Abstract
OBJECTIVE
Physical activity or metformin enhances insulin sensitivity and opposes the progression from prediabetes to type 2 diabetes. The combination may be more effective because each treatment stimulates AMP-activated protein kinase activity in skeletal muscle. We evaluated the effects of exercise training plus metformin on insulin sensitivity in men and women with prediabetes, compared with each treatment alone.
RESEARCH DESIGN AND METHODS
For 12 weeks, men and women with prediabetes were assigned to the following groups: placebo (P), 2,000 mg/day metformin (M), exercise training with placebo (EP), or exercise training with metformin (EM) (n = 8 per group). Before and after the intervention, insulin sensitivity was measured by euglycemic hyperinsulinemic (80 mU/m2/min) clamp enriched with [6,6-2H]glucose. Changes due to intervention were compared across groups by repeated-measures ANOVA.
RESULTS
All three interventions increased insulin sensitivity (P < 0.05) relative to the control group. The mean rise was 25–30% higher after EP than after either EM or M, but this difference was not significant.
CONCLUSIONS
Insulin sensitivity was considerably higher after 12 weeks of exercise training and/or metformin in men and women with prediabetes. Subtle differences among condition means suggest that adding metformin blunted the full effect of exercise training.
Before developing overt diabetes, most individuals spend years in an intermediate condition called prediabetes. Prediabetes is defined by impaired glucose tolerance (IGT), impaired fasting glucose (IFG), or the combination of IGT plus IFG (1). Approximately 79 million individuals in the U.S. have prediabetes and are at risk to develop type 2 diabetes (2). The progression is not inevitable, however. The U.S. Diabetes Prevention Program (DPP) demonstrated that either lifestyle modification (i.e., low-fat diet and increased physical activity) or the antihyperglycemic medication metformin reduced the transition from prediabetes to type 2 diabetes (3).
Habitual exercise and metformin each increase peripheral (mainly skeletal muscle) insulin sensitivity in part by stimulating AMP-activated protein kinase (AMPK) (4–8). Combining exercise plus metformin, compared with either treatment alone, may more effectively activate the key regulatory enzyme AMPK and oppose the transition from prediabetes to type 2 diabetes.
The American Diabetes Association strongly recommends exercise as a cornerstone therapy for diabetes prevention and, recently, suggested that some individuals with prediabetes be considered for metformin treatment (9,10). The efficacy of combining lifestyle modification with metformin has been tested only a few times (11–15). Results suggest 2–5 kg more weight loss with the addition of metformin compared with lifestyle modification alone (11,12), but little (11,14) or no further (15,16) improvement to insulin sensitivity. However, the use of self-reports to estimate physical activity and surrogates (via fasting glucose and insulin concentrations or responses to oral carbohydrate) (11,13–15) rather than direct measurement of insulin sensitivity using the glucose clamp limits our understanding of the interaction between exercise and metformin. There is considerable need to better understand the potential for additive effects when physical activity and metformin are used concurrently because the scope of the public health problem is so pressing. Therefore, the purpose of this study was to determine the effect of combining exercise training with metformin (EM) on insulin sensitivity in individuals with prediabetes, compared with either treatment alone.
RESEARCH DESIGN AND METHODS
Overall summary
Subjects were recruited from the local community via flyers and newspaper advertisements. A total of 32 otherwise healthy individuals with IGT were assigned to placebo (P), metformin (M), exercise training with placebo (EP), or EM (n = 8 per group). Insulin sensitivity was measured by glucose clamp and stable isotope tracer before and after 12 weeks of each intervention.
Subjects
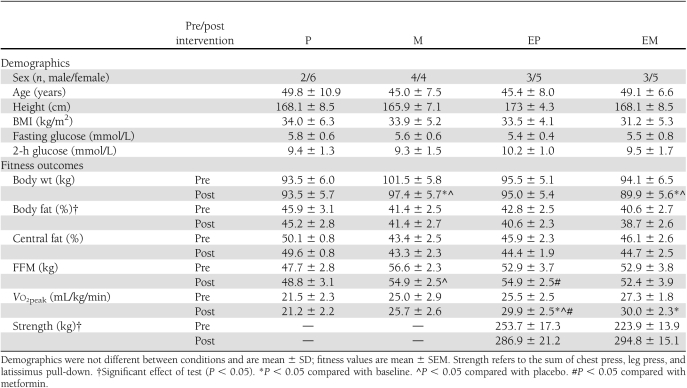
Individuals were nonsmoking, weight stable (<5% weight change over last 3 months), free of cardiovascular disease or diabetes (i.e., chronic illness) or any contraindications to metformin (e.g., respiratory disease, heart failure, or renal or hepatic disease), and not taking dietary supplements (e.g., chromium, niacin) or medications (glucocorticoids, oral contraceptives, sulfonylureas, etc.) likely to affect insulin sensitivity. Subject characteristics are outlined in Table 1. Reflecting the racial/ethnic composition of the local community, 27 individuals were Caucasian, 3 were African American, and 2 were Hispanic. Pre- and postmenopausal women were included in each condition. The number of premenopausal (n = 8) and postmenopausal (n = 12) was balanced across conditions. Premenopausal women were tested before and after the intervention in the same phase of the menstrual cycle (3 in the follicular and 5 in the luteal). Prior to testing, subjects were verbally briefed about the study and signed informed consent documents approved by the institutional review board at the University of Massachusetts Amherst.
Table 1.
Demographics and fitness
Screening
An oral glucose tolerance test was used to determine if potential participants met the inclusion criterion of IGT. After a minimum 5-h fast, blood samples were taken from a forearm vein. Subjects consumed 75 g of glucose and blood was collected 2 h later. Participants with 2-h glucose concentrations between 7.8 and 11.1 mmol/L (140–199 mg/dL), the definition of IGT, were included. Subjects with IFG, defined as fasting glucose concentration between 5.5 and 6.9 mmol/L (100–125 mg/dL), as well as IGT were also included (IFG plus IGT).
Metformin or placebo protocol
In a double-blind design, pills were distributed and subjects were instructed to take metformin or placebo with food to minimize potential side effects. Subjects started treatment with 500 mg/day of metformin. The dose was increased 500 mg/day each week until a clinical dose of 2,000 mg/day was reached by week 4. Subjects remained at this dose for the last 8 weeks of the 12-week protocol. Subjects were instructed to return any pills missed to verify compliance, which was >90% for all conditions.
Preliminary testing
Peak oxygen consumption (Vo2peak) was determined using a continuous progressive exercise test on a cycle ergometer (SensorMedics 800, Yorba Linda, CA). Vo2peak was defined as the highest value obtained during the test using common criteria (i.e., respiratory exchange ratio >1.1, heart rate within 15 bpm of age-predicted maximum, and rise in Vo2 <150 mL/min despite increased workload). In addition, one-repetition maximum (1-RM) tests were conducted for the chest press, latissimus pull-down, leg press, bicep curl, triceps push-down, and upright row. The 1-RM was defined as the highest weight lifted with proper technique through the full range of motion. Dual-energy X-ray absorptiometry (Lunar Prodigy, Madison, WI) was used for determination of body fat, central fat (i.e., from the last floating rib to the top of the iliac crest divided by total body fat mass), and fat-free mass (FFM) (17).
Euglycemic hyperinsulinemic clamp
Subjects were provided food (55% carbohydrate, 30% fat, and 15% protein) 24 h prior to pre- and posttesting. After an overnight fast, subjects reported to the laboratory and indwelling catheters were placed in a superficial vein of each forearm. Baseline blood samples were collected and a priming bolus of 200 mg [6,6-2H]glucose was given followed by a 90-min infusion of [6,6-2H]glucose at 3.0 mg/min by peristaltic infusion pump (Harvard Apparatus Pump 22; Harvard Apparatus, Holliston, MA). Blood samples were collected at 75 and 90 min. Expired breath samples were collected between 80 and 90 min, with the last 2 min used to estimate substrate oxidation. A primed (250 mU/m2/min) constant infusion (80 mU/m2/min) of insulin diluted in saline containing 4% (v/v) of the subject’s own serum was given for 20 min. Next, a 20% glucose solution containing 2% [6,6-2H]glucose was infused at a variable rate to maintain plasma glucose at 5 mmol/L for the remaining 100 min. Blood samples were collected every 5 min for glucose analysis and every 15 min for analysis of insulin, C-peptide, stable isotope enrichment, and nonesterified fatty acids (NEFAs). Expired breath samples were collected between minutes 110 and 120 for determination of insulin-stimulated glucose oxidation. Approximately 28 h before postintervention measurements, subjects in the training groups (EP and EM) exercised on a cycle ergometer at 75% of their pretraining peak heart rate for 45 min. The exercise intensity was not different between EP and EM (mean heart rate for EP = 125.4 ± 4.7 bpm, for EM = 122.2 ± 4.6; P = 0.64).
Exercise training
Exercise was supervised 3 days a week for 60–75 min per session. Subjects performed aerobic and resistance exercise on the 1st and 3rd day of each week. To minimize muscle soreness, only aerobic training was performed on the 2nd day. Participants warmed up on a cycle ergometer for 5 min, followed by cycling at 70% of their pretraining heart rate peak for 45 min. Resistance exercise was performed at 70% of the subject’s 1-RM. Weight was increased ∼5% when two sets of 12 repetitions could be lifted with proper form. Resistance training targeted all major muscle groups noted earlier.
Blood sample collection
Blood was collected in 3-mL syringes, transferred to vacutainers, spun at 3,000 rpm, and aliquoted to cryotubes for storage at −80°C. Samples for analysis of glucose isotopic enrichment, glucose, and lactate contained sodium fluoride to inhibit glycolysis, and those for analysis of insulin, C-peptide, and NEFAs contained the anticoagulant EDTA.
Analysis of metabolites and hormones
Plasma glucose and lactate concentrations were determined enzymatically using glucose oxidase and lactate dehydrogenase assays (GL5 Analyzer; Analox Instruments, Lunenberg, MA). Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Millipore, St. Charles, MO). Plasma NEFA concentrations were measured by enzymatic colorimetry (Wako Chemicals, Richmond, VA). Glucose isotopic enrichment was measured by high-performance liquid chromatography and mass spectrometry as previously described (18).
Calculations
Standard equations were used to determine glucose Ra and Rd (19). Insulin sensitivity was defined as the Rd per unit plasma insulin during the final 30 min of the clamp. Ra or basal hepatic glucose production (HGP) was averaged during minutes 75 and 90 of the resting isotope infusion. Endogenous HGP during the clamp was defined as the difference between HGPclamp and the exogenous glucose infusion rate. The suppression of HGP was defined as [1 − (HGPclamp/HGPfast) × 100%] and used to provide an estimate of hepatic insulin sensitivity. Insulin-stimulated suppression of NEFAs was defined as [1 − (NEFAclamp/NEFAfast) × 100%]. Carbohydrate oxidation was determined by indirect calorimetry using standard equations (20). Nonoxidative glucose disposal (NOGD) was calculated during the final 30 min of the clamp [NOGD (mg/min) = Rd − rate of carbohydrate oxidation].
Statistical analysis
Group means were compared using the R statistical software package (version 2.4.0 [2006]; The R Foundation, Vienna, Austria). Baseline characteristics were compared across groups with a one-way ANOVA. There was no statistical difference in any baseline outcome variable. Outcomes were assessed using a two-way (group by test) repeated-measures ANOVA. Although baseline insulin sensitivity was not statistically different between conditions, there were subtle differences across groups. To account for the influence of subtle (nonsignificant) differences in baseline insulin sensitivity on the magnitude of change, we included it as a covariate. To assess the impact of weight loss and changes in Vo2peak on insulin sensitivity, we also ran the analysis using those factors as covariates. When there was a significant interaction, Tukey post hoc analysis was used to determine differences between groups and paired t tests were used to compare within-group means. Pearson correlation coefficient was used to examine relationships. Significant differences were accepted as α ≤ 0.05.
RESULTS
Anthropometrics and cardiorespiratory fitness
M and EM groups reduced body weight by ∼4 kg compared with P (P < 0.05) and EP (P = 0.07) groups (Table 1). Although both EM and EP reduced body fat (P < 0.01), there was no effect on central fat (P = 0.056) (Table 1). EP increased FFM compared with P (P < 0.02) (Table 1). Only EM and EP increased Vo2peak (P < 0.05) (Table 1).
Fasted state
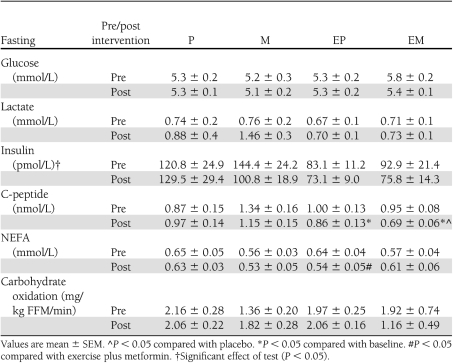
Fasting insulin concentrations were 13–25% lower after all three interventions (P < 0.05) with no differences among the three interventions. Both EM and EP lowered fasting C-peptide (P < 0.05) (Table 2), but M and P did not. Fasting glucose concentrations and carbohydrate oxidation did not change after any treatment (Table 2).
Table 2.
Fasting hormones, metabolites, and substrate use
Peripheral insulin sensitivity and glucose disposal
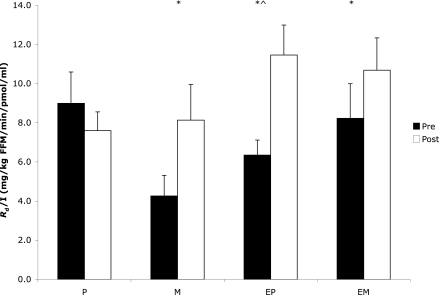
Relative to baseline, M, EP, and EM enhanced insulin sensitivity (P < 0.05) (Fig. 1) and NOGD (P < 0.05) (Table 3). There was no effect of treatment on insulin-stimulated carbohydrate oxidation.
Figure 1.
Insulin (I) sensitivity across conditions. Values are mean ± SEM. *P < 0.05 compared with baseline. ^P < 0.05 compared with placebo.
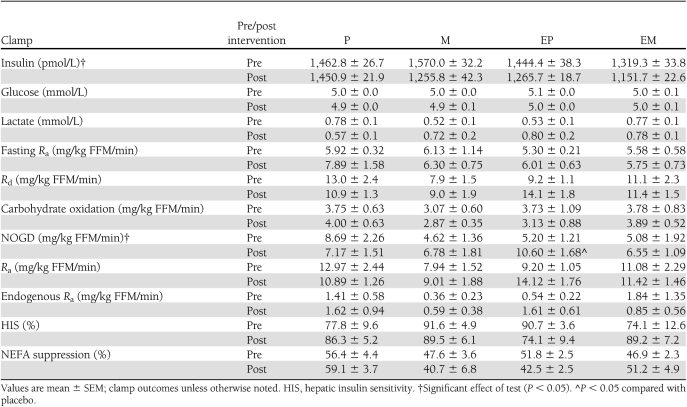
Table 3.
Glucose metabolism, hormones, and metabolites
HGP and insulin sensitivity
HGP in the fasted state (HGPfast) did not change after any treatment (Table 3). Hepatic insulin sensitivity, defined as suppression of HGPfast during the clamp, also was unaffected (Table 3).
Suppression of NEFAs
NEFA concentrations in the fasting state decreased with EP but increased with EM (P < 0.02) (Table 3). Insulin-stimulated suppression of NEFAs, however, did not change after any treatment (Table 3).
Correlations
Increased NOGD (r = 0.85, P < 0.01), Vo2peak (r = 0.57, P < 0.05), and weight loss (r = −0.42, P < 0.05) were correlated with greater insulin sensitivity. Elevated fasting FFA concentrations were correlated with attenuated insulin sensitivity (r = −0.42, P < 0.05).
CONCLUSIONS
Contrary to our original hypothesis, adding metformin did not accentuate the effects of exercise training on whole-body insulin sensitivity in this group of men and women with prediabetes. Consistent with our prior study of acute exercise (18), the addition of metformin may have blunted the response to exercise training alone. In prior studies, combining lifestyle modification with metformin has had inconsistent effects. Atabek and Pirgon (11) reported that lifestyle modification plus metformin reduced BMI, fasting hyperinsulinemia, and 2-h plasma insulin concentrations in obese adolescents compared with lifestyle modification alone. Love-Osborne et al. (12) demonstrated that lifestyle modification plus metformin resulted in more weight loss than lifestyle modification alone, and the weight loss was correlated with lower 2-h blood glucose concentrations. In the Indian Diabetes Prevention Program (IDPP), 500 mg/day of metformin, lifestyle modification, and a combination of the two had equivalent effects to improve insulin sensitivity and reduce the progression from prediabetes to type 2 diabetes (16). The inconsistency in the literature may be related to the outcomes used. In the four studies (including ours) that report no additive effects of metformin and exercise, insulin sensitivity is a key outcome. In the studies that do suggest additive effects, weight loss and a response to an oral glucose tolerance test are the key outcomes. Differences in the outcomes between the studies are also likely related to the effects of metformin and exercise on different tissues. Insulin sensitivity is primarily a measure of skeletal muscle glucose uptake, whereas glucose tolerance also reflects contributions from hepatic glucose output.
There are several potential explanations for the lack of additive effects when metformin and exercise were combined in this study. Metformin is a mild inhibitor of Complex I in the mitochondrial electron transport chain (21) and, thus, it is possible that metformin constrains the cellular adaptations to training. Although we cannot address mitochondrial adaptations to training in this study, there were no statistical differences in Vo2peak after training with or without metformin.
We previously found that short-term metformin treatment blunted the upregulation of AMPK and insulin sensitivity after one bout of moderate-intensity exercise (18). If habitual treatment with metformin does not enhance stimulation of AMPK by training, we would expect few additive effects (22). Direct measurements of AMPK activity will be required to test this hypothesis. Gain of FFM may raise insulin sensitivity, although this observation is not universal (5). Metformin increased FFM in adolescent girls treated during puberty (23), but in the current study, FFM was unaffected by metformin and slightly higher after training alone. Scaling insulin-mediated glucose uptake to FFM did not alter the results, implying that differences between conditions were not attributable to small variations in FFM.
On the basis of results from the DPP and other studies (24), there is a strong relationship between weight loss and greater insulin sensitivity. In the current study, EM and M resulted in 4 kg of weight loss, whereas EP had no effect. Despite the differences in total weight loss, however, both EM and EP lost similar quantities of body fat and “central” fat as estimated from dual-energy X-ray absorptiometry. There is a clear relationship between reduced body fat, especially central visceral fat, and greater insulin sensitivity (25). Equivalent losses of body fat may partly explain why both EM and EP had impressive rises to insulin sensitivity despite no weight loss in the EP group. In our prior study, the addition of metformin to a single bout of exercise raised the plasma concentrations of NEFA, which could have blunted insulin sensitivity (18). In the current study, however, adding metformin to training did not raise NEFA concentrations during the clamp (data not shown), although they were higher in the fasted state. It is possible that elevated fasting NEFA concentrations may be related to the blunted rise in insulin sensitivity when metformin was combined with training.
Although not statistically significant (P ≤ 0.50), the 25–30% blunting effect of adding metformin to training versus M or EP (Fig. 1) has potential clinical relevance and is worth considering. Inclusion of subjects with different subtypes of prediabetes may have increased interindividual variability and obscured a true difference between conditions (1). Like the individuals in the DPP, we included men and women with prediabetes who had IGT, with or without IFG concentrations. The response to all three interventions was accentuated in the individuals with fasting hyperglycemia (i.e., those with IFG plus IGT compared with those who had IGT only) (Supplementary Fig. 1). On the basis of these results and similar outcomes in larger studies (J.M. Hagberg, personal communication), it would be fruitful to study the effects of exercise and/or metformin in the different subgroups of prediabetes to better understand the effects of fasting hyperglycemia.
In summary, exercise training increased insulin sensitivity in individuals with prediabetes. Adding metformin to training did not accentuate improvements in insulin sensitivity, and it may have blunted the full effects of training. The results we observed were independent of weight loss and not explainable by any difference in the effects of training on cardiorespiratory fitness in the presence of metformin. Although acute bouts of exercise can increase insulin sensitivity for up to 48 h, the time-course effects of the combined treatment remain unclear. Despite the lack of additive effects on insulin sensitivity, combining metformin with training, as described in the recent American Diabetes Association clinical recommendations (9), may still be a potentially useful strategy to prevent the transition from prediabetes to diabetes. Further work is required to understand the utility of combining metformin with training in regard to its impact on insulin sensitivity and other aspects of cardiometabolic health.
Acknowledgments
B.B. has received a grant from the National Institutes of Health (5-R56-DK-081038).
No potential conflicts of interest relevant to this article were reported.
S.K.M. contributed to the study design and data collection, was primarily responsible for data analysis and statistical integrity, and wrote the manuscript. R.G. collected data and contributed to data analysis. S.R.C. contributed to the study design and data collection and reviewed and edited the manuscript. B.B. contributed to the study design and data collection and wrote the manuscript.
Parts of this study were presented in abstract form at the 2009 ACSM Conference on Integrative Physiology of Exercise, Miami, Florida, 22–25 September 2009.
The authors thank Kirsten Granados and Richard Viskochil of the Energy Metabolism Laboratory for technical assistance and helpful discussion. The authors also thank John Staudenmeyer, University of Massachusetts, for statistical consulting and the dedicated undergraduate research assistants and participants for their effort.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0925/-/DC1.
References
- 1.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep 2009;9:193–199 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet, 2011[Internet]. Atlanta, GA, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed 13 February 2010.
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol 1993;264:E855–E862 [DOI] [PubMed] [Google Scholar]
- 5.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care 1998;21:1353–1355 [DOI] [PubMed] [Google Scholar]
- 6.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002;51:2074–2081 [DOI] [PubMed] [Google Scholar]
- 7.Musi N, Fujii N, Hirshman MF, et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 2001;50:921–927 [DOI] [PubMed] [Google Scholar]
- 8.Lehtovirta M, Forsén B, Gullström M, et al. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabet Med 2001;18:578–583 [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee MK, Herrick K, Ziemer DC, et al. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2010;33:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab 2008;21:339–348 [DOI] [PubMed] [Google Scholar]
- 12.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr 2008;152:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 14.Clarson CL, Mahmud FH, Baker JE, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine 2009;36:141–146 [DOI] [PubMed] [Google Scholar]
- 15.Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril 2011;95:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snehalatha C, Mary S, Selvam S, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme-1 (IDPP-1). Diabetes Care 2009;32:1796–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CJ, Wu CH, Lu FH, Wu JS, Chiu NT, Yao WJ. Discriminating glucose tolerance status by regions of interest of dual-energy X-ray absorptiometry. Clinical implications of body fat distribution. Diabetes Care 1999;22:1938–1943 [DOI] [PubMed] [Google Scholar]
- 18.Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 2010;298:E815–E823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. New York, Wiley-Liss, 1992 [Google Scholar]
- 20.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 1991;16:23–29 [PubMed] [Google Scholar]
- 21.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607–614 [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 1999;87:1990–1995 [DOI] [PubMed] [Google Scholar]
- 23.Ibáñez L, Potau N, Ferrer A, Rodriguez-Hierro F, Marcos MV, De Zegher F. Anovulation in eumenorrheic, nonobese adolescent girls born small for gestational age: insulin sensitization induces ovulation, increases lean body mass, and reduces abdominal fat excess, dyslipidemia, and subclinical hyperandrogenism. J Clin Endocrinol Metab 2002;87:5702–5705 [DOI] [PubMed] [Google Scholar]
- 24.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000;133:92–103 [DOI] [PubMed] [Google Scholar]
- 25.Després JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ 2001;322:716–720 [DOI] [PMC free article] [PubMed] [Google Scholar]