Abstract
OBJECTIVE
Coronary artery disease (CAD) is the major cause of morbidity and mortality in type 2 diabetic patients. Severe vitamin D deficiency has been shown to predict cardiovascular mortality in type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
We investigated the association among severe vitamin D deficiency, coronary calcium score (CCS), and asymptomatic CAD in type 2 diabetic patients with elevated urinary albumin excretion rate (UAER) >30 mg/24 h. This was a cross-sectional study including 200 type 2 diabetic patients without a history of CAD. Severe vitamin D deficiency was defined as plasma 25-hydroxyvitamin D (p-25[OH]D3) <12.5 nmol/L. Patients with plasma N-terminal pro-brain natriuretic peptide >45.2 ng/L or CCS ≥400 were stratified as being high risk for CAD (n= 133). High-risk patients were examined by myocardial perfusion imaging (MPI; n = 109), computed tomography angiography (n = 20), or coronary angiography (CAG; n = 86). Patients’ p-25(OH)D3 levels were determined by high-performance liquid chromatography/tandem mass spectrometry.
RESULTS
The median (range) vitamin D level was 36.9 (3.8–118.6) nmol/L. The prevalence of severe vitamin D deficiency was 9.5% (19/200). MPI or CAG demonstrated significant CAD in 70 patients (35%). The prevalence of CCS ≥400 was 34% (68/200). Severe vitamin D deficiency was associated with CCS ≥400 (odds ratio [OR] 4.3, 95% CI [1.5–12.1], P = 0.005). This association persisted after adjusting for risk factors (4.6, 1.5–13.9, P = 0.007). Furthermore, severe vitamin D deficiency was associated with asymptomatic CAD (adjusted OR 2.9, 1.02–7.66, P = 0.047).
CONCLUSIONS
In high-risk type 2 diabetic patients with elevated UAER, low levels of vitamin D are associated with asymptomatic CAD.
Coronary artery disease (CAD) is the major cause of morbidity and mortality in patients with type 2 diabetes. Diabetic patients have been shown to have an increased prevalence of subclinical CAD (1). Coronary calcium score (CCS), a noninvasive screening method quantifying the extent of coronary artery calcification (CAC), is generally accepted as a marker of increased cardiovascular risk. CCS has been shown to correlate strongly with histopathologic CAD (2,3) and the development of adverse coronary events (4,5).
Results from cross-sectional studies examining the relation between low vitamin D levels and presence of CAD in the general population are conflicting (6,7). In type 1 diabetic patients, vitamin D deficiency has been shown to independently predict both prevalence and development of CAC (8). However, a study in type 2 diabetic patients with a history of cardiovascular disease (CVD) found a strong inverse association between low vitamin D levels and prevalent coronary, cerebrovascular, or peripheral CVD (9). Furthermore, low vitamin D levels have been associated with increased cardiovascular morbidity and mortality in the general population (10) and in patients with type 1 (8) and 2 (11) diabetes.
To expand our knowledge on the increased all-cause and cardiovascular mortality seen in type 2 diabetic patients with low vitamin D levels, the current study investigated the association between severe vitamin D (plasma 25-hydroxyvitamin D [p-25(OH)D3]) deficiency and the presence of elevated CAC and asymptomatic CAD in type 2 diabetic patients with elevated urinary albumin excretion rate (UAER) >30 mg/24 h.
RESEARCH DESIGN AND METHODS
In a cross-sectional study at Steno Diabetes Center from January 2007 to February 2008, we identified a cohort of 200 type 2 diabetic patients without prior diagnosed CAD or other known cardiac disease but with a high risk of CAD as indicated by persistent UAER >30 mg/24 h. A written invitation to participate in the study was sent to all patients aged 20–70 years from the outpatient clinic (n = 613, 69% were male, patients had a mean [SD] age of 47 [8] years). Seventy-two patients refused to participate. Patients were excluded (n = 341) if one or more of the following characteristics were present: normal UAER or nonpersistent elevated UAER, significant Q-waves in the 12-lead electrocardiogram, and relative contraindications to computed tomography angiography or coronary angiography (CAG), including elevated plasma creatinine above the upper normal limit. All patients received multifactorial treatment for the prevention of CAD. The treatment included statins (94%), aspirin (90%), and blockade of the renin-angiotensin-aldosterone system (98%). The p-25(OH)D3 levels were determined by high-performance liquid chromatography/tandem mass spectrometry. Severe vitamin D deficiency was defined as p-25(OH)D3 <12.5 nmol/L in correspondence with the National Danish guidelines at the time of the study. Plasma N-terminal pro-brain natriuretic peptide (p-NT-proBNP) was analyzed by an immunoassay as previously described (12). Clinical measurements and results of the cardiac examinations have been described (13) and are therefore only outlined briefly in the current article. Coronary calcium scanning was performed during a single breath-hold using a 16 multidetector-row computed tomography scanner with 3-mm slice thickness with quantification of Agatston CCS (Heartbeat-CS, EBW; Philips Medical Systems, Andover, MA) (14). According to levels of p-NT-proBNP and CCS, patients were stratified into groups with high or low risk for CAD in the following manner: 1) Patients with p-NT-proBNP >45.2 ng/L (this cutoff value was the median p-NT-proBNP among the first 50 patients examined) or CCS ≥400 were defined as high risk (n = 133). 2) All other patients were considered low risk, i.e., patients assumed not to have CAD (n = 67). High-risk patients were examined by myocardial perfusion imaging (MPI) (n = 109), computed tomography angiography (n = 20), or CAG (n = 86), according to our study referral algorithm for patients with abnormal results of the noninvasive tests for further invasive examination (13). MPI was performed using a standard 2-day protocol with 99mTc-Tetrofosmin (600–900 MBq) injected at rest and at peak stress with intravenous dipyridamole (0.6 mg/kg over 4 min). CAG was performed according to standard techniques. Coronary artery stenosis was defined as left main stem luminal diameter stenosis >50% or luminal stenosis >70% of the left anterior descending, right, or left circumflex coronary artery. Elevated CAC was defined by CCS ≥400. Significant CAD was defined as the presence of one or more significant myocardial perfusion defects on MPI or one or more significant major epicardial coronary artery stenoses on CAG. All patients had normal p-creatinine levels and no recent illnesses or history of liver disease. The study was approved by the local ethics committee, and all patients gave written informed consent.
Statistical analysis
Variables with skewed distribution are expressed as medians (interquartile range); data for all other variables are expressed as means (SD). Non-normally distributed variables were log10-transformed before analysis. Because CCS was highly skewed with values of zero, log10 (CCS + 1) was used for analysis. The patients were divided into two groups, in accordance with a vitamin D level ≥ or <12.5 nmol/L. Comparisons between groups were performed using an unpaired Student t test. The Pearson χ2 test was used to compare noncontinuous variables. The association of severe vitamin D deficiency with the presence of significant CAD or CCS ≥400 was assessed by logistic regression model and expressed as unadjusted and adjusted odds ratio (OR) with 95% CIs. Covariate adjustments were made for sex, age, and conventional cardiovascular risk factors (systolic blood pressure, p-total cholesterol, p-creatinine, and smoking). Two-tailed P values ≤0.05 were considered statistically significant. All statistical calculations were performed using SPSS for Windows, version 14.0 (SPSS Institute, Inc., Chicago, IL).
RESULTS
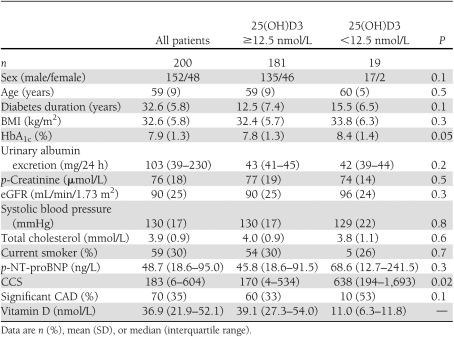
The clinical baseline patient characteristics are shown in Table 1. Median (range) vitamin D level was 36.9 (3.8–118.6) nmol/L. The prevalence of severe vitamin D deficiency was 9.5% (19/200). In linear regression analyses, vitamin D level was negatively associated with BMI (R = −0.26, P < 0.01), and a weak positive association was found with age (R = 0.159, P = 0.025). Vitamin D levels were not associated with sex, blood pressure, HbA1c, estimated glomerular filtration rate (eGFR), and UAER. Furthermore, neither eGFR nor UAER was associated with CCS scores or prevalence of significant CAD.
Table 1.
Clinical characteristics of 200 type 2 diabetic patients without a history of cardiovascular disease
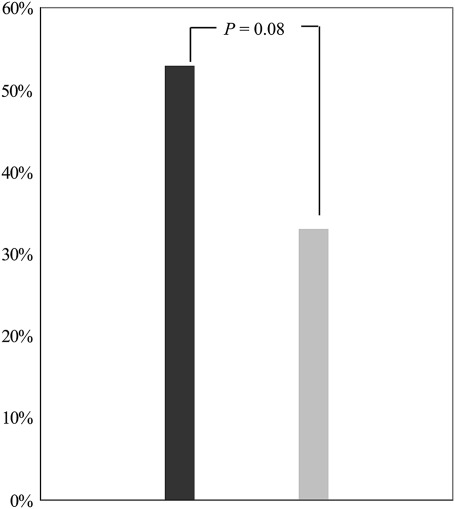
The prevalence of CCS ≥400 was 34% (68/200). Twelve of 68 patients (18%) with elevated CCS had severe vitamin D deficiency. In contrast, only 6 of 132 patients (4.5%) with CCS <400 had severe vitamin D deficiency (P = 0.003). In a logistic regression model, severe vitamin D deficiency was significantly associated with CCS ≥400 (unadjusted OR 4.3, 95% CI 1.5–12.1, P = 0.005). This association persisted after adjusting for age, sex, and conventional cardiovascular risk factors (p-total cholesterol, p-creatinine, HbA1c, systolic blood pressure, and smoking) (4.6, 1.5–13.9, P = 0.007). Among 133 high-risk patients with elevated NT-proBNP or CCS ≥400, significant CAD was demonstrated by MPI or CAG in 70 patients. Of these 70 patients, 10 had severe vitamin D deficiency. The prevalence of significant CAD was increased in the patients with severe vitamin D deficiency, although this was not statistically significant (P = 0.08) (Fig. 1). In a logistic regression model, severe vitamin D deficiency was associated with significant asymptomatic CAD (unadjusted OR 2.24, 0.87–5.81, P = 0.097). After adjustment, the OR was 2.9 (1.02–7.66, P = 0.047). Adjustment for interaction between vitamin D levels and season at time of study did not change the effect of vitamin D on CAD.
Figure 1.
Prevalence of significant CAD among all 200 patients in accordance with vitamin D level. Black bar, 25(OH)D3 <12.5 nmol/L; gray bar, 25(OH)D3 ≥12.5 nmol/L.
Neither moderate vitamin D deficiency (p-25(OH)D3 = 12.5–24.9 nmol/L) nor vitamin D insufficiency (p-25(OH)D3 = 25.0–49.9 nmol/L) was significantly associated with CCS ≥400 or increased prevalence of subclinical CAD.
CONCLUSIONS
In this cross-sectional study including 200 asymptomatic type 2 diabetic patients with elevated UAER and no history of CAD, severe vitamin D deficiency was associated with subclinical CAD evaluated as CCS ≥400 and with significant CAD detected by MPI or CAG. Our findings add to those of our previous prospective study in which low levels of vitamin D were associated with increased cardiovascular mortality in type 2 diabetic patients who had not undergone cardiovascular investigation.
CCS is a continuous measurement of the coronary arteries atherosclerotic burden, and CCS has been shown to be strongly correlated with histopathologic CAD (2,3).
The first large prospective observational study of CCS in diabetic patients and controls was performed by Raggi et al. in 2004 (15), who demonstrated that CCS is highly associated with all-cause mortality. Of note, low CCS in this study was associated with a low rate of mortality similar to that of controls. Later studies have confirmed and extended this association in asymptomatic diabetic patients without suspected CVD (16–18). In addition, in diabetic patients with proteinuria, a threshold (CCS ≥400) for clinical significant disease exists (19). For symptomatic patients, the method is somewhat more controversial (20); however, in these patients, a high CCS has been reported to identify symptomatic patients with a high risk of structural coronary artery stenosis as determined by CAG, whereas a low CCS accurately excluded patients with significant coronary artery stenosis (<1%) (21). Subsequently, CCS was suggested by Beller (22) to be valid in risk assessment and screening for asymptomatic CAD.
The association between a low vitamin D level and CCS ≥400 seen in the current study complements data correlating p-25(OH)D3 to an increased prevalence or incidence of markers of subclinical CVD. A recent study in 1,370 primarily nondiabetic subjects found 25(OH)D3 levels to be inversely associated with the risk of developing CAC (7). The same study found a trend toward an increase in magnitude of this association among participants with impaired kidney function. In 390 consecutive patients with type 2 diabetes, without advanced chronic liver or renal disease, and not taking medications or supplements known to affect vitamin D metabolism, low levels of vitamin D were found to be strongly and independently associated with increased carotid intima-media thickness, a marker of subclinical CVD (23).
Our finding of an association between low vitamin D levels and asymptomatic significant CAD also complements data suggesting an association between hypovitaminosis D and known CVD in the general population (10) and in type 1 diabetic patients (8). In addition, a study of 459 type 2 diabetic patients showed a strong inverse association between 25(OH)D3 levels and prevalent CVD (9). However, some of these patients, unlike the patients in the current study, had a history of CVD. Furthermore, as indicated above, in a prospective observational follow-up study, we found low levels of vitamin D to independently predict cardiovascular mortality among type 2 diabetic patients (11). Unlike in the present cross-sectional study, in which we have analyzed 25(OH)D3 in fresh plasma samples, the samples used for vitamin D measurements in our previous follow-up study had been stored for many years before analysis. These patients were not examined in regard to CVD, and data were not available in regard to comorbidities, level of physical activity, and seasonal variation in 25(OH)D3. We were therefore not able to rule out the possibility that the low 25(OH)D3 levels were due to such possible confounders. The current study has shown that severe vitamin D deficiency is associated with subclinical CAD in 200 asymptomatic and otherwise healthy type 2 diabetic patients. This finding was not affected by adjustment for interaction between the vitamin D levels and the season of blood sampling for 25(OH)D3 analysis (data not shown).
The exact mechanisms of action behind the increased prevalence of asymptomatic CAD among subjects with low vitamin D levels are unclear. Vitamin D is primarily known for its role in the regulation of the calcium homeostasis in the body. A growing body of evidence indicates that vitamin D through activation of the vitamin D receptor (VDR) exerts extraskeletal pleiotropic effects beneficial to cardiovascular health. Activation of the VDR is associated with suppression of the renin-angiotensin-aldosterone system (24), cardiac myocyte hypertrophy (25), and vascular calcification together with atherosclerosis-lowering, anti-inflammatory (26), and immunomodulatory actions. Clinical data show that administration of a VDR activator causes a reduction in serum levels of inflammatory markers involved in the acute inflammatory response (27,28). Secondary hyperparathyroidism triggered by hypovitaminosis D can also elicit an acute phase response and thereby promote development of CVD, providing a plausible explanation for how hypovitaminosis D is a possible risk factor for CVD. Animal models are supportive of these considerations, where vascular calcification is prevented by calcitriol doses sufficient to correct secondary hyperparathyroidism (29). Interactions between bone and vitamin D may also mediate increased deposition of calcium in the arterial wall mediated by molecules and hormones released by bone (30). However, a recent study investigating the mechanism by which vitamin D deficiency may mediate increased risk of CVD found a reduced VDR signaling to be a potential mechanism underlying increased foam cell formation and accelerated CVD in type 2 diabetic patients compared with nondiabetic patients (31). Figure 1 shows the prevalence of significant CAD among all 200 patients according to vitamin D deficiency status in our present study. Although this prevalence is probably a minimum estimate because not all patients underwent extensive examinations aimed at subclinical CAD, the prevalence of significant CAD was considerably higher in the patients with severe vitamin D deficiency, albeit this difference did not reach statistical significance. Furthermore, in the logistic regression analysis, severe vitamin D deficiency was significantly associated with CAD even after adjustment for known confounders, suggesting the possibility of vitamin D playing an independent role in the development of CAD.
In healthy subjects, vitamin D deficiency mainly results from inadequate sunlight exposure, dark skin color, and inadequate nutritional supply of vitamin D–containing foods. Seasonal variations in vitamin D levels occur depending on geographic latitude and sun exposure in particular. A study of the general population in a Northern European country showed a seasonal variation in the prevalence of vitamin D insufficiency of 73 and 29% during winter and summer, respectively. The difference for vitamin D deficiency was likewise found to be 8 and 1%, respectively (32). Furthermore, several conditions, such as obesity, disorders of intestinal absorption, and liver or kidney function, pose an increased risk of developing vitamin D deficiency. However, as seen in Table 1, the mean BMI for our patients with low vitamin D levels was not significantly different compared with the patients with higher vitamin D levels. There was no association between vitamin D level and renal function or UAER, and there was no association between CCS and UAER or eGFR. However, all patients were selected to have normal p-creatinine levels to avoid radiocontrast problems (mean [SD] eGFR of 90 [25] mL/min/1.73 m2). All patients had an elevated UAER, mostly in the microalbuminuric range. Had the range of eGFR or UAER been wider, including normal and abnormal values, it is possible there would have been significant associations.
International consensus is lacking in regard to a definition of plasma vitamin D levels representative of normal, insufficiency, and deficiency. In our study, severe vitamin D deficiency was defined as p-25(OH)D3 <12.5 nmol/L in both men and women in correspondence with the National Danish guidelines at the time of the study, but further studies are warranted to more clearly establish physiologic and pathologic vitamin D levels in health and disease.
Our study has some strengths and limitations. This cohort of type 2 diabetic patients underwent detailed and comprehensive testing including CAG to detect asymptomatic CAD. Therefore, this study contributes to future identification of patients in whom aggressive investigations and treatment aimed at CAD should be considered. Given the cross-sectional design, the current study does not elaborate further on the causative nature of the associations made, but it does add to an increasing amount of data suggesting that vitamin D substitution might be a potential therapeutic mean to prevent cardiovascular disease development or progression. We did not measure parathyroid hormone levels, and therefore we were not able to adjust for this potential confounder in our analysis. Prospective, randomized, placebo-controlled trials administrating a VDR activator are needed to examine causality between vitamin D status and CAD in diabetic patients.
In conclusion, we have shown that low levels of vitamin D are associated with asymptomatic CAD in asymptomatic high-risk type 2 diabetic patients with elevated UAER.
Acknowledgments
The study was supported by the European Foundation for the Study of Diabetes.
No potential conflicts of interest relevant to this article were reported.
C.J. wrote the manuscript and is the guarantor of this study. H.R., P.R.H., N.W., C.L.P., and P.K.J. researched data and reviewed and edited the manuscript. A.S. measured p-25(OH)D3. K.W. measured p-NT-proBNP. H.-H.P. and P.R. reviewed and edited the manuscript.
The authors thank the laboratory technicians at the Steno Diabetes Center for technical assistance.
References
- 1.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care 2001;24:335–338 [DOI] [PubMed] [Google Scholar]
- 2.Simons DB, Schwartz RS, Edwards WD, Sheedy PF, Breen JF, Rumberger JA. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol 1992;20:1118–1126 [DOI] [PubMed] [Google Scholar]
- 3.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157–2162 [DOI] [PubMed] [Google Scholar]
- 4.Raggi P, Callister TQ, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation 2000;101:850–855 [DOI] [PubMed] [Google Scholar]
- 5.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol 2000;36:1253–1260 [DOI] [PubMed] [Google Scholar]
- 6.Michos ED, Streeten EA, Ryan KA, et al. Serum 25-hydroxyvitamin D levels are not associated with subclinical vascular disease or C-reactive protein in the old order Amish. Calcif Tissue Int 2009;84:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 2009;20:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young KA, Snell-Bergeon JK, Naik RG, et al. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care 2011;34:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 2006;29:722–724 [DOI] [PubMed] [Google Scholar]
- 10.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 11.Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving H-H, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care 2010;33:2238–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarnow L, Gall M-A, Hansen BV, Hovind P, Parving H-H. Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia 2006;49:2256–2262 [DOI] [PubMed] [Google Scholar]
- 13.Reinhard H, Hansen PR, Persson F, et al. Elevated NT-proBNP and coronary calcium score in relation to coronary artery disease in asymptomatic type 2 diabetic patients with elevated urinary albumin excretion rate. Nephrol Dial Transplant 2011;26:3242–3249 [DOI] [PubMed]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832 [DOI] [PubMed] [Google Scholar]
- 15.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 16.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 2006;27:713–721 [DOI] [PubMed] [Google Scholar]
- 17.Elkeles RS, Godsland IF, Feher MD, et al. ; PREDICT Study Group Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J 2008;29:2244–2251 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Morgan T, Herrington DM, et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the Diabetes Heart Study. Diabetes Care 2011;34:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu YW, Adler SG, Budoff MJ, Takasu J, Ashai J, Mehrotra R. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int 2010;77:1107–1114 [DOI] [PubMed] [Google Scholar]
- 20.Oudkerk M, Stillman AE, Halliburton SS, et al. ; European Society of Cardiac Radiology; North American Society for Cardiovascular Imaging Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Eur Radiol 2008;18:2785–2807 [DOI] [PubMed] [Google Scholar]
- 21.Knez A, Becker A, Leber A, et al. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol 2004;93:1150–1152 [DOI] [PubMed] [Google Scholar]
- 22.Beller GA. Noninvasive screening for coronary atherosclerosis and silent ischemia in asymptomatic type 2 diabetic patients: is it appropriate and cost-effective? J Am Coll Cardiol 2007;49:1918–1923 [DOI] [PubMed] [Google Scholar]
- 23.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597 [DOI] [PubMed] [Google Scholar]
- 24.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 2005;288:E125–E132 [DOI] [PubMed] [Google Scholar]
- 26.Zehnder D, Quinkler M, Eardley KS, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int 2008;74:1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab 2003;88:4623–4632 [DOI] [PubMed] [Google Scholar]
- 28.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM 2002;95:787–796 [DOI] [PubMed] [Google Scholar]
- 29.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol 2008;19:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 2004;15:1943–1951 [DOI] [PubMed] [Google Scholar]
- 31.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009;120:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kull M, Jr, Kallikorm R, Tamm A, Lember M. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]