factors at age 15 years
There were no notable differences in associations between maternal diabetes and glycosuria with outcomes when comparing age- and sex-adjusted models (model 1) for all participants with these data (range 3,177–4,960) to results presented in Tables 2 and 3.
CONCLUSIONS
In this prospective birth cohort study, we have found that at mean age 15.5 years, maternal gestational diabetes and glycosuria were associated with higher mean fat mass and BMI z scores in simple unadjusted analyses, with the association attenuating somewhat in multivariable models. Offspring of mothers who had existing or gestational diabetes during their pregnancy had higher fasting glucose, but this was not accompanied by an increase in fasting insulin. In contrast, offspring of mothers who experienced glycosuria in pregnancy had higher fasting insulin. We found little evidence of associations between maternal diabetes or glycosuria and a wider range of cardiometabolic risk factors beyond glucose and insulin (i.e., no associations with dBP, sBP, lipids, or CRP).
In a previously published study from the same cohort, we demonstrated that gestational diabetes and glycosuria were associated with greater offspring BMI, waist circumference, and fat mass assessed when the offspring were aged 9–11 years and that although associations attenuated with adjustment for potential confounding factors, including maternal early pregnancy BMI, the positive associations remained (4). It is notable that the point estimates for all models in the current analyses, conducted using data obtained when participants were on average 5 years older (now adolescents), are nearly identical to those in that earlier publication. Thus, although the adjusted (including for maternal BMI) associations in this current study are consistent with the null, it would be incorrect to conclude that any effect of exposure to maternal dysglycemia in utero declines with age. The magnitude of the association appears stable between childhood and adolescence, but in the current study, we have less statistical power as a result of smaller sample size (gestational diabetes and glycosuria, n = 23 and 123, respectively) compared with the previous study for outcomes at age 9–11 years (gestational diabetes and glycosuria, n = 40 and 232, respectively). Although a recent meta-analysis concludes that in European populations, there was no association between pregnancy diabetes and offspring BMI once maternal BMI was taken into account, that conclusion was based on pooling data from just three studies that together included only 244 offspring of mothers with diabetes and were clinically heterogeneous with mothers with either type 1 diabetes, gestational diabetes, or a composite of both (2). A positive association of maternal diabetes with BMI in adolescence that is independent of maternal BMI is supported by results from a recently published large prospective cohort of 280,866 men aged 18 years (n = 1,475 mothers with diabetes in pregnancy) that used within-sibling comparisons to control for fixed-family characteristics (3).
In our population, frank maternal diabetes (existing or gestational) was associated with offspring fasting glucose, whereas glycosuria (a marker of potential gestational hyperglycemia) was associated with offspring fasting insulin but not glucose. It is possible that the former reflects inherited genetic variants associated with higher glucose as well as intrauterine mechanisms. By contrast, the glycosuria association may be driven solely by intrauterine mechanisms—by higher maternal glucose influencing fetal insulin secretion that persists after birth. However, given the issues regarding sensitivity of glycosuria as a marker of hyperglycemia in pregnancy (see below) and the small numbers in this study, these apparent differences may be chance findings and need further replication in larger studies with continuous measurements of fasting (and postload) glucose and insulin in pregnancy.
Our findings regarding measures of glucose metabolism are consistent with the small number of studies that examine these associations in non-Pima populations. Clausen et al. (13) reported higher fasting glucose levels in offspring of gestational diabetic mothers (n = 168) compared with healthy control subjects (n = 128), and three studies report higher levels of insulin resistance in the offspring of mothers with gestational diabetes compared with those without, with the number with gestational diabetes in these studies varying from 68 to 232 (14–16). In one study, there was no strong evidence of an association of gestational diabetes (n = 95) with offspring fasting glucose, but positive associations with fasting insulin and insulin resistance were observed (17).
There may be a number of mechanisms by which gestational glucose status affects offspring fasting glucose and insulin in later life. First, genetic variation associated with type 2 diabetes and/or variation in fasting glucose and insulin could explain the associations that we have observed. Evidence from Pima Indians (sibling and parental comparisons) suggests that genetic inheritance does not fully explain the associations of maternal pregnancy diabetes and offspring fasting glucose/diabetes in that population (18), but we are unable to undertake such familial studies in our cohort owing to lack of information on diabetes in fathers or outcomes in siblings. In addition to the Pima family studies, there is a large body of evidence from different populations showing that maternal type 2 diabetes (not specifically in pregnancy) is more strongly associated with offspring type 2 diabetes risk than paternal type 2 diabetes (19–21). Possible explanations for this include specific intrauterine mechanisms, with women who are prone to type 2 diabetes likely to have higher glucose levels in pregnancy that could program offspring to increased risk of type 2 diabetes later in life, and others such as differences in transmission of diabetes risk factors (i.e., mothers possibly having more impact on offspring diet and physical activity behaviors than fathers), effects of mitochondrial DNA on type 2 diabetes, and genetic imprinting (22).
Second, maternal diabetes during pregnancy is known to result in greater fat mass at birth, with the suggestion that insulin resistance might be present at birth (23). This could persist into later life of the offspring and result in increased levels of glucose and insulin. Third, shared familial lifestyles or genetic variation related to greater adiposity could explain the associations that we have observed because greater adiposity is an important risk factor for gestational diabetes (24) and variation in fasting glucose and insulin. However, in our study, we have shown that offspring adiposity (whether assessed by BMI, waist circumference, or directly assessed fat mass) does not appear to mediate the associations with offspring glucose and insulin that we have observed.
Lastly, intrauterine mechanisms might explain these associations. Exposure to greater maternal circulating levels of glucose in pregnancy results in increased fetal insulin secretion in utero (11), and this greater activity of the fetal pancreas may have lasting effects on offspring glucose/insulin metabolism throughout their life. A recent sibling study in a non-Pima population suggests an intrauterine mechanism for the association with offspring greater adiposity (2), and Pima sibling and parental comparison studies also support this for fasting glucose and insulin (18), but we are unaware of any non-Pima studies that have relevant data, including fasting glucose and insulin in offspring siblings, and sufficient statistical power to determine that this is the case in these populations.
Strengths and limitations
The main strength of this study is that we were able to examine associations of maternal diabetes/glycosuria with a range of offspring cardiometabolic risk factors, which have rarely been examined before. The main limitation is the small number of participants with gestational diabetes and the possibility that the lack of universal screening with a blood glucose test at the time the mothers in this study were pregnant may mean that some women with gestational diabetes were not identified as such. This is supported by the relatively low prevalence of gestational diabetes in this cohort (0.5%) compared with prevalence of 1.2% in a study population of U.K. pregnant women who were also assessed in the early 1990s but who underwent screening using oral glucose tolerance tests as part of a research study (25). However, comparing our study with that study is complicated by the fact that it was conducted in a relatively deprived, multiethnic group of women who would be expected to have higher rates of gestational diabetes than our population who are by comparison more affluent and largely (98%) of white British ethnicity. It is notable that the prevalence of preexisting diabetes is in line with the expected prevalence of 1 in 250. It is possible that women defined as having glycosuria in this study included a mixture of women with high circulating glucose levels (but below the threshold that would be used to define gestational diabetes) and also women with undiagnosed gestational diabetes. Any such misclassification would be nondifferential with respect to the outcomes that we have assessed because clinicians providing antenatal care could not possibly know what the measures of fat mass or blood glucose, insulin, and lipids of offspring would be 15–16 years later.
Despite these issues, we have previously shown that glycosuria is related to macrosomia in this cohort, giving face validity to its use as an assessment of hyperglycemia (4).
Loss to follow-up in our study is consistent with other prospective cohort studies, and although it can affect statistical power, it is unlikely to have importantly biased the association results. This is because loss to follow-up is likely to be nondifferential with respect to outcomes (participants would not be aware of their fasting glucose, insulin, or lipid levels) and because to importantly bias the findings, we would have to assume that the associations we have found were null or in the opposite direction (i.e., maternal diabetes in pregnancy or glycosuria being associated with lower offspring glucose and insulin) in those individuals lost to follow-up.
Our results suggest that maternal diabetes in pregnancy (existing or gestational) is associated with greater offspring fasting glucose in adolescence and maternal hyperglycemia (as indicated by glycosuria) is associated with greater offspring fasting insulin in adolescence. These associations do not appear to be mediated by birth weight or offspring adiposity at the time of glucose and insulin assessment. Results from this study add to the relatively small body of evidence to date suggesting that maternal diabetes or hyperglycemia in pregnancy are related to offspring diabetes risk factors. However, further large studies are required to replicate these findings, and methods such as sibling comparisons and long-term follow-up of trials assessing the effectiveness of treatments for gestational diabetes/hyperglycemia are needed to determine whether these associations are at least in part driven by intrauterine mechanisms or are fully explained by shared familial genetic or environmental characteristics. Of note, our study suggests that beyond associations with adiposity and fasting glucose and insulin, maternal hyperglycemia/diabetes in pregnancy is not associated with a broader range of cardiometabolic risk factors.
Acknowledgments
This study was funded by grants from the Wellcome Trust (WT087997) and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-077659). The U.K. Medical Research Council (MRC; grant G074882), the Wellcome Trust (grant WT076467), and the University of Bristol provided core funding support for ALSPAC. The U.K. MRC (grant G0600705) and the University of Bristol provided core funding for the MRC Centre of Causal Analyses in Translational Epidemiology. S.P. has received European Union FP7 Grant 241604. A.F. has received a U.K. MRC research fellowship (grant 0701594).
No potential conflicts of interest relevant to this article were reported.
S.P. proposed the statistical protocol, researched data, and wrote the first draft of the manuscript. A.F., G.D.S., R.S.L., N.S., and S.M.N. revised later drafts of the manuscript, contributed to the discussion, and edited the manuscript. D.A.L. proposed the study objectives, proposed the statistical protocol, and revised drafts of the manuscript.
S.P. acts as the guarantor for this article.
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting the families, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1633/-/DC1.
The views expressed in this article are those of the authors and not necessarily those of any funding body, or others whose support is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes 1991;40(Suppl. 2):126–130 [DOI] [PubMed] [Google Scholar]
- 2.Philipps LH, Santhakumaran S, Gale C, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 2011;54:1957–1966 [DOI] [PubMed] [Google Scholar]
- 3.Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011;123:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89–97 [DOI] [PubMed] [Google Scholar]
- 5.Golding J, Pembrey M, Jones R; ALSPAC Study Team ALSPAC–The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74–87 [DOI] [PubMed] [Google Scholar]
- 6.Lind T, Hytten FE. The excretion of glucose during normal pregnancy. J Obstet Gynaecol Br Commonw 1972;79:961–965 [DOI] [PubMed] [Google Scholar]
- 7.Buhling KJ, Elze L, Henrich W, et al. The usefulness of glycosuria and the influence of maternal blood pressure in screening for gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2004;113:145–148 [DOI] [PubMed] [Google Scholar]
- 8.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmet P, Alberti G, Kaufman F, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention of Diabetes The metabolic syndrome in children and adolescents. Lancet 2007;369:2059–2061 [DOI] [PubMed] [Google Scholar]
- 10.McCarthy HD, Jarrett KV, Crawley HF. The development of waist circumference percentiles in British children aged 5.0-16.9 y. Eur J Clin Nutr 2001;55:902–907 [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 12.Levy-Marchal C, Arslanian S, Cutfield W, et al. ; ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE, Insulin Resistance in Children Consensus Conference Group Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010;95:5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464–2470 [DOI] [PubMed] [Google Scholar]
- 14.Boerschmann H, Pflüger M, Henneberger L, Ziegler AG, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 2010;33:1845–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egeland GM, Meltzer SJ. Following in mother’s footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet Med 2010;27:257–265 [DOI] [PubMed] [Google Scholar]
- 16.Keely EJ, Malcolm JC, Hadjiyannakis S, Gaboury I, Lough G, Lawson ML. Prevalence of metabolic markers of insulin resistance in offspring of gestational diabetes pregnancies. Pediatr Diabetes 2008;9:53–59 [DOI] [PubMed] [Google Scholar]
- 17.Vääräsmäki M, Pouta A, Elliot P, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol 2009;169:1209–1215 [DOI] [PubMed] [Google Scholar]
- 18.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 19.Karter AJ, Rowell SE, Ackerson LM, et al. Excess maternal transmission of type 2 diabetes. The Northern California Kaiser Permanente Diabetes Registry. Diabetes Care 1999;22:938–943 [DOI] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Moss SE, Cruickshanks KJ. Parental history of diabetes in a population-based study. Diabetes Care 1996;19:827–830 [DOI] [PubMed] [Google Scholar]
- 21.Thomas F, Balkau B, Vauzelle-Kervroedan F, Papoz L; CODIAB-INSERM-ZENECA Study Group Maternal effect and familial aggregation in NIDDM. The CODIAB Study. Diabetes 1994;43:63–67 [DOI] [PubMed] [Google Scholar]
- 22.Rampersaud E, Mitchell BD, Naj AC, Pollin TI. Investigating parent of origin effects in studies of type 2 diabetes and obesity. Curr Diabetes Rev 2008;4:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring SJ, Oken E. Obesity and diabetes in mothers and their children: can we stop the intergenerational cycle? Curr Diab Rep 2011;11:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koukkou E, Taub N, Jackson P, Metcalfe G, Cameron M, Lowy C. Difference in prevalence of gestational diabetes and perinatal outcome in an innercity multiethnic London population. Eur J Obstet Gynecol Reprod Biol 1995;59:153–157 [DOI] [PubMed] [Google Scholar]