Abstract
OBJECTIVE
Elevated glucose levels are common after an acute myocardial infarction (AMI) and increase the risk of death. Prior trials of glucose control after AMI have been inconsistent in their ability to lower glucose levels and have reported mixed effects on mortality. We developed a paper-based glucose-lowering algorithm and assessed its feasibility and safety in the setting of AMI.
RESEARCH DESIGN AND METHODS
A total of 287 participants with an acute ST segment elevation myocardial infarction (STEMI) and a capillary glucose level ≥8.0 mmol/L were randomly allocated to glucose management with intravenous glulisine insulin using this algorithm in the coronary care unit (CCU), followed by once-daily subcutaneous insulin glargine for 30 days versus standard glycemic approaches. The primary outcome was a difference in mean glucose levels at 24 h. Participants were followed for clinical outcomes through 90 days.
RESULTS
At 24 h, the mean glucose level was 1.41 mmol/L (95% CI 0.69–2.13) lower in the insulin (6.53 vs. 7.94 mmol/L). Differences in glucose levels were maintained at 72 h and 30 days. A total of 22.7% of the insulin group versus 4.4% of the standard group had biochemical hypoglycemia (with neither signs nor symptoms) in the CCU because of lower glycemic goals. However, there were no differences in symptomatic hypoglycemia or clinical outcomes between the groups.
CONCLUSIONS
A paper-based insulin algorithm targeting glucose levels of 5.0–6.5 mmol/L (90–117 mg/dL) can be feasibly implemented in the CCU. A cardiovascular outcomes trial using this approach can determine whether targeted glucose lowering improves patient outcomes.
Large surveys in several countries have shown that approximately two-thirds of people admitted to a coronary care unit (CCU) have impaired fasting glucose, impaired glucose tolerance, newly detected diabetes, or established diabetes (1,2). Prospective studies have also consistently reported that these dysglycemic patients have a higher mortality and in-hospital complications than euglycemic patients and that mortality is correlated to the degree of dysglycemia (3–8). Furthermore, patients with acute myocardial infarction (AMI) whose glucose levels normalize after admission experience lower mortality than patients with persistent hyperglycemia. These epidemiologic relationships suggest that strategies to lower glucose levels in the setting of an AMI may reduce mortality (4). However, clinical trials that targeted glucose control with intravenous insulin in the CCU (with or without postdischarge insulin therapy) have yielded mixed results to date, with some studies suggesting a strong clinical benefit and others suggesting no benefit (9–14). Reasons for this heterogeneity remain unclear and have been attributed to a number of possibilities. These include the following: 1) different approaches to glucose lowering; 2) varying glucose targets; 3) inability to clearly achieve a substantial contrast in glucose concentrations between the study groups; 4) heterogeneous study designs; 5) underrecruitment; and 6) the absence of a simple, easily implementable, reproducible approach to glucose control in the CCU (13). Despite this heterogeneity, a meta-analysis of clinical trials in which insulin therapy was used to target lower glucose levels suggested that there may be a mortality benefit of such an approach in the CCU (15). These findings suggest that a simple, safe, and easily implemented approach to target normal glucose levels in the CCU may have clinically important benefits.
To address these challenges, we developed an insulin-based approach that targeted normal glucose levels of 5.0–6.5 mmol/L (90–117 mg/dL) while minimizing hypoglycemia. This approach comprised a simple intravenous glulisine insulin (rapid-acting insulin) algorithm (i.e., an algorithm that did not require any calculations or a computer to implement) while the patient was in the CCU, followed by once-daily subcutaneous glargine insulin (long-acting insulin) for up to 30 days after randomization. We then tested the feasibility, safety, and effectiveness of this approach in the AMI setting in the RECREATE (REsearching Coronary REduction by Appropriately Targeting Euglycemia) pilot trial.
RESEARCH DESIGN AND METHODS
The RECREATE pilot trial enrolled patients with an AMI, who presented to the hospital within 24 h of onset of ischemic symptoms; had either ST segment elevation (≥1 mm) in two or more contiguous leads or a new left bundle branch block on initial electrocardiogram; and had a blood glucose level of ≥8.0 mmol/L (144 mg/dL) at presentation to the hospital. Patients with or without diabetes were eligible for randomization, provided that they were not taking or requiring insulin therapy at the time of hospital presentation. Exclusion criteria included the following: type 1 diabetes, history of severe hypoglycemia within the past 2 years, known or suspected end-stage liver disease, cardiogenic shock at presentation, documented pregnancy, life expectancy of <90 days, anticipated poor adherence or loss to follow-up, and prior enrollment in this trial or current enrollment in another trial of AMI.
Study protocol
All patients were managed according to the best judgment of their treating physician, including both medical therapy and interventions (primary percutaneous coronary intervention [PCI] and/or thrombolytic therapy). In addition, consenting participants were randomly allocated in a 1:1 fashion to either receive or not receive open-label intensive insulin therapy. Allocation was achieved using a central computerized system. The insulin arm comprised the immediate initiation of a rapid-acting insulin (glulisine/Apidra) infusion that was titrated to target glucose levels between 5.0 and 6.5 mmol/L (90 and 117 mg/dL) while in the CCU. Once transferred to the ward, insulin arm participants were started on once-daily insulin (glargine/Lantus) injections to target a fasting fingerstick glucose level between 4.0 and 5.5 mmol/L (72 and 99 mg/dL). In the standard therapy arm, treating physicians were free to add insulin therapy if they felt it was warranted to treat high glucose levels. All participants had plasma glucose levels measured at 10, 24, 48, and 72 h after admission, on the day of discharge from the hospital, and at 30 days.
At discharge from the hospital, participants in the insulin arm were continued on once-daily glargine until 30 days postrandomization and were instructed on the use of glucose meters, self-administration of insulin, and recognition and treatment of hypoglycemia. Participants monitored fingerstick glucose values fasting daily and when symptomatic with hypoglycemia. Participants were also given a diary to record daily fasting fingerstick values, hypoglycemia, and any symptoms of concern. Participants in both arms were counseled regarding healthy cardiovascular lifestyle modifications (dietary and physical activity) before hospital discharge. Participants were seen 30 days after randomization and were contacted at 90 days after randomization to ascertain vital status.
Insulin algorithm
Insulin arm participants were started on an intravenous infusion of glulisine insulin mixed in normal saline. The insulin infusion rate was adjusted using a paper-based insulin algorithm (Supplementary Data) that suggested insulin doses based on both the participants’ current glucose level and the change in glucose level in response to therapy. The insulin infusion was continued throughout the participant’s stay in the CCU. This insulin algorithm required minimal calculations and fingerstick glucose measurements every 1–4 h. It was derived from a more complex algorithm that required more frequent blood glucose monitoring and that was previously demonstrated to be safe and effective in the setting of an AMI (16).
When transferred to the ward, participants were transitioned to long-acting subcutaneous insulin, as per a suggested glargine algorithm. The initial dose of glargine insulin was determined by calculating the total dose of intravenous glulisine insulin used within the preceding 24 h and reducing this total dose by 10% or more, as per the judgment of the treating physician. Further increments or decrements of 1–2 units/day in the glargine dose were made until the participant’s fasting blood glucose levels were at target (4.0 and 5.5 mmol/L [72 and 99 mg/dL]). The glargine dose was adjusted by the research team daily while in the hospital, at discharge, at 2 weeks after discharge, and more frequently at their discretion.
Outcomes
The primary outcome for this pilot trial was the difference in mean venous plasma glucose levels between the two study groups at 24 h. Secondary outcomes included the following: the difference in mean venous plasma glucose between the study groups at hospital discharge or 7 days (whichever came first) and 30 days, clinical cardiovascular outcomes, mortality, and rehospitalization. Hypoglycemia, hypokalemia (potassium level <3.5 mmol/L or mEq/L), and any serious adverse events were documented. All outcomes were adjudicated blinded to the treatment allocation. Laboratory values were measured locally at the participating centers.
Data were collected using standardized case report forms by local investigators and were managed through the National Coordinating Offices for each country and the Global Coordinating Project Office (Population Health Research Institute [PHRI] at McMaster University, Hamilton, Ontario, Canada). An independent data safety monitoring board monitored clinical outcomes and safety end points throughout the trial. In addition, a glucose monitoring committee analyzed the glucose data at specific time intervals to ensure the safety and efficacy of the insulin algorithms. Each country obtained regulatory approval for study drugs. All sites received local ethics board approval, and all participants provided informed consent.
Statistical analysis
All analyses were performed using the intention-to-treat approach. Continuous variables were summarized using means and SDs and/or medians with interquartile range (IQR), and counts with percentages were used for binary variables. The effect of the insulin intervention on the primary outcome of change in mean glucose at 24 h was calculated using the difference at 24 h with 95% CIs. Similar calculations were performed at days 7 and 30. For the effect of the intervention on glucose levels over the course of the trial, repeated-measures ANOVA was performed using a one-way ANOVA, adjusting for baseline glucose levels. Clinical outcomes were tabulated; however, no statistical comparisons of clinical outcomes were planned, since the trial was not designed or powered to detect differences in clinical outcomes.
It was estimated that a sample size of 500 participants would detect a mean difference of 1.3 mmol/L (23.5 mg/dL) between the insulin and standard therapy arm glucose values at 24 h, assuming a standard deviation of 4.2 mmol/L (75 mg/dL) (17), 90% power, two-sided α level of 0.05, and 6% loss to follow-up. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
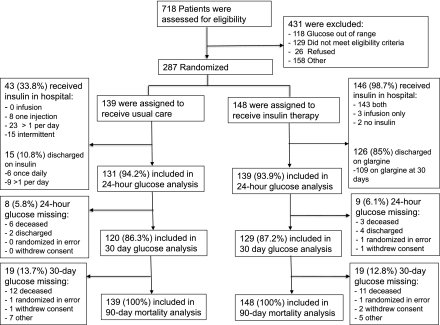
Overall, 287 participants were enrolled in the RECREATE trial (273 in India, 13 in Canada, and 1 in Argentina). A total of 148 participants were randomized to insulin therapy and 139 to standard therapy. As outlined in the study flow diagram (Fig. 1), all participants were accounted for at the 90-day follow-up period.
Figure 1.
RECREATE trial flow diagram.
Baseline characteristics
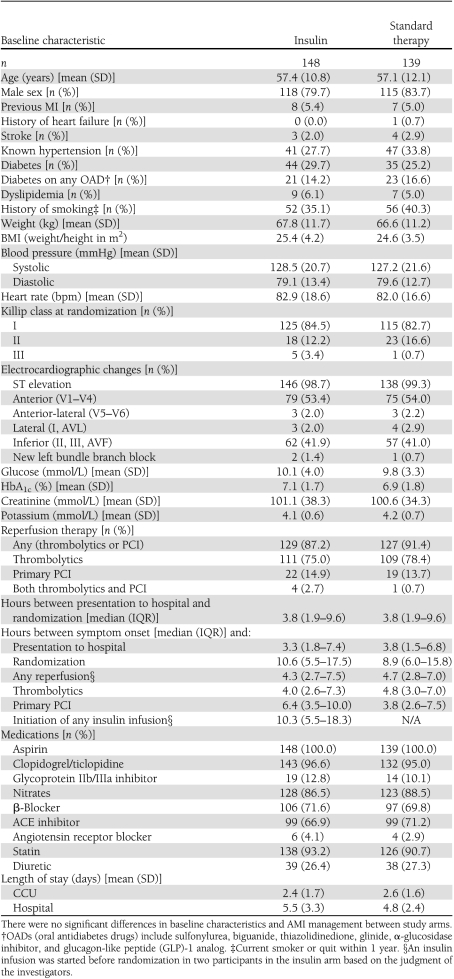
Baseline patient characteristics were similar in the two groups (Table 1). The mean age was 57.3 years, and 82% of participants were male. At randomization, 27% were known to have diabetes, 31% had hypertension, and 37.6% reported smoking. Five percent of participants had a previous history of MI, only 1% had a previous history of congestive heart failure, and 54% had anterior ST segment elevation myocardial infarction (STEMI). The mean glucose at randomization was 10.14 mmol/L (SD 3.99) [182.5 mg/dL (SD 72)] in the insulin arm and 9.80 (SD 3.31) [176.4 mg/dL (SD 60)] in the standard therapy arm (P = 0.42).
Table 1.
Baseline patient characteristics and AMI management
Course in hospital
The median time from presentation to randomization was identical in both arms at 3.8 h (IQR 1.92–9.6), and the median time from randomization to initiation of the insulin infusion was 4.25 h (IQR 2.0–10.3) (Table 1). Of those randomized to the insulin arm, 98% received the insulin infusion while in the CCU and 96.6% received both the insulin infusion in the CCU and the long-acting glargine insulin after transfer from the CCU. Glargine was continued at discharge and through 30 days in 85 and 74% of participants, respectively, in the insulin arm. Only two (1.4%) participants randomized to the insulin arm did not receive any insulin, since both died before insulin initiation. The mean dose of glulisine per kilogram used in the first 24 h was 0.78 (SD 0.48). The mean dose of glargine was 0.4 units/kg (SD 0.22) after discontinuation of the glulisine infusion and 0.35 units/kg (SD 0.22) at 30 days.
No patients in the standard therapy arm received an insulin infusion. While in hospital, 33.8% of standard therapy participants received subcutaneous insulin: 8 (5.76%) received only one insulin injection; 23 (16.55%) received more than one injection per day; and 16 (11.5%) had intermittent use. At discharge, 15 (10.8%) participants in the standard therapy arm were on insulin, 6 (4.3%) were on one injection daily, and 9 (6.5%) were on more than one injection per day.
Both groups received similar medical therapies for AMI, including antiplatelet agents, β-blockers, diuretics, ACE inhibitors or angiotensin receptor blockers, and statins. Rates of thrombolytic therapy (78 vs. 75%, P = 0.49) and primary PCI (13.7 vs. 14.9%, P = 0.77) were also similar between both arms (Table 1). In the insulin arm, reperfusion therapy was started before initiation of the insulin infusion in 90% of patients who received thrombolytics and 81% of patients who underwent primary PCI.
Effects on glucose outcomes
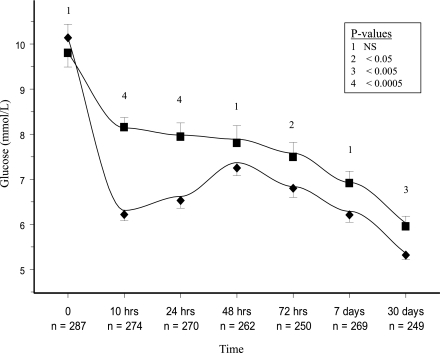
At 24 h, the mean glucose level (mmol/L) was 6.53 (SD 2.8) [117.5 mg/dL (SD 50)] in the insulin arm and 7.94 (SD 3.23) [142.9 mg/dL (SD 58)] in the standard therapy arm, with a mean unadjusted difference of −1.41 mmol/L (95% CI −2.3 to −0.69) [–25.4 mg/dL (95% CI −41.4 to −12.4)]. There was no evidence of a different effect of the insulin infusion in Indian versus non-Indian participants (P for heterogeneity 0.68). Figure 2 shows that the glucose levels over the course of the trial were significantly lower in the insulin arm. At 24 h, the glulisine algorithm lowered the glucose level (mmol/L) to ≤4.6 in 29%, ≤5.8 in 53%, and ≤7.7 in 76% of participants in the insulin arm. In the standard therapy arm at 24 h, the glucose was ≤4.6 in 19%, ≤5.8 in 47%, and ≤7.7 in 63% of participants (P < 0.05 for all comparisons). At 30 days, hemoglobin A1C (%) was similar in both the insulin and standard therapy arms 6.59 (1.3) and 6.77 (1.5), respectively.
Figure 2.
Glucose levels (repeated-measures analysis). The point estimates show the actual values achieved. ■, Standard therapy group values; ♦, insulin group values. The lines are based on modeled data using repeated-measures analysis that take into account the dependence among all values within participants. Bars represent the 95% CIs of the point estimates.
Effects on clinical outcomes
There was only one (0.7%) episode of severe hypoglycemia (defined as glucose <3.0 mmol/L [54 mg/dL] and requiring treatment by a person other than the patient) in the insulin arm. This episode occurred while on insulin infusion in the CCU. There were no episodes of severe hypoglycemia while on once-daily glargine therapy (both inpatient and outpatient). There were two (1.4%) episodes of symptomatic nonsevere hypoglycemia (defined as the presence of clinical signs or symptoms of hypoglycemia detected by the participant who is able to self-correct) in the insulin arm and none in the standard therapy arm. A total of 32 (22.7%) people in the insulin arm and 6 (4.4%) people in the standard arm had a plasma glucose value <3.9 mmol/L while in the CCU (P < 0.05) with no signs or symptoms.
There were similar rates of hypokalemia in both the insulin and standard therapy arms (23.7 and 25.9%, respectively). Other adverse events were infrequent, occurred at similar rates between arms, and were unrelated to insulin, including fever, altered level of consciousness, arrhythmia, atypical chest pain, hematemesis, and forearm superficial thrombosis in the insulin arm and fever, altered mood, nephropathy, and pleuritic chest pain in the standard therapy arm.
There were similar numbers of cardiovascular and other outcomes (reinfarction, stroke, congestive heart failure, and rehospitalization) in the two arms. All-cause mortality was 12 (8.6%) in the standard therapy arm and 13 (8.8%) in the insulin arm at 90 days. All deaths were adjudicated as cardiovascular in nature.
CONCLUSIONS
The RECREATE trial demonstrated that a paper-based insulin algorithm can be easily implemented, is effective in lowering glucose levels, and appears to be safe in individuals with acute STEMI. After 24 h, the insulin algorithm achieved a mean glucose level of 6.53 mmol/L (SD 2.8) [117.5 mg/dL (SD 50)], whereas the standard therapy achieved a mean glucose level of 7.94 mmol/L (SD 3.23) [142.9 mg/dL (58)]. This reduction in glucose levels was associated with only one episode of severe hypoglycemia but more frequent biochemical asymptomatic hypoglycemia, defined as a plasma glucose <3.9 mmol/L (with or without symptoms). Similar rates of hypokalemia and cardiovascular outcomes were observed in both arms.
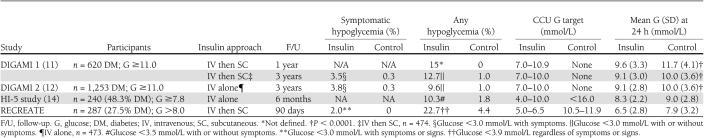
The intravenous insulin algorithm used in the RECREATE trial achieved lower glucose levels than other published clinical trials of insulin therapy in AMI (Table 2). Possible explanations for the lower achieved glucose levels in the RECREATE trial include the following: tighter glycemic targets, the use of insulin mixed in normal saline instead of a glucose-based solution, and a lower percentage of participants with preexisting diabetes (27.5 vs. 48–100%). Despite RECREATE’s tighter glycemic targets, symptomatic hypoglycemia was infrequent and occurred in fewer participants in the RECREATE trial (2.0%) compared with other AMI studies (3.5–3.8%) (Table 2) (11,12,14). Conversely, the rate of biochemical hypoglycemia appeared to be similar to that observed in these other trials, after accounting for the fact that lower glucose cutoffs (i.e., below 3.0 or 3.5 mmol/L) were used to define biochemical hypoglycemia in these other studies (Table 2).
Table 2.
Summary of studies of intravenous insulin therapy in AMI
The main strength of the RECREATE pilot trial is the use of a paper-based insulin algorithm that did not require frequent glucose measurements or a computer. The intravenous insulin algorithm was easily and successfully implemented across a number of international centers. Overall, there was a high rate of adherence to the study protocol and high rates of participant follow-up (94% at 24 h, 87% at 30 days, and 100% survival data at 90 days). The rates of symptomatic hypoglycemia, hypokalemia, and other adverse events were low and were similar in both arms. Finally, the fact that participants recruited from different parts of the world responded similarly to the intervention suggests that it is broadly applicable and implementable.
Although fewer patients were recruited than originally planned, a clear and significant difference between glucose levels of 1.41 mmol/L (25 mg/dL) was achieved (with 97.7% power), which has been associated with a 22% decrease in mortality in previously published epidemiologic analysis (6). However, this trial was clearly not designed to assess morbidity or mortality, and whether this glucose strategy improves clinical outcomes in patients hospitalized with AMI remains a key unanswered question. This continuing uncertainty regarding optimal glucose levels during AMI underscores the need for a definitive clinical trial of targeted glucose control strategies, such as the one tested in our study.
In conclusion, the RECREATE pilot trial showed that an insulin-based approach comprising a paper-based insulin algorithm targeting normal glucose levels followed by subcutaneous insulin for 1 month can effectively lower glucose levels with minimal symptomatic hypoglycemia but more biochemical hypoglycemia in patients with an acute STEMI. It is therefore feasible to test the clinical risks and benefits of this approach in a large outcomes trial that could be globally conducted in a wide variety of settings.
Acknowledgments
This trial was funded by an unrestricted grant by sanofi-aventis. sanofi-aventis had no role in the design and conduct of the trial; in site monitoring; in data collection, analysis, and interpretation; or in preparation of the manuscript.
K.A.N. received a salary from the Canadian Institutes of Health Research RCT Mentorship award under supervision from H.C.G. S.R.M. received consulting fees from sanofi-aventis, and his institution received research grants from sanofi-aventis. M.K. is a consultant for Medtronic MiniMed and sanofi-aventis. H.C.G. holds the Population Health Research Institute Chair in Diabetes (established by a donation from sanofi-aventis). He received consulting and lecture fees from sanofi-aventis, the manufacturer of glulisine and glargine, and his institution received grants from sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
K.A.N., A.G., D.X., S.R.M., R.D., S.Y., and H.C.G. designed the study. K.A.N., D.X., A.S., S.R.M., and R.D. acquired the data. J.N. performed statistical analysis. K.A.N., A.G., J.N., and H.C.G. performed analysis and interpreted the data. K.A.N., A.G., M.K., and H.C.G. developed the insulin algorithms. All authors had an active role in manuscript preparation. K.A.N. was the guarantor.
Parts of this study were presented in poster form (936-P) at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
Clinical trial reg. no. NCT00640991, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0706/-/DC1.
References
- 1.Bartnik M, Rydén L, Ferrari R, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: The Euro Heart Survey on diabetes and the heart. Eur Heart J 2004;25:1880–1890 [DOI] [PubMed] [Google Scholar]
- 2.Hu D-Y, Pan C-Y, Yu J-M; China Heart Survey Group The relationship between coronary artery disease and abnormal glucose regulation in China: the China Heart Survey. Eur Heart J 2006;27:2573–2579 [DOI] [PubMed] [Google Scholar]
- 3.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 2005;111:3078–3086 [DOI] [PubMed] [Google Scholar]
- 4.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med 2009;169:438–446 [DOI] [PubMed] [Google Scholar]
- 5.Suleiman M, Hammerman H, Boulos M, et al. Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction: a prospective study. Circulation 2005;111:754–760 [DOI] [PubMed] [Google Scholar]
- 6.Goyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J 2006;27:1289–1297 [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Mehta SR, Díaz R, et al. Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation 2009;120:2429–2437 [DOI] [PubMed] [Google Scholar]
- 8.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–778 [DOI] [PubMed] [Google Scholar]
- 9.Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008;29:177–184 [DOI] [PubMed] [Google Scholar]
- 10.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999;99:2626–2632 [DOI] [PubMed] [Google Scholar]
- 11.Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65 [DOI] [PubMed] [Google Scholar]
- 12.Malmberg K, Rydén L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 13.Goyal A, Nerenberg KA, Gerstein HC, Umpierrez G, Wilson PW. Insulin therapy in acute coronary syndromes: an appraisal of completed and ongoing randomised trials with important clinical end points. Diab Vasc Dis Res 2008;5:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006;29:765–770 [DOI] [PubMed] [Google Scholar]
- 15.Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2004;164:2005–2011 [DOI] [PubMed] [Google Scholar]
- 16.Kosiborod M, Inzucchi SE, Hamburg M, et al. Feasibility, effectiveness and safety of intensive glucose control in critically ill hyperglycemic patients hospitalized with acute coronary syndromes (Abstract). Circulation 2007;116:799 [Google Scholar]
- 17.Mehta SR, Yusuf S, Díaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 2005;293:437–446 [DOI] [PubMed] [Google Scholar]