Abstract
OBJECTIVE
Subjects who are normal glucose tolerant (NGT) are considered at low risk, even if a plasma glucose value ≥155 mg/dL for the 1-h postload plasma glucose during an oral glucose tolerance test (OGTT) is able to identify NGT subjects at high risk for type 2 diabetes and subclinical organ damage. Hyperuricemia is associated with several risk factors for cardiovascular diseases such as hypertension, insulin resistance, and diabetes. However, it is unknown whether uric acid (UA) is able to affect 1-h postload plasma glucose in hypertensive NGT subjects.
RESEARCH DESIGN AND METHODS
From a cohort of ∼1,200 uncomplicated hypertensive outpatients who underwent OGTT, we selected 955 subjects (548 men and 407 women) aged 45.6 ± 10.1 years. Laboratory evaluations were performed, and estimated glomerular filtration rate was assessed by using the new equation proposed by investigators in the Chronic Kidney Disease Epidemiology Collaboration.
RESULTS
Considering different stepwise multivariate linear regression models, UA was the major predictor of 1-h postload glucose in the entire population, with NGT ≥155 subjects, impaired glucose tolerant, and type 2 diabetic patients accounting for 26.0% (P < 0.0001), 25.3% (P < 0.0001), 13.5% (P < 0.0001), and 13.5% (P = 0.003) of its variation in the respective models.
CONCLUSIONS
We documented that in hypertensive NGT ≥155 subjects, UA is strongly associated with 1-h postload glucose, similarly to what is observed in impaired glucose tolerant and diabetic patients.
Uric acid (UA), the end product of purine metabolism, possesses both antioxidant and pro-oxidant properties, depending on its chemical microenvironment. Hyperuricemia predisposes to disease through the formation of urate crystals that cause gout, but it is also associated with hypertension and diabetes, all risk factors for atherosclerosis (1). Hyperuricemia is present in patients with the metabolic syndrome (2) and in subjects with insulin resistance (3). Obesity is also positively associated with hyperuricemia, which decreases with body weight loss (4–6). Hyperuricemia is frequently documented in subjects with cardiovascular diseases (7) and is recognized as an independent predictor for myocardial infarction and stroke (8).
Similarly, type 2 diabetes is known to be an independent risk factor for cardiovascular disease (9–11) and heart failure, even in the absence of coronary artery disease or hypertension (12). In addition, subjects with impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG) are characterized by an unfavorable cardiovascular risk profile (13).
Recently, a cut-off point of 155 mg/dL for the 1-h postload plasma glucose value during the oral glucose tolerance test (OGTT) is able to identify subjects with normal glucose tolerance (NGT) at high risk of type 2 diabetes (14). Moreover, the 1-h postload plasma glucose value is strongly associated with increased cardiac mass values and left ventricular hypertrophy (15) as well as carotid intima-media thickness (16), which are indicators of subclinical organ damage and independent predictors for cardiovascular events (12,13,17).
Thus, on the basis of this evidence, we suggested the usefulness of reconsidering the concept that NGT subjects are a homogeneous group with a low cardiovascular risk. This recommendation has clinical relevance because it emphasizes the importance of the phenotypic characterization in stratifying the profile of cardiovascular or cardiometabolic risk in each subject. However, it is unknown whether UA is able to affect 1-h postload plasma glucose in hypertensive NGT subjects. Taken together, we designed this study to evaluate, in a group of newly diagnosed hypertensive subjects, the possible relationship between UA and postload plasma glucose.
RESEARCH DESIGN AND METHODS
Study population
From a cohort of ∼1,200 uncomplicated hypertensive outpatients undergoing OGTT, we selected 955 subjects (548 men and 407 women) aged 45.6 ± 10.1 who were participating in the CAtanzaro MEtabolic RIsk factors Study (CATAMERIS). All subjects were Caucasian and underwent physical examination and review of their medical history with regard to family history of diabetes or gout. Causes of secondary hypertension were excluded by appropriate clinical and biochemical tests. Other exclusion criteria were history or clinical evidence of coronary and/or valvular heart disease, congestive heart failure, hyperlipidemia, hyperuricemia, peripheral vascular disease, chronic gastrointestinal diseases associated with malabsorption, or chronic pancreatitis; history of malignant disease, alcohol or drug abuse, or liver or kidney failure; and treatments able to modify glucose or UA metabolism. No subjects had ever been treated with antihypertensive drugs. All subjects underwent evaluation for weight, height, and BMI.
After 12-h fasting, a 75-g OGTT was performed with 0-, 30-, 60-, 90-, and 120-min sampling for plasma glucose and insulin. Glucose tolerance status was defined on the basis of OGTT using the World Health Organization criteria. Insulin sensitivity was evaluated using the Matsuda index (insulin sensitivity index [ISI]), calculated as 10,000/square root of [fasting glucose (mmol/L) × fasting insulin (mU/L)] × [mean glucose × mean insulin during OGTT]. The Matsuda index is strongly related to the euglycemic hyperinsulinemic clamp, which represents the gold standard test for measuring insulin sensitivity (18). The ethical committee approved the protocol, and informed written consent was obtained from all participants. All the investigations were performed in accordance with the principles of the Declaration of Helsinki.
Blood pressure measurements
Readings of clinic blood pressure (BP) were obtained in the left arm of supine patients, after 5 min of quiet rest, with a mercury sphygmomanometer. A minimum of three BP readings were taken on three separate occasions at least 2 weeks apart. Systolic and diastolic BP (SBP, DBP) was recorded at the first appearance (phase I) and the disappearance (phase V) of Korotkoff sounds. Baseline BP values were the average of the last two of the three consecutive measurements obtained at intervals of 3 min. Patients with a clinic SBP >140 mmHg and/or DBP >90 mmHg were defined as hypertensive.
Laboratory determinations
All laboratory measurements were performed after a fast of at least 12 h. Plasma glucose was determined immediately by the glucose oxidase method (Glucose Analyzer, Beckman Coulter S.p.A., Milan, Italy), with an intra-assay coefficient of variation (CV) of 2.2% and interassay CV of 3.8%. Triglyceride and total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol concentrations were measured by enzymatic methods (Roche Diagnostics GmbH, Mannheim, Germany). Serum insulin was determined in duplicate by a highly specific radioimmunoassay using two monoclonal antibodies (intra-assay CV, 2.1%; interassay CV, 2.9%). Serum creatinine and UA were measured in the routine laboratory by an automated technique based on the measurement of Jaffe chromogen and by the URICASE/POD method (Boehringer Mannheim, Mannheim, Germany) implemented in an autoanalyzer. C-reactive protein (CRP) was measured by a high-sensitivity turbidimetric immunoassay (Behring, Marburg, Germany). Values of estimated glomerular filtration rate (e-GFR; mL/min/1.73 m2) were calculated by using the new equation proposed by investigators in the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration. This equation was developed from a large cohort of patients, including healthy and CKD individuals. We preferred this equation because it is more accurate in subjects with a GFR >60 mL/min/1.73 m2, which our patients were assumed to have considering the creatinine value of <1.5 mg/dL (19).
Statistical analysis
ANOVA for clinical and biologic data were performed to test the differences among groups, and the Bonferroni post hoc test for multiple comparisons was further performed. The χ2 test was used for categorical variables. Correlational coefficients were calculated according to the Pearson method. Linear regression analysis was performed to correlate 1-h postload glucose with the covariates of age, BMI, SBP, DBP, UA, lipemic parameters, Matsuda index, high-sensitivity CRP (hs-CRP), creatinine, and e-GFR. Variables reaching statistical significance and sex, as dichotomous values, were inserted in a stepwise multivariate linear regression model to determine the independent predictors of 1-h postload plasma glucose. Correlational analysis was performed for entire study population and according to different groups of glucose tolerance. Data are reported as mean ± SD. Differences were assumed to be significant at P < 0.05. All comparisons were performed using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL).
RESULTS
Study population
Of 955 patients examined by OGTT, 676 had NGT, 214 had IGT, and 62 had newly diagnosed type 2 diabetes. A 1-h postload plasma glucose cutoff of 155 mg/dL during the OGTT was used to stratify NGT subjects into two groups: 488 with 1-h postload plasma glucose <155 mg/dL (NGT<155) and 191 with 1-h postload plasma glucose ≥155 mg/dL (NGT≥155).
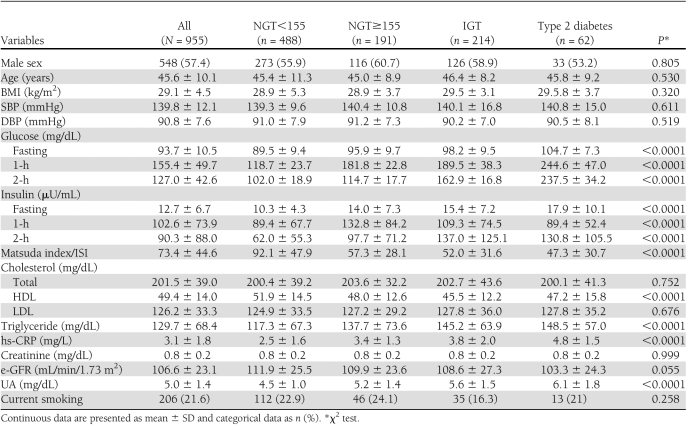
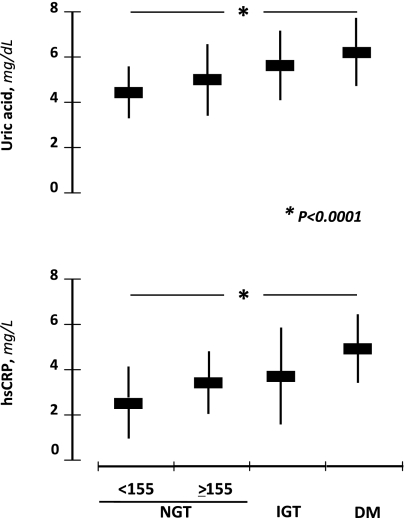
In Table 1 we report the demographic, clinical, and biochemical characteristics of the four study groups. There were no significant differences among the groups in sex distribution (P = 0.805), age (P = 0.530), BMI (P = 0.320), SBP (P = 0.611), DBP (P = 0.519), total (P = 0.752) and LDL (P = 0.676) cholesterol, creatinine (P = 0.999), e-GFR (P = 0.055), and smokers (P = 0.258). From the first to the fourth group, there was a significant increase in triglyceride (P < 0.0001), hs-CRP (P < 0.0001), UA (P < 0.0001), and a decrease in HDL (P < 0.0001). Obviously, a progressive increase of fasting, 1-h, and 2-h postload glucose, as well as fasting and 2-h insulin, paralleled the worsening of glucose tolerance (P < 0.0001) and accounted for the reduction of the Matsuda index/ISI. Moreover, the NGT≥155 subjects had significantly (P < 0.0001) reduced insulin sensitivity and increased UA and hs-CRP values compared with NGT<155; in addition, their metabolic and inflammatory profiles were similar to IGT individuals. The mean values of UA and hs-CRP in the study population according to glucose tolerance are presented in Fig. 1.
Table 1.
Anthropometric, hemodynamic, and biochemical characteristics of the study population according to glucose tolerance
Figure 1.
UA and hs-CRP mean values in subjects with different glucose tolerance.
Correlational analysis
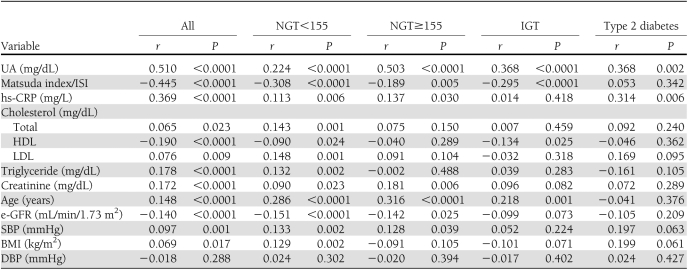
Results of linear regression analysis, performed to test the correlation between 1-h postload glucose and different covariates, are reported in Table 2. In the entire study population, 1-h postload glucose was directly correlated with UA (P < 0.0001), hs-CRP (P < 0.0001), triglyceride (P < 0.0001), creatinine (P < 0.0001), and age (P < 0.0001) and was inversely related with the Matsuda index (P < 0.0001), HDL, and e-GFR (P < 0.0001). A weak relationship was also observed with SBP, LDL and total cholesterol, and BMI.
Table 2.
Univariate linear regression analysis between 1-h postload plasma glucose and different covariates in the entire study population and in groups with different glucose tolerance
In NGT<155 subjects, 1-h postload glucose was directly related with UA (P < 0.0001), hs-CRP (P = 0.006), triglyceride (P = 0.002), age (P < 0.0001), SBP (P = 0.002), total and LDL cholesterol (P = 0.001), and BMI (P = 0.002) and was inversely related with Matsuda index (P < 0.0001) and e-GFR (P < 0.0001). A weak correlation was observed with HDL and creatinine.
In NGT≥155 subjects, a strong relationship was observed between UA and 1-h postload glucose (r = 0.503; P < 0.0001). Other positive covariates were hs-CRP (P = 0.030), creatinine (P = 0.006), age (P < 0.0001), and SBP (P = 0.039); similarly to the other groups, the Matsuda index (P = 0.005) and e-GFR (P = 0.025) were inversely related with 1-h postload glucose.
In IGT patients, 1-h postload glucose was linearly correlated with UA (P < 0.0001) and age (P = 0.001) and was inversely correlated with the Matsuda index (P < 0.0001) and HDL (P = 0.025). Finally, in diabetic patients, 1-h postload glucose was linearly correlated only with UA (P = 0.002) and hs-CRP (P = 0.006).
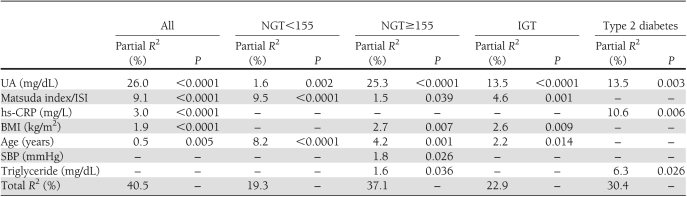
Thus, variables reaching statistical significance and sex, as a dichotomous value, were inserted in a stepwise multivariate linear regression model to determine the independent predictors of 1-h postload glucose variation (Table 3). In the entire population, UA was the major predictor of 1-h postload glucose, explaining 26.0% of its variation (P < 0.0001). Other independent predictors were the Matsuda index, hs-CRP, BMI, and age explaining another 14.5% of its variation. In NGT<155 subjects, the first covariate retained in the final model was the Matsuda index, which explains 9.5% of the 1-h postload plasma glucose variation; age and UA explain another 9.8% of its variation. Of interest, in NGT≥155, IGT, and type 2 diabetic patients, UA was the strongest predictor of 1-h postload glucose, accounting for 25.3% (P < 0.0001), 13.5% (P < 0.0001), and 13.5% (P = 0.003) of its variation in the respective models. In NGT≥155 subjects, age, BMI, SBP, triglyceride, and the Matsuda index were retained as independent predictors of postload glucose, explaining another 11.8% of its variation. In IGT patients, the Matsuda index, BMI, and age were entered in the final model, accounting for 22.9% of postload glucose variation. In type 2 diabetic patients, hs-CRP and triglyceride were also significantly included in the final model, accounting for a further 10.6% (P = 0.006) and 6.3% (P = 0.026), respectively.
Table 3.
Stepwise multiple regression analysis on 1-h postload plasma glucose as the dependent variable in the entire study population and in groups with different glucose tolerance
CONCLUSIONS
The main and novel finding obtained in this study is that in hypertensive NGT≥155 subjects, UA is strongly associated with 1-h postload glucose, similarly to what was observed in IGT and diabetic patients. Even though evidence from several studies has demonstrated the prognostic value of UA in developing new diabetes (1,3,20–22), our data for the first time demonstrate that UA, in nonelevated levels, is associated with 1-h postload glucose, confirming the strong role of UA in the development of insulin resistance. This finding has clinical relevance because 1-h postload glucose in NGT subjects is associated, as we demonstrated, with subclinical organ damage (15,16,23,24) that represents an intermediate stage in the continuum of vascular disease and a determinant of overall cardiovascular risk. In addition, because these findings were obtained in a hypertensive population, it is also plausible that hypertension should not be considered a simple hemodynamic disorder but a cluster of metabolic and hemodynamic alterations that interact in a multiplicative manner in the appearance and progression of cardiovascular disease and outcomes.
Our data are not surprising from a pathophysiologic point of view, because there are evidences showing the association between UA and insulin resistance (1,3,22) that, for many years, precedes the clinical appearance of diabetes. This association may be explained according to the pro-oxidant and proinflammatory effects of UA that interfere with glucose uptake, impairing blood flow to skeletal muscles and peripheral tissues (25,26). In addition, UA reduces endothelial NO bioavailability in humans (27) as well as in cell culture and in animal models (28,29), increasing oxygen free radicals (30,31) and CRP production (29) and exerting a direct scavenging effect (32), all factors interfering with insulin sensitivity and resulting in clinical manifestation of diabetes (33). In keeping with this, NGT≥155 subjects, in baseline conditions and after the 1-h glucose challenge, are more insulin resistant and have higher CRP and insulin levels compared with other groups, including IGT and diabetic patients. All these metabolic abnormalities agree with the hypothesis that measurement of glycemia alone, during an OGTT, allows for a partial stratification of the global cardiovascular risk. In fact, many NGT subjects, characterized by high postload insulin levels and increased inflammatory burden, as documented by CRP values, would be considered at low cardiovascular risk despite the concomitant presence of subclinical organ damage.
Another clinical question supported by our data is that NGT≥155 subjects progress toward new diabetes, as recently demonstrated by others (14). This is an open question, because only 30–40% of subjects with IGT develop diabetes, whereas 40% of subjects who develop new diabetes are NGT at baseline (14,34), and our data contribute to explain the underlying mechanism. All these emphasize the importance of precociously identifying subjects at high risk for diabetes in order to offer them an intervention program that will prevent or slow their progression to overt diabetes. In keeping with this and on the basis of the strong relationship between UA and 1-h postload plasma glucose, it is plausible to hypothesize that simply measuring the UA concentration may also be considered a good predictor for future diabetes in NGT subjects. Baseline and postchallenge glucose and insulin concentrations seem to justify the biologic importance of global metabolic abnormalities and UA specifically. Thus, it should be useful in clinical practice to also routinely use the UA evaluation given that even nonelevated levels can be useful in identifying hypertensive people who are at risk for insulin resistance and diabetes. In fact, up to 50% of people with essential hypertension have insulin resistance, and this is one way to look for it without the need for an OGTT. In addition, the UA assessment is easy to determine and is less expensive.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
F.P. wrote the manuscript and is the guarantor of the article. A.S., M.P., and F.A. reviewed and edited the manuscript. P.E.S. and M.Q. researched the data. G.S. wrote the manuscript.
References
- 1.So A, Thorens B. Uric acid transport and disease. J Clin Invest 2010;120:1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med 1993;44:121–131 [DOI] [PubMed] [Google Scholar]
- 3.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991;266:3008–3011 [PubMed] [Google Scholar]
- 4.Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism 1997;46:1162–1165 [DOI] [PubMed] [Google Scholar]
- 5.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol 1998;8:250–261 [DOI] [PubMed] [Google Scholar]
- 6.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165:742–748 [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000;36:1072–1078 [DOI] [PubMed] [Google Scholar]
- 8.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 2006;37:1503–1507 [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med 2000;342:1040–1042 [DOI] [PubMed] [Google Scholar]
- 10.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansson SP, Andersson DK, Svärdsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes Care 2010;33:551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34 [DOI] [PubMed] [Google Scholar]
- 13.Ilercil A, Devereux RB, Roman MJ, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am Heart J 2001;141:992–998 [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciacqua A, Miceli S, Carullo G, et al. One-hour postload plasma glucose levels and left ventricular mass in hypertensive patients. Diabetes Care 2011;34:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Succurro E, Marini MA, Arturi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009;207:245–249 [DOI] [PubMed] [Google Scholar]
- 17.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr; Cardiovascular Health Study Collaborative Research Group Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiik BP, Larstorp ACK, Høieggen A, et al. Serum uric acid is associated with new-onset diabetes in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Am J Hypertens 2010;23:845–851 [DOI] [PubMed] [Google Scholar]
- 21.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008;31:361–362 [DOI] [PubMed] [Google Scholar]
- 22.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 2009;30:96–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Succurro E, Arturi F, Lugarà M, et al. One-hour postload plasma glucose levels are associated with kidney dysfunction. Clin J Am Soc Nephrol 2010;5:1922–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciacqua A, Miceli S, Greco L, et al. One-hour post-load plasma glucose levels and diastolic function in hypertensive patients. Diabetes Care 2011;34:2291–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol 1998;274:E692–E699 [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C, Maio R, Tripepi G, Mallamaci F, Perticone F. Inflammation as a mediator of the link between mild to moderate renal insufficiency and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 2006;17(Suppl. 2):S64–S68 [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 2006;17:1466–1471 [DOI] [PubMed] [Google Scholar]
- 28.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005;67:1739–1742 [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005;16:3553–3562 [DOI] [PubMed] [Google Scholar]
- 30.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 2008;26:269–275 [DOI] [PubMed] [Google Scholar]
- 31.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–C596 [DOI] [PubMed] [Google Scholar]
- 32.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol 2004;94:932–935 [DOI] [PubMed] [Google Scholar]
- 33.Perticone F, Maio R, Sciacqua A, et al. Endothelial dysfunction and C-reactive protein are risk factors for diabetes in essential hypertension. Diabetes 2008;57:167–171 [DOI] [PubMed] [Google Scholar]
- 34.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]