Abstract
OBJECTIVE
We examined associations of birth weight and weight gain in infancy and early childhood with type 2 diabetes (DM) risk in five cohorts from low- and middle-income countries.
RESEARCH DESIGN AND METHODS
Participants were 6,511 young adults from Brazil, Guatemala, India, the Philippines, and South Africa. Exposures were weight at birth, at 24 and 48 months, and adult weight, and conditional weight gain (CWG, deviation from expected weight gain) between these ages. Outcomes were adult fasting glucose, impaired fasting glucose or DM (IFG/DM), and insulin resistance homeostasis model assessment (IR-HOMA, three cohorts).
RESULTS
Birth weight was inversely associated with adult glucose and risk of IFG/DM (odds ratio 0.91[95% CI 0.84–0.99] per SD). Weight at 24 and 48 months and CWG 0–24 and 24–48 months were unrelated to glucose and IFG/DM; however, CWG 48 months–adulthood was positively related to IFG/DM (1.32 [1.22–1.43] per SD). After adjusting for adult waist circumference, birth weight, weight at 24 and 48 months and CWG 0–24 months were inversely associated with glucose and IFG/DM. Birth weight was unrelated to IR-HOMA, whereas greater CWG at 0–24 and 24–48 months and 48 months–adulthood predicted higher IR-HOMA (all P < 0.001). After adjusting for adult waist circumference, birth weight was inversely related to IR-HOMA.
CONCLUSIONS
Lower birth weight and accelerated weight gain after 48 months are risk factors for adult glucose intolerance. Accelerated weight gain between 0 and 24 months did not predict glucose intolerance but did predict higher insulin resistance.
Recently, Whincup et al. (1) concluded that birth weight is inversely associated with the development of type 2 diabetes (DM) and that this association is strengthened after adjusting for adult BMI. Studies from high-income countries have shown that rapid weight gain in childhood or adult life is associated with an increased incidence of DM and insulin resistance (2). Therefore, impaired fetal growth and excess postnatal weight gain are both potential precursors to adult DM.
Four-fifths of all individuals with DM live in low- and middle-income countries (LMICs) (3). Many of these countries are undergoing swift nutritional and economic transitions, exposing individuals to environmental conditions that promote weight gain. The combination of early-life undernutrition and overnutrition in adulthood may be fueling the epidemic of DM in LMICs (4).
Few studies have examined childhood weight gain in relation to adult diabetes in LMICs. Gestation and the first 2 postnatal years (the first “1,000 days”; http://www.thousanddays.org) are the time when children’s growth in LMICs falls most rapidly below international reference values (5) and, hence, provide a significant window of opportunity for improved infant survival, cognitive development, and adult economic status (6,7). A critical public health question for LMICs is whether promoting early-life weight gain to achieve improvements in human capital could have adverse effects on adult diabetes risk. Data from birth cohorts in the U.K. and Finland indicate that, as with birth weight, lower weight at 1 year is associated with an increased risk of DM (8,9). Other studies have shown that greater weight or weight gain at this age is associated with an increased risk of obesity (10,11), which could increase diabetes risk.
To clarify relationships between early-weight dynamics and adult diabetes risk, we pooled data from five birth cohort studies in LMICs and investigated associations of weight at birth, 24 months, 48 months, and young adulthood and conditional weight gain (CWG) between these ages, with adult-fasting glucose concentrations, risk of glucose intolerance, and insulin resistance. We hypothesized that lower birth weight and infant CWG but higher CWG after infancy would predict increased risk.
RESEARCH DESIGN AND METHODS
Study populations
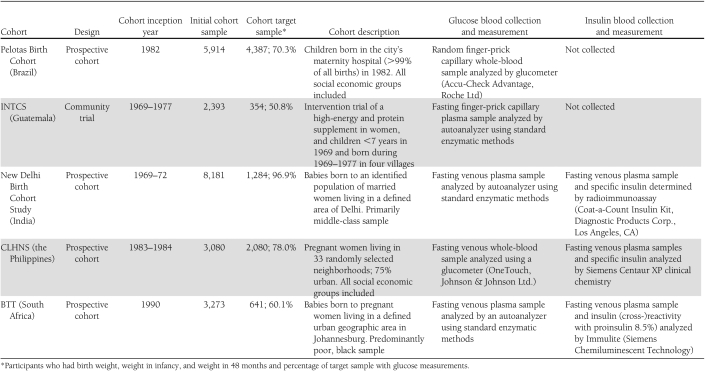
The five cohorts (Table 1) include the 1982 Pelotas Birth Cohort (Brazil) (12), the Institute of Nutrition of Central America and Panama Nutrition Trial Cohort Study (INTCS, Guatemala) (13), the New Delhi Birth Cohort Study (India) (14), the Cebu Longitudinal Health and Nutrition Survey (CLHNS, the Philippines) (15), and the Birth to Twenty cohort (BTT, South Africa) (16). All cohort participants were adults at the time the outcome variables were measured, with mean (SD) ages of 22.7 (0.4), 32.4 (4.1), 29.2 (1.4), and 21.2 (0.9) years for Brazil, Guatemala, India, and the Philippines, respectively, with the exception of the South Africans, who were aged 15.5 (0.3) years. For all cohorts, appropriate institutional research ethics committee approval was granted, and informed consent was obtained from participants or their parents, as appropriate.
Table 1.
Characteristics of the five cohorts, glucose and insulin sample collection, and measurement protocols
Exposures: weights at birth, 24 months, and 48 months
In Brazil, India, and Guatemala, birth weight was measured by research teams. In the Philippines, birth weight was measured by birth attendants using hanging scales for home births and was obtained from hospital records for hospital births. In South Africa, birth weight was measured by birth attendants in the hospitals, and these were obtained from hospital birth records. In all sites, postnatal weights were measured by research teams using standardized methods. Weight at 24 months was available at all sites. Weight at 48 months was available in three cohorts (Brazil, Guatemala, and India). In South Africa and the Philippines, weights obtained at the nearest possible age (60 and 102 months, respectively) were used.
Gestational age
Gestational age was based on the date of the mother’s last menstrual period, except in the Philippines, where Ballard scores obtained by clinical assessment of the newborn’s neuromuscular and physical characteristics (17) were used for participants with low birth weight.
Adult anthropometry
Adult height and weight were measured using standardized techniques. Waist circumference was measured using a measuring tape to the nearest 0.1 cm at the umbilicus (Guatemala, the Philippines, and South Africa), narrowest part of the trunk (Brazil), or midway between the lower costal margin and superior iliac crest laterally (India).
Outcomes: adult glucose and insulin
We considered three adult outcomes as follows: fasting plasma glucose concentration, the combined prevalence of impaired fasting glucose or DM (IFG/DM), and insulin resistance. In all cohorts, with the exception of Brazil, the research team obtained a fasting sample and determined glucose using site-specific procedures (Table 1). In Brazil, random blood samples were taken, and values were adjusted for time since the last meal (18). Because glucometers overestimate glucose concentrations in whole venous blood compared with standard laboratory methods (19,20), we subtracted 0.97 mmol/L from the values in the Philippines cohort to obtain the best equivalent to venous plasma as analyzed by a laboratory autoanalyzer (19). We defined DM as glucose concentration ≥7.0 mmol/L and IFG as glucose ≥6.1 and <7.0 mmol/L (21). Three sites (Delhi, the Philippines, and South Africa) measured insulin (Table 1). Insulin resistance homeostasis model assessment (IR-HOMA) was calculated using the HOMA 2 calculator (22).
Analytic sample
We included 8,746 participants with complete data for weight at birth, 24 months, 48 months, and adulthood (Table 1). Of these, 6,511 had adult plasma glucose concentrations, and 6,503 had glucose concentrations and waist circumference. Models for IR-HOMA were based on 3,202 participants from India, the Philippines, and South Africa.
Data management and statistical methods
Fasting glucose and IR-HOMA had skewed distributions and were log-transformed. Measures of weight were converted into sex-specific SD scores using the World Health Organization Growth Standards and further standardized within each site to create variables with a mean of 0 and a SD of 1. We imputed 48-month SD scores for participants in South Africa and the Philippines, assuming a linear change in the SD score from 24 to 60 or 102 months, respectively. Small for gestational age (SGA) was defined as a birth weight below the age and sex-specific 10th percentile of the birth weight distribution published by Williams et al. (23).
To focus on weight gain in the three intervals of 0–24 months, 24–48 months, and 48 months–adulthood, we used CWG variables. These represent how much a child has exceeded or fallen short of expected weight at a particular age, given his or her earlier weight trajectory and the weight trajectories of the whole population. They are calculated separately for each site and sex as the standardized residual from a linear regression model in which all weights measured up to the beginning of the interval were used as predictors. By construction, birth weight and the three CWG measures are all uncorrelated (24,25).
Analyses were conducted using SPSS 18.0 software (IBM SPSS, Armonk, NY). We used χ2 and t tests to assess differences between the included and excluded participants. Associations between weight and CWG in early life (exposures) and adult outcomes were assessed within each of the 10 combinations of site and sex, and then in pooled data, adjusted for site, sex, and adult age. Additional models were adjusted sequentially for adult waist circumference, BMI, and height. We used linear regression for continuous outcomes and logistic regression for binary outcomes. We tested for heterogeneity across the 10 site-sex strata using F tests from nested linear models and χ2 tests based on the difference in deviance from nested logistic regression models. Because the study in Guatemala was an intervention trial, we checked for heterogeneity of effects on outcomes between the intervention and control arms of the trial by testing for interactions between early-life weights and intervention group.
RESULTS
Characteristics of participants in the five cohorts
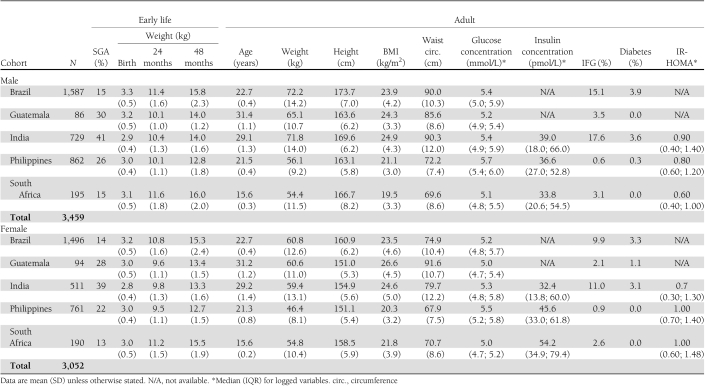
Participants included in the analysis sample were similar to those excluded with respect to sex, birth weight, infant weight, 48-month weight, adult weight, and adult waist circumference (all P > 0.10). Indian participants had the lowest birth weights and the highest prevalence of SGA births (Table 2). Brazilian participants had the highest birth weights and adult heights. South African participants were the heaviest at 24 and 48 months. The prevalence of IFG/DM was highest in India and lowest in the Philippines.
Table 2.
Characteristics of the study sample by sex and cohort location
Weight in early life and adult glucose concentrations and prevalence of IFG/DM
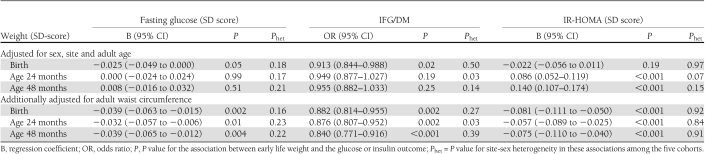
Table 3 shows the cross-sectional analyses for associations of early weights with adult glucose concentrations and prevalence of IFG/DM and includes P values for the tests for site-sex heterogeneity. There was minimal evidence of site-sex heterogeneity, and consequently, all cohort data were pooled. There was no evidence of heterogeneity between trial groups in Guatemala; therefore, both groups were considered together. Birth weight was inversely associated with adult fasting glucose and with the prevalence of IFG/DM. Fasting glucose decreased by 0.025 SD and the risk of developing IFG/DM was reduced by 9% per SD (∼0.5 kg) increase in birth weight. There were no associations between 24- or 48-month weight and glucose or IFG/DM (Table 3).
Table 3.
Cross-sectional analyses of the associations of weight at birth, and at ages 24 and 48 months with adult fasting glucose concentration, presence of IFG/DM, and insulin resistance
Weights at birth, 24 months, and 48 months were positively correlated with BMI (all P < 0.001) and waist circumference (all P < 0.001). Adult BMI and waist circumference were positively related to glucose concentrations (both P < 0.001). After adjusting for adult waist circumference, the inverse associations of birth weight with plasma glucose and IFG/DM were strengthened (Table 3), and there were inverse associations between 24- and 48-month weight and these outcomes. These findings were little changed after further adjusting for adult BMI and height (data not shown).
CWG and adult glucose concentrations and IFG/DM
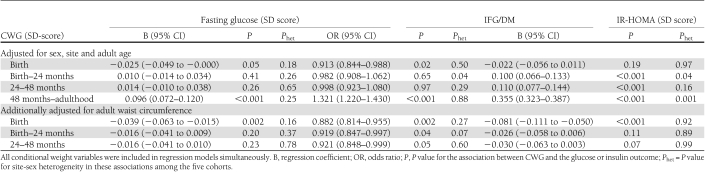
The longitudinal analyses found minimal evidence of site-sex heterogeneity, and the data were pooled. CWGs between birth and 48 months were not associated with fasting glucose or the prevalence of IFG/DM (Table 4). CWG between 48 months and adulthood was strongly positively associated with adult glucose and IFG/DM. In models further adjusting for waist circumference, birth weight and CWG birth–48 months were inversely associated with fasting glucose and/or IFG/DM; whereas CWG 24–48 months was not associated. These findings were unaltered with adjustment for adult BMI and height (data not shown).
Table 4.
Longitudinal analyses of the associations of weight at birth and conditional weight gain from birth to 24 months, 24 to 48 months, and 48 months to adulthood, with adult fasting glucose concentration, presence of IFG/DM, and insulin resistance
Weight in early life and adult insulin resistance
There was minimal evidence of site-sex heterogeneity in the associations between weight in early life and IR-HOMA (Table 3). In pooled models, birth weight was unrelated to IR-HOMA, whereas 24- and 48-month weights were positively associated with IR-HOMA. IR-HOMA was positively associated with adult BMI and waist circumference (both P < 0.001). After adjusting for adult waist circumference, the associations of birth weight and 24- and 48-month weight with adult insulin resistance were inversed (Table 3).
CWG and adult insulin resistance
There was one significant P value for site-sex heterogeneity (CWG from 48 months to adulthood; Table 4). This was explained by heterogeneity in the association between CWG from 48 months to adulthood and adult waist circumference. This association was strongest in India, where waist circumference rose by 10.2 (SE 0.3) cm in women and 9.9 (0.3) cm in men per SD increase in CWG compared with 4.9 (0.2) and 4.6 (0.2) cm in the Philippines, and 5.8 (0.5) and 5.0 (0.5) cm in South Africa. The heterogeneity was much reduced with adjustment for adult waist circumference (Table 4).
In the pooled analysis, there were positive associations of CWG with IR-HOMA in all three periods (Table 4). After further adjusting for adult waist circumference or for BMI and height (data not shown), the CWG variables in early life were not associated with insulin resistance.
SGA and the associations of weight gain with outcomes
We examined interactions of SGA (as a binary variable) and birth weight (as a continuous variable) with each CWG variable. There were no significant interactions with CWG at any age (all P values > 0.06). This indicates that CWG in infancy and childhood had similar associations with adult outcomes across the range of birth weights, whether or not the individual was SGA at birth.
CONCLUSIONS
In a pooled analysis of five cohorts from LMICs, lower birth weight was associated with higher adult glucose concentrations and an increased risk of glucose intolerance. Weight at 24 months and 48 months and CWG between birth and 48 months were unrelated to glucose concentrations and IFG/DM. In contrast, CWG between 48 months and adulthood was strongly and positively related to both outcomes. Adult waist circumference was positively associated with all early weights, as well as with adult glucose concentration and IFG/DM. After adjusting for adult waist circumference, birth weight, infant weight, and infant CWG were all inversely associated with glucose and prevalence of IFG/DM. Birth weight showed no association with insulin resistance (estimated in three cohorts, Table 3), whereas greater CWG in any period was associated with higher insulin resistance. After adjusting for waist circumference, insulin resistance, as with glucose and IFG/DM, was inversely associated with birth weight (Table 3). SGA status did not modify the associations between CWG and outcomes.
Birth weight
The inverse associations of birth weight with adult glucose concentrations and risk of IFG/DM were consistent with previous studies and with a recent systematic review, mainly from high-income populations (1). The size of the effect on IFG/DM (9% [95% CI 1–16] reduction in risk per SD increase in birth weight of ∼500 g) was similar to that reported for DM alone in the systematic review (25 [19–30] reduction in risk per kilogram of birth weight). Our findings are compatible with the hypothesis that environmental factors that influence fetal growth (e.g., maternal size and nutritional status) have long-term effects on glucose homeostasis. Insulin resistance later in life was not related to birth weight unadjusted, but was inversely associated with birth weight after adjusting for adult adiposity.
Infant and childhood weight and weight gain
There were no associations of 24- or 48-month weight, or CWG through 48 months, with adult glucose or IFG/DM. In contrast, CWG between 48 months and adulthood was strongly associated with these outcomes. This suggests that infancy and early childhood may be an important window of opportunity to promote weight gain in LMIC populations to improve survival and adult human capital without exacerbating adult DM risk. However, the findings for insulin resistance suggest that this conclusion still needs to be treated with caution. Insulin resistance was positively related to infant weight and CWG, and it is possible that this will result in a higher risk of diabetes at older ages. The strong association between CWG from 48 months to adulthood is consistent with other studies (9,14) and suggests that accelerated weight gain or upward crossing of weight percentiles during childhood should be avoided.
Interpretation of the findings after adjustment for adult adiposity
Infant weight, 48-month weight, and CWGs were correlated with adult weight and with adult BMI and waist circumference. Thus, weight at these ages, or some component(s) of it, makes a contribution to adult adiposity and a strong risk factor for diabetes. Without adjustment for adult waist circumference, we found no association of 24- and 48-month weight/CWG with adult glucose and IFG/DM. After adjusting for adult waist circumference, we found inverse associations of birth and/or 24-month weight, and CWG between birth and 24 months, with all three outcomes. Our interpretation is that for any given adult waist circumference, higher birth weight and/or infant weight and weight gain are associated with a lower adult insulin resistance and DM risk. This suggests that there is a component of early weight/weight gain that is not associated with larger adult waist circumference and that may be protective against later disease. An example may be lean tissue or muscle mass. Our data suggest that the positive associations of 24- and 48-month weight, and CWG at all ages, with adult insulin resistance are driven by the component(s) associated with larger adult waist circumference.
Strengths and limitations of the study
The major strength of this study was the prospective serial measurements of weight from a large number of individuals in LMICs. Our choice of time points was limited due to differing ages of follow-up in the five sites; measurements earlier in infancy and later in childhood would have been valuable. Interpolation was required in two cohorts to estimate 48-month weight; however, weight gain between the end of infancy and the onset of puberty tends to be linear.
Another limitation was loss to follow-up, especially in the older (India and Guatemala) cohorts. However, comparison of the analysis sample with the original full cohorts showed that their early weights were similar. Bias would be introduced only if the associations between early size and glucose tolerance differ between those who were and were not included in the analysis.
Additional limitations were heterogeneity in the age of the participants among the five cohorts and the methods used for measuring birth weight, gestational age, and plasma glucose concentrations. Even though three cohorts used one approach and two cohorts used another to measure birth weight, both methods are acceptable means of obtaining birth weight. Only one of the five cohorts determined gestational age in an alternative way to the other four cohorts, and again used a recognized method.
We had only single plasma glucose concentrations (no glucose tolerance test data). The site in Brazil collected nonfasting blood glucose but validated the equation to correct these values to a fasting state (18). The differences in glucose measurements between whole blood and plasma are well defined (22). The differences between laboratory and glucometer measurements are less well known, but laboratory and glucometer values in venous and capillary samples, and in whole blood and plasma, have been compared extensively in the literature, including direct comparisons of the methods used in our study (20). Despite these differences in methodologies we are struck by the consistency of results across the five sites. This speaks to the robustness of our findings.
In conclusion, lower birth weight is a risk factor for glucose intolerance and has important implications for LMICs, where poor birth outcomes are common. Greater CWG between 48 months and adolescence/adulthood (15–32 years) is also a risk factor for glucose intolerance, providing more evidence that upward crossing of weight percentiles after 48 months should be avoided in LMICs. Our analysis showed no increased risk of IFG/DM associated with greater infant and early childhood weight gain, which suggests that it may be possible to promote weight gain at this stage of life to accrue benefits for survival, growth faltering, and human capital, without increasing adult diabetes risk. However, the cohorts are still young, and the associations of above-average weight gain in infancy with increased adult waist circumference and insulin resistance may predict a risk of diabetes in the future.
Acknowledgments
COHORTS is supported by Wellcome Trust (U.K.) and the Bill and Melinda Gates Foundation. Funding for the individual cohorts was as follows: INTCS (Guatemala)—U.S. National Institutes of Health and U.S. National Science Foundation; Pelotas Birth Cohort (Brazil)—Wellcome Trust; New Delhi Birth Cohort Study (India)—Indian Council of Medical Research, U.S. National Center for Health Statistics, Medical Research Council (U.K.), and British Heart Foundation; BTT (South Africa)—Wellcome Trust, Human Sciences Research Council, South African Medical Research Council, South-African Netherlands Programme on Alternative Development, Anglo American Chairman’s Fund, and University of the Witwatersrand; and CLHNS (the Philippines)—U.S. National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
The COHORTS group designed the research. S.A.N. and C.H.D.F. conducted the research, analyzed data, wrote the manuscript, read and approved the final manuscript, and had primary responsibility for final content. C.O. conducted the research, analyzed data, read and approved the final manuscript, and had primary responsibility for final content. D.G., C.W.K., L.R., N.R.L., M.R.-Z., and L.M.R. conducted the research and read and approved the final manuscript. A.D.S. conducted the research, wrote the manuscript, and read and approved the final manuscript. N.T. conducted the research and read and approved the final manuscript.
The authors thank the following colleagues from each site. Pelotas Birth Cohort (Brazil): Rosangela Lima; CLHNS (the Philippines): Sororro Gultiano, Josephine Avila, Lorna Perez, and Thomas McDade; New Delhi Birth Cohort Study (India): Shanti Ghosh, I.M. Moriyama, Vinod Kapani, Rajeshwari Verma, Bhaskar Singh, Arti Mishra, K.D. Gupta, K. Belwal, Dileep Gupta, and Shikha Sinha; INTCS (Guatemala): Rafael Flores, Usha Ramakrishnan, Kathryn Yount, Ruben Grajeda, Paul Melgar, Humberto Mendez, Luis Fernando Ramirez, Jere Behrman, John Hoddinott, Agnes Quisumbing, Alexis Murphy, and John Maluccio; BTT (South Africa): John Pettifor and Noel Cameron.
APPENDIX
The COHORTS group members are as follows: Cesar G. Victora, Pedro C. Hallal, Fernando C. Barros, and Bernardo L. Horta (Universidade Federal de Pelotas, Brazil); Reynaldo Martorell (Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia); Santosh K. Bhargava (Sunderlal Jain Hospital, New Delhi, India); Harshpal Singh Sachdev (Sitaram Bhartia Institute of Science and Research, New Delhi, India); Linda Adair (University of North Carolina at Chapel Hill, Chapel Hill, North Carolina); Judith Borja (Office of Population Studies, University of San Carlos, Cebu City, Philippines); Darren Dahly (University of Leeds, Leeds, U.K.); and Mathew Mainwaring (Developmental Pathways for Health Research Unit, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
A complete list of the COHORTS group members can be found in the appendix.
References
- 1.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–2897 [DOI] [PubMed] [Google Scholar]
- 2.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev 2008;6:241–247 [PubMed] [Google Scholar]
- 3.International Diabetes Federation IDF Diabetes Atlas. 4th ed. Brussels, Belgium, IDF, 2009 [Google Scholar]
- 4.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res 2004;56:311–317 [DOI] [PubMed] [Google Scholar]
- 5.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125:e473–e480 [DOI] [PubMed] [Google Scholar]
- 6.Martorell R, Horta BL, Adair LS, et al. ; Consortium on Health Orientated Research in Transitional Societies Group Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low- and middle-income countries. J Nutr 2010;140:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet 2008;371:411–416 [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia 2003;46:190–194 [DOI] [PubMed] [Google Scholar]
- 10.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stettler N, Iotova V. Early growth patterns and long-term obesity risk. Curr Opin Clin Nutr Metab Care 2010;13:294–299 [DOI] [PubMed] [Google Scholar]
- 12.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol 2006;35:237–242 [DOI] [PubMed] [Google Scholar]
- 13.Stein AD, Melgar P, Hoddinott J, Martorell R. Cohort Profile: the Institute of Nutrition of Central America and Panama (INCAP) Nutrition Trial Cohort Study. Int J Epidemiol 2008;37:716–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adair LS. Size at birth and growth trajectories to young adulthood. Am J Hum Biol 2007;19:327–337 [DOI] [PubMed] [Google Scholar]
- 16.Richter L, Norris SA, Pettifor J, Yach D, Cameron N. Cohort Profile: Mandela’s children: the 1990 Birth to Twenty study in South Africa. Int J Epidemiol 2007;36:504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr 1979;95:769–774 [DOI] [PubMed] [Google Scholar]
- 18.Horta BL, Gigante DP, Victora CG, Barros FC, Oliveira I, Silveira V. [Early determinants of random blood glucose among adults of the 1982 birth cohort, Pelotas, Southern Brazil]. Rev Saude Publica 2008;42(Suppl 2):93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar G, Sng BL, Kumar S. Correlation of capillary and venous blood glucometry with laboratory determination. Prehosp Emerg Care 2004;8:378–383 [DOI] [PubMed] [Google Scholar]
- 20.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta 2008;396:10–13 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland, WHO, 1999 [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol 1982;59:624–632 [PubMed] [Google Scholar]
- 24.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol 2005;58:1320–1324 [DOI] [PubMed] [Google Scholar]
- 25.Adair LS, Martorell R, Stein AD, et al. ; COHORTS group Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter? Am J Clin Nutr 2009;89:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]