Abstract
OBJECTIVE
Early after Roux-en-Y gastric bypass (RYGB), there is improvement in type 2 diabetes, which is characterized by insulin resistance. We determined the acute effects of RYGB, with and without omentectomy, on hepatic and peripheral insulin sensitivity. We also investigated whether preoperative diabetes or postoperative diabetes remission influenced tissue-specific insulin sensitivity after RYGB.
RESEARCH DESIGN AND METHODS
We studied 40 obese (BMI 48 ± 8 kg/m2) participants, 17 with diabetes. Participants were randomized to RYGB alone or in conjunction with omentectomy. Hyperinsulinemic-euglycemic clamps with isotopic-tracer infusion were completed at baseline and at 1 month postoperatively to assess insulin sensitivity.
RESULTS
Participants lost 11 ± 4% of body weight at 1 month after RYGB, without an improvement in peripheral insulin sensitivity; these outcomes were not affected by omentectomy, preoperative diabetes, or remission of diabetes. Hepatic glucose production (HGP) and the hepatic insulin sensitivity index improved in all subjects, irrespective of omentectomy (P ≤ 0.001). Participants with diabetes had higher baseline HGP values (P = 0.003) that improved to a greater extent after RYGB (P = 0.006). Of the 17 participants with diabetes, 10 (59%) had remission at 1 month. Diabetes remission had a group × time effect (P = 0.041) on HGP; those with diabetes remission had lower preoperative and postoperative HGP.
CONCLUSIONS
Peripheral insulin sensitivity did not improve 1 month after RYGB, irrespective of omentectomy, diabetes, or diabetes remission. Hepatic insulin sensitivity improved at 1 month after RYGB and was more pronounced in patients with diabetes. Improvement in HGP may influence diabetes remission early after RYGB.
Of the estimated 26 million people in the U.S. with type 2 diabetes and the 79 million with prediabetes (1), ∼80% are overweight or obese. Roux-en-Y gastric bypass (RYGB) surgery for treatment of obesity leads to long-term diabetes remission in ∼80% of patients (2), whereas very limited effects are observed with lifestyle intervention (3). Improvements in diabetes are reported to occur immediately after RYGB (4), with ∼30% of patients being discharged from the hospital with discontinuation of all diabetes medications (5).
Impaired insulin sensitivity (insulin resistance) at the liver and in the periphery (primarily skeletal muscle) is an underlying mechanism of and precursor to diabetes (6). Several studies have described a long-term improvement in peripheral and hepatic insulin sensitivity 6 months to 1 year after RYGB (7–9), which is considered to occur secondary to weight loss. The mechanisms of the immediate improvement in diabetes after RYGB, before substantial weight loss, are not well delineated. Improvements in insulin sensitivity are reported early after RYGB, by hyperglycemic clamp at 1 and 4 weeks postoperatively (10) or by intravenous glucose tolerance tests (11) and homeostasis model assessment (HOMA) during the first week postoperatively (11,12). These approaches to measure insulin sensitivity, however, cannot distinguish between peripheral and hepatic insulin sensitivity. The hyperinsulinemic-euglycemic clamp method in conjunction with isotopically labeled tracer infusion allows measurement of peripheral (primarily skeletal muscle) insulin sensitivity as well as hepatic glucose production (HGP) and hepatic insulin sensitivity. Two studies have used this technique to assess insulin sensitivity early after RYGB, and interestingly, did not find a significant improvement in peripheral insulin sensitivity 2 to 4 weeks after RYGB; however, hepatic insulin sensitivity was not assessed (13,14). Recently, Camastra et al. (7) reported that peripheral and hepatic insulin sensitivity did not change at 2 weeks after RYGB in patients with and without diabetes.
Increased visceral fat is considered an important risk factor for diabetes and insulin resistance (15). We recently reported, however, that surgical removal of the greater omentum (omentectomy) in conjunction with RYGB did not augment the improvement in insulin sensitivity long-term after RYGB (8). Here we report the acute effects of RYGB with or without omentectomy on hepatic and skeletal muscle insulin sensitivity. This was assessed by hyperinsulinemic-euglycemic clamp with tracer infusion at 1 month after RYGB. We also tested the hypothesis that an early remission of diabetes after RYGB is associated with improved hepatic and peripheral insulin sensitivity.
RESEARCH DESIGN AND METHODS
Study protocol and participants
The study was approved by the Vanderbilt Institutional Review Board, and written informed consent was obtained from all participants. From March 2005 through November 2009, we recruited patients aged between 18 and 60 years from the Vanderbilt Center for Surgical Weight Loss (Nashville, TN) after approval for RYGB. Study recruitment was concluded when the analysis of primary outcome measures revealed no long-term effect of omentectomy (8). Exclusion criteria included prior gastric operations, metabolic acidosis, positive pregnancy test, or medications that can affect metabolism other than diabetes medications.
Participants were randomly assigned to RYGB, with or without omentectomy (Supplementary Fig. 1), using a computer-generated randomization code with a permuted block size of four. The study coordinator held the randomization code, and all participants and study personnel were blinded to omentectomy status. Hyperinsulinemic-euglycemic clamps with glucose tracer infusions were completed preoperatively and at 33 ± 7 days (range 21–49) postoperatively in 40 subjects (5 men and 35 women) aged 42 ± 9 years.
At 1 month after RYGB, patients were counseled by a bariatric surgery dietitian to consume a diet of pureed or soft foods with a caloric intake of ∼600 to 800 kcal/day. Subjects with type 2 diabetes discontinued oral diabetes medications and long-acting insulin 5 days before a study visit, and therapy with short-acting insulin was initiated to temporarily control hyperglycemia, if needed.
The primary outcome measure was the effect of omentectomy on hepatic and peripheral insulin sensitivity. We further analyzed the data for differential improvements in insulin sensitivity in patients with and without preoperative diabetes and the relationship between early postoperative remission of diabetes and improvements in hepatic and peripheral insulin sensitivity.
Study procedures
Participants were admitted to the Vanderbilt Clinical Research Center the evening before the study, given a standard meal, and fasted overnight. The next morning, a catheter was inserted into a forearm vein to infuse high-performance liquid chromatography purified [3-3H]glucose (PerkinElmer, Waltham, MA), potassium, dextrose, and insulin. A second catheter was inserted into a contralateral superficial forearm or hand vein, which was heated, to obtain arterialized blood samples. A primed (33 μCi), continuous (0.14 μCi/min) infusion of [3-3H]glucose was maintained for 2.5 h. Then, a primed (4 mU · kg−1 · min−1 for 8 min), continuous (2.4 mU · kg−1 · min−1) insulin infusion was maintained for 2 h. Similar plasma insulin levels were achieved during the preoperative and postoperative clamps (363.5 ± 142 and 355.3 ± 123.4 μU/mL, P = 0.656). Euglycemia (90–100 mg/dL) was maintained by a variable infusion of 20% dextrose. Blood samples were obtained during the final 30 min of the basal and insulin infusion periods to determine plasma glucose specific activity and insulin concentrations.
After completing the first clamp procedure, participants underwent open (n = 3) or laparoscopic (n = 37) RYGB surgery, with or without omentectomy, as described (8). No significant postoperative complications occurred. The study coordinator notified the surgeon of the randomization assignment at the beginning of the procedure, and 756 ± 334 g (range 400–1,675) of greater omentum was surgically removed.
Sample collection and analysis
Glucose was measured using the glucose oxidase method (Beckman Glucose Analyzer; Beckman Coulter, Inc., Fullerton, CA). Glycosylated hemoglobin (HbA1c) was determined using high-performance liquid chromatography and the Variant II Hemoglobin Testing System (Bio-Rad Laboratories Diagnostic Group, Hercules, CA). Plasma concentrations of insulin were determined by radioimmunoassay (Millipore, Billerica, MA). Plasma glucose specific activity ([3-3H]glucose/glucose) was determined by measuring glucose radioactivity in plasma on Somogyi filtrate (1:10 with 4.5% barium hydroxide and 4.5% zinc sulfate) after evaporation to remove radioactive water.
Diabetes status
Medical records for all participants were reviewed by an endocrinologist (J.P.D.), including the 1-month postoperative visit required by the Vanderbilt Center for Surgical Weight Loss. Preoperative diabetes diagnosis was based on participants’ report and medical record review. Diabetes remission was determined by 1) lack of ongoing requirement for diabetes medications (as dictated by the participants’ primary care physician or endocrinologist at the postoperative clinic visits); 2) no reports or documentations of elevated blood glucose; and 3) no elevations in HbA1c or fasting glucose on the morning of the postoperative study. A normal range HbA1c (<6.0%) was not used to determine diabetes remission, but an elevated HbA1c (≥6.5%) was used to exclude remission.
Calculations
During basal insulin conditions, HGP was calculated as tracer infusion rate/tracer specific activity. Hepatic insulin sensitivity was evaluated with the hepatic insulin sensitivity index (HISI), calculated as the inverse of the product of HGP and fasting plasma insulin concentration (16). The glucose disposal rate, or M value, was calculated as the average amount of glucose infused during the last 30 min of the clamp period and is an index of maximal muscle glucose utilization. The ratio of glucose disposal to plasma insulin (M/I) was calculated to account for variations in steady-state insulinemia.
Statistical analysis
Effect sizes were previously reported (8). Data are reported as the mean ± SD and were analyzed with IBM SPSS Statistics 18 software (IBM, Armonk, NY). Differences between groups at baseline were assessed with an independent-samples t test. The main effects of time and group and the group × time interaction for outcome measures were determined with linear mixed-effects models. Omentectomy assignment, preoperative diabetes, and postoperative diabetes remission were the group terms used in three separate models. Pearson correlations were used to determine the association between preoperative glucose disposal and the postoperative change in glucose disposal.
RESULTS
Acute impact of RYGB and omentectomy on insulin sensitivity
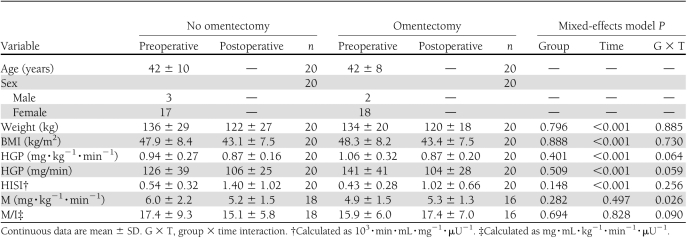
Baseline comparisons between participants who did or did not undergo omentectomy revealed no differences in weight, BMI, or any measure of glucose kinetics (Table 1). Overall, weight decreased at 1 month after surgery (11 ± 4%), but there was no additional benefit of omentectomy. HGP and HISI improved after RYGB, and we did not detect an effect of omentectomy. Overall, peripheral insulin sensitivity did not change after RYGB. There was an effect of omentectomy over time on the M value; however, both groups had similar postoperative values, and this effect was not observed with the M/I value.
Table 1.
Measurements of insulin sensitivity before and 1 month after RYGB by omentectomy assignment
Effect of a preoperative diagnosis of diabetes on post-RYGB insulin sensitivity
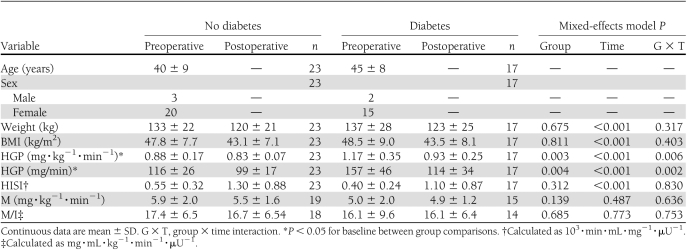
Baseline comparisons between participants with and without a preoperative diagnosis of diabetes found similar weights and BMIs, and there was no effect of preoperative diabetes on these outcomes after RYGB (Table 2). Baseline HGP was significantly higher in participants with preoperative diabetes than in those without diabetes. These two groups had different trajectories in HGP after RYGB; the decrease in HGP after surgery was greater in participants with preoperative diabetes compared with those without diabetes. Baseline HISI and M values were not significantly different between groups, and preoperative diabetes did not influence postoperative outcomes.
Table 2.
Measurements of insulin sensitivity before and 1 month after RYGB by preoperative diabetes diagnosis
Relationship between diabetes remission and insulin sensitivity after RYGB
Of the 17 participants with diabetes, preoperative therapies included (n participants) diet alone (2), insulin alone (1), insulin and other antidiabetes agents (2), one oral medication (5), and two or more antidiabetes agents preoperatively (9); noninsulin, antidiabetes agents used preoperatively included metformin (10), sulfonylureas (6), thiazolidinediones (6), and exenatide (3). In the first weeks after surgery, the participants with diabetes were only treated with insulin therapy for glycemic control; thus, no participants were discharged from the hospital on noninsulin, antidiabetes agents. Of the 17 patients with preoperative diabetes, 11 (65%) were discharged from the hospital off all diabetes medications, and the remaining 6 were discharged on insulin therapy. RYGB resulted in remission of diabetes in 10 of 17 patients (59%) within 1 month after the surgical procedure; the 3 who required insulin preoperatively still required insulin at follow-up.
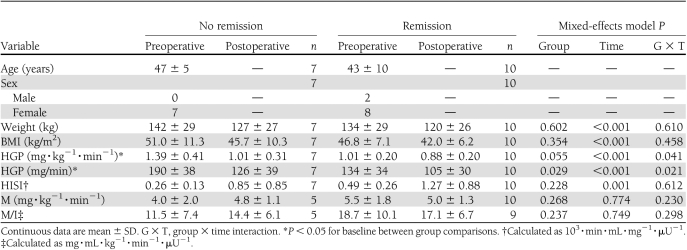
Participants with and without remission of diabetes at 1 month after RYGB were of similar age, body weight, and BMI before RYGB (Table 3). Preoperative HbA1c was 8.1 ± 1.2% in those who did not achieve remission compared with 6.2 ± 0.6% in those with remission (P < 0.001). The participants who achieved early remission of diabetes had lower preoperative HGP and HISI compared with those who did not have remission. Remission of diabetes also had an effect on HGP over time. Both groups had improvements in their HGP at 1 month. Patients who did not achieve remission had higher preoperative HGPs that decreased to a greater extent compared with those with remission (27 vs. 13%, respectively). Nonetheless, those with diabetes remission at 1 month had lower absolute HGP after surgery. Preoperative and postoperative glucose disposals were similar for participants with and without diabetes remission at 1 month after RYGB.
Table 3.
Measurements of insulin sensitivity before and 1 month after RYGB by diabetes remission
Variable responses in glucose disposal at 1 month after RYGB
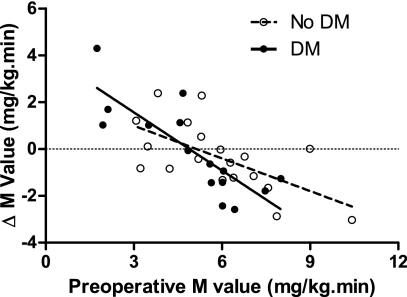
During the studies and data analyses, we observed that glucose disposal improved in some participants and worsened in others, and that the baseline glucose disposal was influencing the direction of change at 1 month. We therefore examined the relationship between preoperative glucose disposal and the degree of postoperative change. In all group comparisons, there were strong negative relationships: 1) whole cohort, r2 = 0.507, P < 0.001; 2) with omentectomy, r2 = 0.515, P = 0.002; and without omentectomy, r2 = 0.540, P = 0.001; and 3) preoperative diagnosis of diabetes, r2 = 0.644, P = 0.004; and without preoperative diagnosis of diabetes, r2 = 0.392, P < 0.001 (Fig. 1). That is, a high preoperative M value predicted a decline in the M value at 1 month after RYGB, and a low preoperative M value predicted an increase in the M value at 1 month after RYGB.
Figure 1.
Preoperative glucose disposal predicts the direction of change in glucose disposal at 1 month after RYGB in subjects with and without a preoperative diagnosis of diabetes. A high preoperative M was associated with a worsened M postoperatively and vice versa. Pearson correlation coefficients: entire cohort r2 = 0.507, P = 0.001; subjects with a preoperative diagnosis of diabetes, r2 = 0.644, P = 0.004; and subjects without a preoperative diagnosis of diabetes, r2 = 0.392, P < 0.001. DM, diabetes.
CONCLUSIONS
Rapid, clinical improvements in type 2 diabetes occur after RYGB (4,5), yet little is known regarding the relative contribution of insulin-sensitive tissues. To determine this, we measured hepatic and muscle insulin sensitivity with a hyperinsulinemic-euglycemic clamp. Our major finding was that at 1 month after RYGB, hepatic insulin sensitivity improved whereas skeletal muscle insulin sensitivity did not. In addition, hepatic and peripheral insulin sensitivity was not influenced by surgical removal of the omentum, as postoperative values were similar in the groups who did and did not undergo omentectomy. Lima et al. (13) reported that omentectomy did not affect peripheral insulin sensitivity at 1 month after RYGB, but they did not directly assess hepatic insulin sensitivity. We have previously reported that omentectomy does not influence metabolic outcomes of RYGB after long-term follow-up (8).
The rapid and sustained improvement in diabetes after RYGB has contributed to the American Diabetes Association recommending bariatric surgery as a therapeutic option (17). We report a 59% remission rate of diabetes at 1 month after RYGB. We found that a preoperative diagnosis of diabetes neither influenced preoperative nor early post-RYGB glucose disposal, supporting that peripheral tissues (predominately skeletal muscle) do not contribute to the early metabolic improvements after surgery in patients with or without diabetes. Conversely, patients with a preoperative diagnosis of diabetes had higher HGP before surgery that improved after surgery, whereas those without diabetes had limited improvements; therefore, at least in patients with diabetes, early metabolic improvements appear to be predominantly due to improved hepatic insulin sensitivity. Furthermore, the subjects who achieved diabetes remission at 1 month after RYGB had postoperative hepatic insulin sensitivity similar to subjects without diabetes, whereas those who did not have remission continued to have elevated HGP. These data support that hepatic insulin sensitivity has a role in early remission of diabetes. A limitation in these analyses is the absence of information regarding the duration of diabetes in these subjects and its potential impact on short-term remission of diabetes after RYGB. The amount of weight loss has been shown to predict diabetes remission from 3 to 24 months postoperatively (18), but we did not find an effect of weight loss on acute diabetes remission. Pancreatic β-cell function also exhibits improvement early after RYGB (9), and early improvements in diabetes may also reflect changes in β-cell function (19).
We have recently reported that the improvements in insulin sensitivity in the first week after RYGB are primarily due to caloric restriction. HOMA improved similarly in the first week after RYGB compared with equally obese subjects who underwent a matched diet (12). HOMA is based on fasting levels of glucose and insulin, and although it does correlate well with whole-body glucose disposal during a hyperinsulinemic-euglycemic clamp, it is considered a better representation of HGP and insulin secretion (20). Caloric restriction at a level similar to the first week after RYGB has been shown to decrease HGP without altered whole-body glucose disposal (21,22), and this reduction in HGP is due to reduced glycogenolysis (23). Therefore, the improvements in insulin resistance in the first week after RYGB that we (12) and others (11) have reported may reflect decreased HGP resulting from caloric restriction. Only one other study measured acute hepatic responses to RYGB (2 weeks) and found no change (7).
We determined muscle insulin sensitivity with high insulin levels that completely suppress HGP (24), allowing us to show that, overall, skeletal muscle insulin sensitivity does not improve at 1 month after surgery; greater weight loss is likely needed for this effect (8,14). We achieved similar insulin levels during insulin infusions before RYGB and at 1 month after RYGB, supporting that a lack of change in glucose disposal is not due to differences in steady-state insulin concentrations from baseline to post-RYGB. This is consistent with other reports that glucose disposal is not altered early after RYGB (7,13,14); these studies also used hyperinsulinemic-euglycemic clamps but at a lower insulin dose (40 mU · m−2 · min−1) than our study. Interestingly, Kashyap et al. (10) describes an increase in the M/I value during a hyperglycemic clamp at 1 month after RYGB. Although this method is generally used to assess insulin secretion and β-cell function, insulin sensitivity measures have been shown to correlate with the hyperinsulinemic-euglycemic clamp (25). Caloric restriction has been shown to have an inconsistent effect on glucose disposal in obese subjects (21,22,26). Perhaps greater weight loss is necessary to achieve a consistent improvement in peripheral insulin sensitivity after RYGB (8,14).
We found that the acute effect of RYGB on peripheral insulin sensitivity was variable among individuals, similar to the observation by Lima et al. (13). Because of our large sample size (n = 40), we were also able to notice that the preoperative glucose disposal value predicts the direction of change after surgery. Subjects who were the most insulin resistant before surgery improved postoperatively, whereas those with higher preoperative insulin sensitivity worsened after surgery. This relationship was strong in participants with and without diabetes and cannot be attributed to incomplete medication withdrawal in those with diabetes. Furthermore, the degree of weight loss was not associated with postoperative changes in peripheral insulin sensitivity. Although major improvements in peripheral insulin sensitivity are likely related to weight loss (14), whole-body glucose disposal has been reported to decrease in lean subjects (27) and to be unaffected (21,22) or increased in obese subjects (26) with short-term caloric restriction. Thus, the most insulin-sensitive obese individuals in our cohort may be responding to RYGB similarly to calorically restricted lean individuals by decreasing glucose disposal. Although our data support that the response of peripheral insulin sensitivity to caloric restriction/moderate weight loss is dependent on baseline insulin sensitivity, the verification and relevance of these findings requires further study in an independent cohort.
Our cumulative work supports caloric restriction as a mediator of early metabolic improvements after RYGB, and reducing visceral fat by omentectomy is not beneficial acutely or long-term after RYGB. At this early time after surgery, skeletal muscle insulin sensitivity did not improve overall or influence remission of diabetes. Importantly, we report that at 1 month after RYGB, glucose production decreased and hepatic insulin sensitivity improved, which emerges as a potential mechanism for early diabetes remission.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants UL1-RR-024975 from the National Center for Research Resources (Vanderbilt Clinical and Translational Science Award), DK-20593 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; Vanderbilt Diabetes Research and Training Award), DK-058404 from the NIDDK (Vanderbilt Digestive Disease Research Center), K12-ESO15855 from the National Institute of Environmental Health Sciences (Vanderbilt Environmental Health Science Scholars Program) to J.P.D., and DK-70860 from the NIDDK to N.N.A.
No potential conflicts of interest relevant to this article were reported.
J.P.D. acquired data, analyzed and interpreted data, wrote the manuscript, and critically revised and approved the manuscript. N.N.A. obtained funding; conceived of, directed, and supervised studies; interpreted data; and critically revised and approved the manuscript. I.B. acquired data, analyzed and interpreted data, and critically revised and approved the manuscript. P.A.M.-S. acquired data, provided administrative support, and critically revised and approved the manuscript. C.R.F. and K.J. acquired data and critically revised and approved the manuscript. I.D.F. performed statistical analysis and critically revised and approved the manuscript. R.A.T. acquired data, directed and supervised studies, analyzed and interpreted data, wrote the manuscript, critically revised and approved the manuscript, and is the guarantor of the study.
The authors appreciate the participants who volunteered for this study and thank the following Vanderbilt colleagues for their assistance with this study: Erik N. Hansen, James M. Isbell, and Jabbar Saliba for clinical support and intellectual contributions; Marcy Buckley, Joan Kaiser, and the Vanderbilt Clinical Research Center nursing staff for outstanding nursing support; and Phillip Williams, Nadine Saliba, and Ed Yoo for invaluable technical support.
Footnotes
Clinical trial reg. no. NCT00212160, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1383/-/DC1.
References
- 1.National Diabetes Education Program. National diabetes fact sheet: general information and national estimates on diabetes in the United States [Internet], 2007. Bethesda, MD, Centers for Disease Control and Prevention. Available from: http://ndep.nih.gov/diabetes-facts/index.aspx Accessed 8 April 2011
- 2.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256 [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 4.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350; discussion 350–332 [DOI] [PMC free article] [PubMed]
- 5.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467–484; discussion 484–485 [DOI] [PMC free article] [PubMed]
- 6.Gastaldi G, Russell A, Golay A, et al. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia 2007;50:2348–2355 [DOI] [PubMed] [Google Scholar]
- 7.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010;139:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009;58:693–700 10.2337/db08-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on [beta]-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2019;34:462–471 [DOI] [PMC free article] [PubMed]
- 11.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481 [DOI] [PubMed] [Google Scholar]
- 12.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima MM, Pareja JC, Alegre SM, et al. Acute effect of Roux-en-Y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3871–3875 10.1210/jc.2010-0085 [DOI] [PubMed] [Google Scholar]
- 14.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010;14:15–23 10.1007/s11605-009-1060-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl. 1):16S–19S 10.1038/oby.2006.277 [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 10.2337/dc11-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis 2009;5:305–309 10.1016/j.soard.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 19.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372–E1379 10.1210/jc.2011-0446 [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 21.Jazet IM, Pijl H, Frölich M, Romijn JA, Meinders AE. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism 2005;54:705–712 10.1016/j.metabol.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 22.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552–1560 10.1053/j.gastro.2009.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christiansen MP, Linfoot PA, Neese RA, Hellerstein MK. Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes 2000;49:1691–1699 10.2337/diabetes.49.10.1691 [DOI] [PubMed] [Google Scholar]
- 24.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630–E639 [DOI] [PubMed] [Google Scholar]
- 25.Mitrakou A, Vuorinen-Markkola H, Raptis G, et al. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemia clamp. J Clin Endocrinol Metab 1992;75:379–382 10.1210/jc.75.2.379 [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993;77:1287–1293 10.1210/jc.77.5.1287 [DOI] [PubMed] [Google Scholar]
- 27.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest 1995;96:2735–2743 10.1172/JCI118342 [DOI] [PMC free article] [PubMed] [Google Scholar]