Abstract
OBJECTIVE
To determine whether short-time, real-time continuous glucose monitoring (RT-CGM) has long-term salutary glycemic effects in patients with type 2 diabetes who are not on prandial insulin.
RESEARCH DESIGN AND METHODS
This was a randomized controlled trial of 100 adults with type 2 diabetes who were not on prandial insulin. This study compared the effects of 12 weeks of intermittent RT-CGM with self-monitoring of blood glucose (SMBG) on glycemic control over a 40-week follow-up period. Subjects received diabetes care from their regular provider without therapeutic intervention from the study team.
RESULTS
There was a significant difference in A1C at the end of the 3-month active intervention that was sustained during the follow-up period. The mean, unadjusted A1C decreased by 1.0, 1.2, 0.8, and 0.8% in the RT-CGM group vs. 0.5, 0.5, 0.5, and 0.2% in the SMBG group at 12, 24, 38, and 52 weeks, respectively (P = 0.04). There was a significantly greater decline in A1C over the course of the study for the RT-CGM group than for the SMBG group, after adjusting for covariates (P < 0.0001). The subjects who used RT-CGM per protocol (≥48 days) improved the most (P < 0.0001). The improvement in the RT-CGM group occurred without a greater intensification of medication compared with those in the SMBG group.
CONCLUSIONS
Subjects with type 2 diabetes not on prandial insulin who used RT-CGM intermittently for 12 weeks significantly improved glycemic control at 12 weeks and sustained the improvement without RT-CGM during the 40-week follow-up period, compared with those who used only SMBG.
The prevalence of diabetes is projected to rise from the current 11% of the U.S. population aged ≥20 years (1) to as much as 33% by 2050 (2). Those with type 2 diabetes represent ~90% of all people with diabetes. Poor glycemic control remains a problem for many people. Forty-four percent of people with type 2 diabetes have a hemoglobin A1C higher than the generally accepted target for most patients of 7% (3). Despite the emergence of several new classes of pharmacologic agents, the introduction of medication-use guidelines and algorithms by major professional organizations (4–6), the improvement in the quality and user-friendliness of devices for self-monitoring of blood glucose (SMBG), and the development of numerous care- and self-management tools for providers and patients that provide some decision support and facilitate the sharing of information, a significant number of type 2 diabetic subjects remain suboptimally controlled. This state of affairs suggests the need for additional approaches to glycemic management for people with type 2 diabetes. Such an approach might be real-time continuous glucose monitoring (RT-CGM).
RT-CGM provides patients with a glucose reading every 5 min, typically on a visual display that shows the glucose trend and whether it is above, below, or within preset ranges. RT-CGM has been shown to improve glycemic control and/or reduce the frequency of hypoglycemic episodes in pediatric and adult patients with type 1 diabetes and in adult patients with type 2 diabetes taking prandial insulin (7–14), although age and frequency of sensor use affect the magnitude of the A1C reduction (15). RT-CGM has not been used as a tool for diabetes management in patients with type 2 diabetes who are not taking prandial insulin, by far the largest subgroup of people with type 2 diabetes. Their glycemic monitoring relies on SMBG at a frequency and time of day commensurate with their treatment regimen.
We sought to determine whether RT-CGM could play an adjunctive role in the management of patients with type 2 diabetes who are not on prandial insulin. The rationale for this investigation was that the volume of information from RT-CGM and the visual display of that information as a trend could provide important feedback to participants on their glucose status. We recently reported that RT-CGM over 12 weeks was associated with a clinically significant reduction in A1C (1.0%) over the same period of time compared with SMBG before meals and before bedtime (0.5%) (16). We now report the legacy effects of this 12-week intervention on glycemic control and other diabetes-related outcomes in the same cohort over a total of 52 weeks.
RESEARCH DESIGN AND METHODS
The study recruited military health care beneficiaries from the Walter Reed Health Care System. Patients were eligible for the study if they were aged ≥18 years, had a diagnosis of type 2 diabetes for at least 3 months, had an initial A1C ≥7 but ≤12%, were treated with diet and exercise alone or other glucose-lowering therapies except prandial insulin, were able to independently measure and read fingerstick blood glucose levels, and were willing to perform SMBG four times daily. All subjects attended an American Diabetes Association–recognized diabetes self-management education program. Patients who were pregnant, lactating, or attempting pregnancy and those on glucocorticoids, amphetamines, and anabolic or weight-reducing medications were excluded. All subjects gave written, informed consent. Recruiting began 1 June 2007, and ultimately 530 subjects were screened (Supplementary Fig. S1). One hundred subjects were enrolled by 13 January 2010. Subjects were followed for 1 year.
This was an investigator-initiated study sponsored by DexCom Corp. The design and intervals of measurement have been previously described (16). In brief, this was a 52-week, prospective, two-arm, randomized controlled study comparing the short-term (12 weeks) and long-term (52 weeks) relative effectiveness of RT-CGM with frequent SMBG. Those subjects randomly assigned to RT-CGM used a DexCom SEVEN (DexCom, San Diego, CA), which was calibrated according to the manufacturer’s recommendations. RT-CGM occurred in four cycles (2 weeks/1 week off) for 3 months. Those in the RT-CGM group also were requested to perform SMBG before meals, at bedtime, and at the time of symptoms of hypo- or hyperglycemia. Alarms were set to activate at <70 and >180 mg/dL. After the initial 12 weeks, the RT-CGM group continued with SMBG for the duration of the study, as recommended by their usual provider.
Those subjects randomly assigned to SMBG were asked to test before meals and at bedtime for 12 weeks, as well as during times associated with the symptoms of hypo- or hyperglycemia. After the initial 12 weeks, they performed SMBG for the duration of the study, as recommended by their usual provider. All subjects were provided with and instructed in the use of the AccuChek Aviva glucometer (Roche Diagnostics, Indianapolis, IN).
The study staff did not provide any care management. Subjects in both groups continued usual care for their type 2 diabetes and were instructed to contact their primary care provider for all treatment decisions. Follow-up study visits were performed at 3-week intervals during the first 12 weeks and every 3 months during the follow-up phase. The study was approved by the human use committee/institutional review board at the Walter Reed Army Medical Center. All subjects gave their written, informed consent to participate.
Outcomes
The primary outcome of the study was A1C over the course of the study, which was measured at baseline and quarterly thereafter. A1C was measured using a Roche/Hitachi Cobas c system with a Tina-quant Hemoglobin A1C Gen.2 assay in the Walter Reed Clinical Laboratory. The a priori sample size calculation assumed that the analyses would use all five time points of A1C data (i.e., a repeated-measures analysis to characterize and explain A1C over the course of the study). It further assumed a “medium” effect of 0.25, α = 0.05 (0.025 for a two-tailed test), power = 0.8, and a correlation of 0.40 between the repeated measurements of A1C. The sample size calculation also assumed that the correlation of the A1C measures taken close together in time would be larger than the correlation between A1C measures taken far apart in time (i.e., nonsphericity); the correction for nonsphericity used for the sample size calculation was 0.25 (1/[repetitions − 1]). The total sample size required was 76 subjects, prior to inflating the estimate by 4 subjects per covariate included in the analyses. We used G*Power 3.0.10 to obtain this estimate.
Secondary outcomes included blood glucose assessed by RT-CGM and SMBG, weight, blood pressure, and change in diabetes-related stress. SMBG data were analyzed using common metrics for the first 12 weeks (the active intervention phase) and the entire 52 weeks of the study, namely the mean and percentage of readings <70, >180, and >240 mg/dL and within the target range of 70–180 mg/dL. Weight was measured on a Scale-Tronix 5005 Series scale, and blood pressure was taken using a Welch Allyn Vital Sign 300 Series monitor. Diabetes-related distress was measured using the Problem Areas in Diabetes (PAID) questionnaire (17). The PAID questionnaire is a self-administered questionnaire consisting of 20 items that cover a range of emotional problems frequently reported in diabetes. Each item was coded to indicate the severity of the problem (0 = not a problem to 4 = serious problem). We summed the 20 items and multiplied by 1.25 to yield a final score between 0 and 100. SMBG data were collected by the health care providers and accessed by study staff. Weight, blood pressure, and the PAID questionnaire scores were measured at 0, 12, and 52 weeks. We assessed absolute weight change, and we also categorized weight changes between 0–12 and 0–52 weeks as follows: no change (≤3 pounds), weight gain (> +3 pounds), and weight loss (> −3 pounds). We carried the last observation forward for missing data, unless otherwise indicated in our results. With respect to the primary outcome, 67 subjects had an A1C test at all five time points, 14 had an A1C test at four time points, 13 had an A1C test at three time points, and 6 had one to two A1C tests.
Independent variables
In addition to treatment group, the main independent variable, the study also characterized “usage” of the real-time continuous glucose monitor among participants in this group to differentiate between those who followed the protocol and those who did not. The protocol called for 56 days of usage. Assuming scheduling difficulties or the preference to remove the sensor prior to a holiday or weekend, we determined that the minimum time to wear the sensor over the full 12 weeks to be considered per protocol should be 48 days. Thus, we created a usage variable with the following categories: no usage (SMBG group), <48 days of usage, and ≥48 days of usage. We similarly differentiated subjects in the SMBG group, creating an indicator that separated those who tested less than once per day from those who tested one or more times per day. We also analyzed the number of days of RT-CGM as a continuous variable.
Confounding variables
The study collected data for a variety of additional predictors of change in A1C and/or successful implementation of RT-CGM, including age, sex, type of therapy at baseline (diet and exercise only, oral medications only, oral medications plus exenatide, or basal insulin alone or in combination), and whether the subject started basal and/or prandial insulin during the course of the study. In addition, we recorded all diabetes medications at baseline, including whether subjects were taking insulin or another injectable medication for diabetes (i.e., exenatide or pramlintide) and whether changes were made in those medications over time (i.e., additions of new medications, discontinuations, or dose changes).
Statistical analyses
First, we tested for the group’s equality with respect to selected baseline characteristics, medications at baseline and over time, and outcome variables over time, using t tests, χ2 tests, Fisher exact tests, and repeated-measures ANOVA.
Second, we calculated the change from baseline in A1C, weight, blood pressure, and the PAID questionnaire scores by subtracting the baseline values from the values obtained at the follow-up visits and then graphed the mean change from baseline over time and by group to illustrate the study subjects’ trajectories.
Third, for A1C, weight, blood pressure, and the PAID questionnaire scores, we conducted multilevel models for longitudinal data (also called mixed models, using PROC MIXED in SAS 9.2). These models allowed us to examine all of the data over time to get an overall sense of change and stability, not just mean changes from baseline at each individual time point. In addition, the models included treatment group as a fixed effect and generated a result for this effect, which represented the mean difference of the outcome between the two groups at baseline; in other words, the model adjusted for possible baseline differences in the outcome between the groups. The multilevel models included a variable for time; a transformation of the time variable (1/time2) to reflect the deceleration of change in the outcome over time that we predicted (given that the active intervention was 12 weeks and the study lasted for 52 weeks), age, sex, diabetes therapy, and initiation of basal or prandial insulin during the study. The models specified the Toeplitz covariance structures for the matrix of the within-subject residuals.
Fourth, the analyses compared the groups’ SMBG results, testing for group differences in mean SMBG values using t tests and in percentages above and below the aforementioned thresholds and in percentages within the target range using χ2 tests. All tests were two-tailed, unless otherwise specified.
In addition, because the PAID questionnaire scores at each time point were not normally distributed, we also conducted nonparametric analyses of them. Specifically, we conducted the Wilcoxon-Mann-Whitney test of the total PAID questionnaire scores at baseline and Friedman two-way nonparametric ANOVA in which each subject’s PAID questionnaire scores were rank ordered, and the ranked values were analyzed.
RESULTS
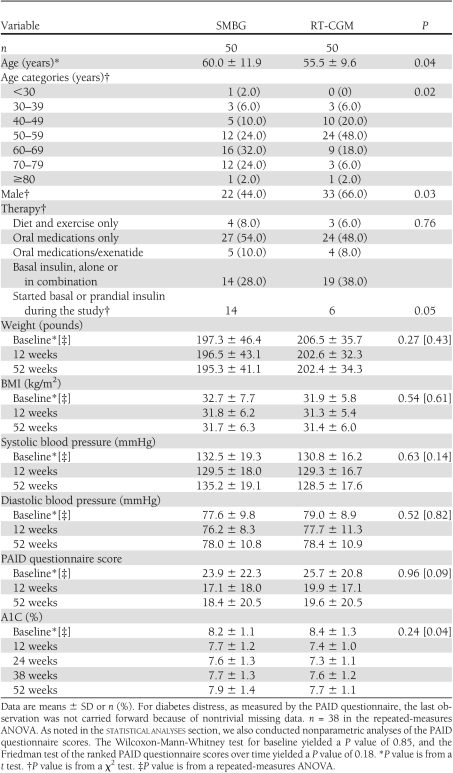
The subjects’ characteristics with respect to age, diabetes therapy, and perceived level of stress at baseline and over time for the outcome variables are shown in Table 1. Subjects in the RT-CGM group were slightly younger, on average, and there were more men in the RT-CGM group than in the SMBG group. Baseline therapies between the two groups were similar. There were no differences between the RT-CGM and SMBG groups in mean weight, systolic or diastolic blood pressure, or PAID questionnaire scores at baseline or over time (for parametric and nonparametric analyses). However, A1C patterns over time differed for the two groups (P = 0.04).
Table 1.
Characteristics of the study subjects by treatment group
The groups did not differ at the 0-, 12-, and 52-week visits with their providers in terms of the numbers of oral hypoglycemia medications, numbers of noninsulin injectables, and whether they were taking insulin (Supplementary Table S2). Although subjects in both groups had an overall intensification of their medication regimen over the course of the study, fewer subjects in the RT-CGM group were started on insulin (6 vs. 14 subjects in the SMBG group) between baseline and the last visit (P = 0.05).
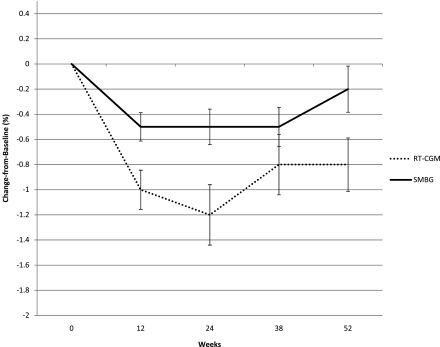
The unadjusted means ± SD A1C decreased by 1.0 ± 1.1%, 1.2 ± 1.7%, 0.8 ± 1.7%, and 0.8 ± 1.5% in the RT-CGM group vs. 0.5 ± 0.8%, 0.5 ± 1.0%, 0.5 ± 1.1%, and 0.2 ± 1.3% in the SMBG group at 12, 24, 38, and 52 weeks, respectively (Fig. 1). After statistical adjustment for age, sex, baseline therapies, and whether the subject was started on insulin over the study, the rates of change in A1C were 1.16 × (1/time2) (P < 0.0001) for the RT-CGM group and 0.51 × (1/time2) (P = 0.002) for the SMBG group. In other words, the adjusted decline in A1C for the RT-CGM versus SMBG group was 0.9 vs. 0.4% from baseline to 12 weeks, 1.0 vs. 0.5% from baseline to 24 weeks, 1.1 vs. 0.5% from baseline to 38 weeks, and 1.1 vs. 0.5% from baseline to 52 weeks. Age, taking only oral hypoglycemia medications, insulin, noninsulin injectable medications at baseline (vs. diet and exercise), and starting insulin were significant predictors of an increase in A1C over time.
Figure 1.
Mean A1C change from baseline by treatment group. Change equals later A1C minus baseline A1C. This figure shows the raw mean changes and SEMs. A separate multilevel model of the actual A1C values, with a transformation of the time variable to reflect the deceleration of change over time (1/time2, with time defined as 1–5), showed that the decline in A1C over the course of the study differed between the groups net of other factors known to cause A1C change: age, sex, diabetes therapy, and initiation of insulin during the study. Specifically, the results of a multilevel model found that the decline for the SMBG group was 0.51% (P = 0.002) and the decline for the RT-CGM group was 1.16% (P < 0.0001). These estimates must be multiplied by 1/time2 to obtain the change in A1C, which occurred at each time point.
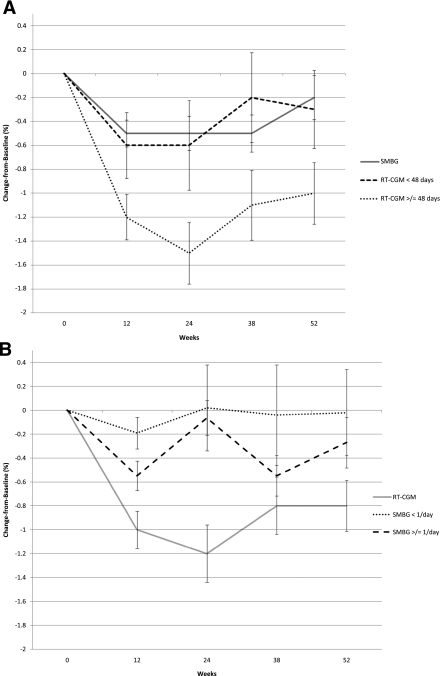
Consideration of the usage of the real-time continuous glucose monitor showed that subjects who wore the sensor for ≥48 days had the greatest drop in mean, unadjusted A1C compared with those who wore it for <48 days (i.e., 1.2 ± 1.1% vs. 0.6 ± 1.1%, 1.5 ± 1.5% vs. 0.6 ± 1.5%, 1.1 ± 1.7% vs. 0.2 ± 1.5%, and 1.0 ± 1.5% vs. 0.3 ± 1.3% at 12, 24, 38, and 52 weeks [Fig. 2A]). When adjusted for potential confounders, subjects in the RT-CGM group who wore the sensor for at least 48 days experienced the following decline in A1C: 1.0% from baseline to 12 weeks, 1.2% from baseline to 24 weeks, and 1.3% from baseline to 38 and 52 weeks. By comparison, subjects in the RT-CGM group who did not wear the sensor per protocol experienced a decline in A1C of 0.7% at 12 weeks, with no further decline for the duration of the study. Age, sex, diabetes therapy at baseline, and starting insulin during the study were not significant in this model. In our analysis of the total number of days of RT-CGM and A1C over the course of the study, we found that for each single day of RT-CGM (a continuous variable), A1C declined by 0.02 (P = 0.02).
Figure 2.
Mean A1C change from baseline per subject adherence to the study protocol, within the treatment groups. Change equals later A1C minus baseline A1C. This figure shows the raw mean changes and SEMs. In the RT-CGM group, 16 subjects wore the technology <48 days and 34 wore it ≥48 days. In the SMBG group, 9 subjects tested less than one time per day and 41 tested one or more times per day. A: The line for the SMBG group is indicated as a reference only; these participants were not included in the multilevel model. B: The line for the RT-CGM group also is indicated as a reference only. Two separate multilevel models of the actual A1C values, with a transformation of the time variable to reflect the deceleration of change over time (1/time2), showed that the decline in A1C over the course of the study differed between the usage groups net of other factors known to cause A1C change: age, sex, diabetes therapy, and initiation of insulin during the study. Specifically, the results of multilevel models found that the decline for the group that took part in RT-CGM for <48 days was 0.76% (P = 0.008), the decline for the group that took part in RT-CGM for ≥48 days was 1.31% (P < 0.0001), the decline for the group that performed SMBG less than one time per day was 0.18% (P < 0.58), and the decline for the group that performed SMBG one or more times per day 0.67% (P < 0.001). These estimates must be multiplied by 1/time2 to obtain the change in A1C that occurred at each time point.
The raw means for the subjects in the SMBG group who tested their blood glucose less than once per day versus those who tested one or more times per day during the first 12 weeks are shown in Figure 2B. These data suggest that the more frequent testers derived the greater benefit (i.e., their A1C declined 0.6 ± 0.8% during the 0- to 12-week interval [vs. −0.2 ± 0.4% for the less frequent testers] and remained steady until A1C levels began to increase, making their overall decline in A1C for the 0- to 52-week period 0.3 ± 1.4% vs. 0.0 ± 1.1% for the less frequent testers). The adjusted rate of change from the multilevel model showed that A1C for the frequent SMBG testers declined faster than that of their counterparts [0.67 × (1/time2)] (P < 0.001), but the frequent SMBG testers did not attain the depth and sustainment of decline in A1C as that seen in the RT-CGM subjects.
Summary SMBG statistics for both the RT-CGM and SMBG groups showed that, on average, 3.6 vs. 2.5% of the total number of SMBG readings obtained from subjects over the course of the study were <70 mg/dL for the RT-CGM and SMBG groups, respectively (P = 0.06) (Supplementary Appendix 3). The improvement in A1C occurred without a notable increase hypoglycemia.
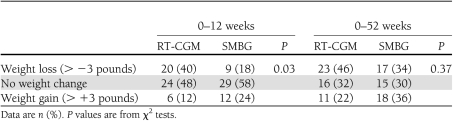
The mean weight change during the 0- to 12-week and 0- to 52-week time periods did not differ between the groups. However, more people in the RT-CGM group experienced weight loss of >3 pounds in the 0- to 12-week time period compared with the SMBG group (P = 0.03) (Table 2). The categorical weight change was similar between the two groups in the 0- to 52-week time periods (P = 0.2).
Table 2.
Weight change by treatment group
CONCLUSIONS
RT-CGM provides important feedback about glycemic trends in response to meals, exercise, and insulin in type 1 diabetic subjects and type 2 diabetic subjects on prandial insulin with resultant improvement in overall glycemic control (7–14). Accordingly, we thought that RT-CGM would be similarly effective for people with type 2 diabetes who are not taking prandial insulin. RT-CGM provides not only point data but also a graphical display of glucose trends superimposed on preset targets. In fact, our previous report demonstrated that episodic, short-term RT-CGM was effective in improving glycemic control over 12 weeks in subjects with type 2 diabetes who were not on prandial insulin (16). We also postulated that short-term exposure to RT-CGM might have lasting effects beyond the active intervention phase. To our knowledge, ours is the first study that has used this technology in a population that is reflective of the majority of people with type 2 diabetes (18).
The 52-week data suggest that there is a lasting effect on A1C from short-term (12 weeks) exposure to RT-CGM. The trend data suggest that there is continued improvement until 24 weeks. After 24 weeks, the effect of RT-CGM attenuates, but A1C does not return to baseline by 52 weeks. This finding raises the possibility that periodic use of the technology every few months might be beneficial.
Similar to the findings in studies of RT-CGM in patients with type 1 diabetes, the frequency of usage is an important variable in its success. Although the intention-to-treat analysis demonstrated a significant benefit of the technology, those who used the technology per protocol had an even greater benefit.
The improvement in A1C occurred in a patient population whose demographics and medication use is typical of that seen in the U.S. (18). The study team did not make any recommendations about medication usage; medication changes were managed by the subjects’ usual providers. Both groups were similar with regard to the number of oral hypoglycemic and injectable diabetes medications at baseline. Although the burden of diabetes medications increased in both groups over the year, there was no difference between the groups in the number of medications at any point throughout the study, except that fewer subjects in the RT-CGM group were started on insulin.
RT-CGM was not associated with changes in blood pressure or diabetes distress over the course of the study. However, it was associated with weight loss during the active intervention phase, although not over the course of the full study. It is unclear at this time why these often-related comorbidities did not also improve, or, as in the case of weight change, the differential improvement was not sustained. One of the limitations of this study is that we did not investigate the behavioral changes concomitant with RT-CGM. In addition, we are unable to determine how long the real-time continuous glucose monitor must be used to achieve the legacy effects. Additional studies may be able to identify what specific factors yielded the glycemic improvement and how many cycles or days of RT-CGM are necessary to achieve an effect.
The improvement in glycemic control that we observed with RT-CGM compares favorably with the improvements achieved by pharmacologic interventions with additional oral agents and/or insulin. Two recent reports showed an approximate A1C reduction of 1.0% with the addition of a second agent to metformin (19,20), and the addition of a third agent causes a further reduction of 0.64–0.97% (21). However, the addition of more drugs can result in well-recognized adverse effects (e.g., weight gain with insulin, thiazolidinediones, and sulfonylureas; hypoglycemia with insulin and sulfonylureas; and heart failure with thiazolidinediones). We did not find that the improvement in A1C was associated with more hypoglycemia either by SMBG or clinically, although the study was not designed to directly test this possibility.
We also found no difference in SMBG results between those in the RT-CGM group and the SMBG group at 3 and 12 months despite the difference in A1C. Two reasons might account for this: 1) the frequency of SMBG readings is only a small fraction of the total number of blood glucose determinations in RT-CGM; and 2) patients were asked to perform SMBG metrics before meals and at bedtime. This suggests the possibility that the improvement resulted from reduced postprandial glycemic excursions.
Although this study was not designed to test the efficacy of SMBG in patients with type 2 diabetes, it seems that testing more than once per day provides some benefit compared with testing less than once per day. These observations inform the debate about whether SMBG is beneficial in most patients. The use of SMBG in patients with type 2 diabetes who are not taking insulin is controversial. Two meta-analyses of randomized controlled trials found that SMBG decreased A1C by 0.39 and 0.42% (22,23). Other meta-analyses found a lower effect of 0.24 and 0.26% (24,25). The most recent systematic review failed to show a clinically significant reduction of SMBG in A1C (26). Nevertheless, our SMBG results are consistent with the trials showing a statistically and clinically significant effect of SMBG.
One of the limitations of this study is that we did not have the patients perform paired testing (pre- and postprandially), which would have been the most direct comparator. Recently published studies suggest that this may be an effective strategy (27–29). Nevertheless, there has been no clear guidance to date from the major professional organizations about the timing and frequency of SMBG in this patient population (30–32).
In summary, intermittent RT-CGM over 12 weeks significantly improved glycemic control in a population of patients with type 2 diabetes not taking prandial insulin both during and for up to 1 year following the intervention. The magnitude of the improvement was comparable to the reported “add-on” pharmacotherapy, and it did so without any greater intensification of pharmacotherapy compared with the SMBG group. Additional studies will be needed to confirm these results as well as determine the mechanism by which the improvement occurred, the minimum time for RT-CGM to be effective, and the effect/timing of refresher courses of this intervention.
Acknowledgments
The authors acknowledge the support of DexCom Corporation for providing funding and in-kind support for this work. No other potential conflicts of interest relevant to this article were reported.
R.A.V. designed the study, oversaw the conduct of the study, wrote the manuscript, and is the guarantor. S.J.F. assisted with writing the manuscript and carried out the statistical analysis. M.C. was the study project officer and contributed to writing the manuscript. M.S.W. contributed to the study design and to writing the manuscript. N.M.E. wrote the study protocol and contributed to the data interpretation and to writing the manuscript.
Parts of this article were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank the patients who volunteered to participate in this study.
Footnotes
Clinical trial reg. no. NCT00529815, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1438/-/DC1.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the U.S. Army or the U.S. Department of Defense.
References
- 1.National Diabetes Information Clearinghouse. National diabetes statistics [article online], 2011. Available from http://diabetes.niddk.nih.gov/dm/pubs/statistics/#diagnosed20 Accessed 29 May 2011
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologist/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Veterans Affairs. VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care (2010) [article online], 2010. Available from http://www.healthquality.va.gov/diabetes_mellitus.asp Accessed 29 May 2011
- 7.Tamborlane WV, Beck RW, Bode BW, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Hirsch IB, Laffel L, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 10.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther 2007;9:203–210 [DOI] [PubMed] [Google Scholar]
- 11.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 [DOI] [PubMed] [Google Scholar]
- 12.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zick R, Petersen B, Richter M, Haug C; SAFIR Study Group Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther 2007;9:483–492 [DOI] [PubMed] [Google Scholar]
- 14.Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003;26:1153–1157 [DOI] [PubMed] [Google Scholar]
- 15.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 7 July 2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 16.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale: an evaluation of its clinical utility. Diabetes Care 1997;20:760–766 [DOI] [PubMed] [Google Scholar]
- 18.National Diabetes Information Clearinghouse. National diabetes statistics [article online], 2011. Available from http://diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm#treatment Accessed 29 May 2011
- 19.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410–1418 [DOI] [PubMed] [Google Scholar]
- 20.Bennett WL, Wilson LM, Bolen S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. In Comparative Effectiveness Review no. 27 Rockville, MD, Agency for Healthcare Research and Quality (AHRQ), March 2011. Available from http://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productID=644 Accessed 10 November 2011 (Prepared by Johns Hopkins University Evidence-based Practice Center under contract no. 290-02-0018. AHRQ publ. no. 11-EHC038-EF)
- 21.Gross JL, Kramer CK, Leitão CB, et al. ; Diabetes and Endocrinology Meta-analysis Group (DEMA) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011;154:672–679 [DOI] [PubMed] [Google Scholar]
- 22.Welschen LMC, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 2005;28:1510–1517 [DOI] [PubMed] [Google Scholar]
- 23.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966-2004). Curr Med Res Opin 2005;21:173–184 [DOI] [PubMed] [Google Scholar]
- 24.Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther 2009;11:775–784 [DOI] [PubMed] [Google Scholar]
- 25.Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: a Bayesian meta-analysis of direct and indirect comparisons. Curr Med Res Opin 2006;22:671–681 [DOI] [PubMed] [Google Scholar]
- 26.Clar C, Barnard K, Cummins E, Royle P, Waugh N; Aberdeen Health Technology Assessment Group Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess 2010;14:1–140 [DOI] [PubMed] [Google Scholar]
- 27.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program Study. Diabetes Care 2011;34:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franciosi M, Lucisano G, Pellegrini F, et al. ; ROSES Study Group ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin: a pilot randomized clinical trial. Diabet Med 2011;28:789–796 [DOI] [PubMed] [Google Scholar]
- 29.Durán A, Martín P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes 2010;2:203–211 [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes: 2011 2011;34Suppl. 1:S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handelsman Y, Mechanick JI, Blonde L, et al. ; AACE Task Force for Developing Diabetes Comprehensive Care Plan American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 2011;17(Suppl. 2):1–53 [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Clinical Excellence. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update) [article online], 2008. Available from http://www.nice.org.uk/nicemedia/live/11983/40803/40803.pdf Accessed 2 September 2011