Abstract
OBJECTIVE
Although associated with adverse outcomes in other cardiopulmonary conditions, the prognostic value of elevated glucose in patients with acute pulmonary embolism (PE) is unknown. We sought to examine the association between glucose levels and mortality and hospital readmission rates for patients with PE.
RESEARCH DESIGN AND METHODS
We evaluated 13,621 patient discharges with a primary diagnosis of PE from 185 acute care hospitals in Pennsylvania (from January 2000 to November 2002). Admission glucose levels were analyzed as a categorical variable (≤110, >110–140, >140–170, >170–240, and >240 mg/dL). The outcomes were 30-day all-cause mortality and hospital readmission. We used random-intercept logistic regression to assess the independent association between admission glucose levels and mortality and hospital readmission, adjusting for patient (age, sex, race, insurance, comorbid conditions, severity of illness, laboratory parameters, and thrombolysis) and hospital (region, size, and teaching status) factors.
RESULTS
Elevated glucose (>110 mg/dL) was present in 8,666 (63.6%) patients. Patients with a glucose level ≤110, >110–140, >140–170, >170–240, and >240 mg/dL had a 30-day mortality of 5.6, 8.4, 12.0, 15.6, and 18.3%, respectively (P < 0.001). Compared with patients with a glucose level ≤110 mg/dL, the adjusted odds of dying were greater for patients with a glucose level >110–140 (odds ratio 1.19 [95% CI 1.00–1.42]), >140–170 (1.44 [1.17–1.77]), >170–240 (1.54 [1.26–1.90]), and >240 mg/dL (1.60 [1.26–2.03]), with no difference in the odds of hospital readmission.
CONCLUSIONS
In patients with acute PE, elevated admission glucose is common and independently associated with short-term mortality.
Elevated serum glucose level at admission during acute illness is common and associated with poor outcomes in acute cardiopulmonary diseases, such as acute myocardial infarction (1), heart failure (2), pneumonia (3), and stroke (4). During the past decade, the association between stress-induced elevated serum glucose level and the outcome of acutely ill patients has received considerable attention because of the potential benefits and risks of tight glycemic control (5).
Acute pulmonary embolism (PE) is a major health problem. In 2005, 140,000 patients were discharged with a primary diagnosis of PE from U.S. hospitals (6), with an average mortality rate of 9% (7). Evidence suggests that acute and chronic hyperglycemia is associated with elevated coagulation factors and impaired fibrinolysis (8) and an increased risk of developing venous thromboembolism (8–10). Whether admission hyperglycemia has a negative impact on prognosis in patients with acute PE is unknown. Given the potential treatment implications of such a finding, our goal was to examine whether an independent association exists between elevated admission glucose levels and 30-day mortality using a large, statewide database of unselected patients with acute PE. A secondary objective of our study was to examine the association between elevated serum glucose levels and hospital readmission.
RESEARCH DESIGN AND METHODS
Patient identification and eligibility
We identified all patients with PE discharged from nongovernmental acute care hospitals in Pennsylvania (from 1 January 2000 to 30 November 2002) using the Pennsylvania Health Care Cost Containment Council (PHC4) database. This database contains information on demographic characteristics, insurance status, ICD-9 Clinical Modification (ICD-9-CM) diagnosis and procedure codes, hospital region and number of beds, and length of hospital stay for all patients.
We included inpatients aged ≥18 years who were discharged with a primary diagnosis of PE based on the following ICD-9-CM codes: 415.1, 415.11, 415.19, and 673.20–24 (7). To ensure that we identified the most severely ill patients with PE as the primary reason for hospitalization, we also included inpatients with a secondary diagnosis code for PE and one of the following primary codes that may represent complications or treatments of this condition: respiratory failure (518.81), cardiogenic shock (785.51), cardiac arrest (427.5), secondary pulmonary hypertension (416.8), syncope (780.2), thrombolysis (99.10), and intubation or mechanical ventilation (96.04, 96.05, 96.70–96.72) (7).
We excluded all other patients who had a secondary ICD-9-CM code for PE or those who were transferred from another health care facility because the latter group of patients is more likely to have PE as a complication of hospitalization, and we did not know whether PE was diagnosed and treated before the patient was transferred. We excluded follow-up records for patients who were subsequently transferred to other hospitals, who had no identifiers required for linkage to the necessary clinical data, and for whom 30-day mortality information was not available. For this analysis, we also excluded patients without a documented serum glucose level at the time of presentation. The institutional review board at the University of Pittsburgh approved this study.
Patient and hospital characteristics
Patient demographic characteristics (age, sex, and race) and insurance status were abstracted from the PHC4 database (7). Baseline clinical variables were obtained by linking eligible patients to the Atlas database (MediQual, Marlborough, MA), which comprises clinical findings and laboratory parameters (including serum glucose level) at the time of presentation for all inpatients treated at nongovernmental acute care hospitals in Pennsylvania (7). The PHC4 and Atlas databases were matched by PHC4 staff using unique patient identifiers (patient date of birth, sex, and social security number); we had no access to personal patient identifiers. We quantified severity of illness using the Pulmonary Embolism Severity Index (PESI), a prognostic model for patients with PE that was developed and validated using these clinical data from the PHC4 and Atlas databases (7). On the basis of the PESI, each patient is classified into one of five severity classes (I–V), with 30-day mortality ranging from 1.1 to 24.5%. To ascertain whether patients received thrombolytic therapy, we used ICD-9-CM procedure codes (99.10) from the PHC4 and Atlas databases (7).
We abstracted the hospital region within Pennsylvania, number of beds per hospital site, and annual number of PE admissions for each site from the PHC4 database. We defined hospital teaching status based on data from the Council of Teaching Hospitals of the Association of American Medical Colleges. Because 76% of teaching hospitals, but only 12% of nonteaching hospitals, had at least 350 hospital beds, we created a composite hospital-level variable for our statistical modeling based on teaching status and size (i.e., small nonteaching hospitals with fewer than 350 beds, large nonteaching hospitals with at least 350 beds, and teaching hospitals).
Admission serum glucose level and diabetes mellitus
We categorized admission serum glucose levels into five categories (≤110, >110–140, >140–170, >170–240, and >240 mg/dL) according to previously published thresholds (11). Prior studies demonstrate that admission serum glucose levels >110 mg/dL are significantly associated with mortality in patients with acute myocardial infarction and pneumonia (3,11). We used the Atlas database to ascertain whether patients had known diabetes mellitus.
Study outcomes
Our primary study outcome was all-cause mortality within 30 days of presentation. We obtained mortality data by linking patients to the National Death Index with unique patient identifiers, including social security number, name, date of birth, and sex (12–14). The National Death Index has a sensitivity and specificity of >97% for identifying mortality (14). To ascertain our secondary outcome, hospital readmission for any reason to any acute care hospital in Pennsylvania within 30 days of presentation, we used the PHC4 database.
Statistical analysis
To compare patient baseline characteristics across the five categories of serum glucose (≤110, >110–140, >140–170, >170–240, and >240 mg/dL), we performed χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. P values for comparisons of baseline characteristics were adjusted using the Holm method (15).We used survival analyses and the log-rank test to compare the cumulative 30-day mortality by serum glucose level. Surviving patients were censored at 30 days.
We used multivariable logistic regression to examine the independent association between categories of serum glucose and 30-day mortality, after adjusting for demographics (age, sex, race, and insurance type), comorbid diseases (history of cancer, chronic lung disease, heart failure, and diabetes mellitus), physical examination findings (systolic arterial blood pressure <100 mmHg, pulse ≥110/min, respiratory rate ≥30 breaths/min, body temperature <36°C, arterial oxygen saturation <90%, and altered mental status), laboratory parameters (hemoglobin level <12 g/dL for women and <13 g/dL for men, sodium ≤135 mmol/L, creatinine >1.5 mg/dL, and troponin ≥0.1 ng/mL), administration of thrombolytics, and hospital characteristics (region within Pennsylvania, annual volume of PE, size, and teaching status). To account for patient clustering within hospital, we used random-intercept logistic regression with the two levels defined by patient and hospital site. To assess whether glucose-associated mortality risk differed in patients with and without known diabetes mellitus, we repeated multivariable analyses in patients with and without this condition.
We used the same logistic regression model to examine the association between serum glucose level and readmission within 30 days in patients discharged alive. Patients who were still hospitalized 30 days after admission and those without a documented readmission status were excluded from this analysis. All analyses were performed using Stata 11.0 (StataCorp, College Station, TX).
RESULTS
Of the 17,733 patient discharges that met our inclusion criteria, we excluded 323 with only a secondary code indicative of PE (1.8%), 767 patient transfers from another hospital (4.3%), 265 subsequent transfers to another hospital (1.5%), 777 discharges without a match to key clinical findings (4.4%), 70 without a linkage to the National Death Index (0.4%), and 1,910 (10.8%) with an undocumented or erroneous admission serum glucose level. The study cohort comprised 13,621 patient discharges with a diagnosis of PE from 185 Pennsylvania hospitals. On admission, 8,666 (63.6%) patients had an elevated serum glucose level (>110 mg/dL). Diabetes mellitus was known on admission in 1,889 (13.9%) of all patients and in 1,685 (19.4%) patients with an elevated serum glucose level.
Compared with the 13,621 enrolled patients, the 1,910 excluded because of an undocumented serum glucose level were significantly younger (median age 63 vs. 67 years; P < 0.001) and were less likely to have known diabetes mellitus (7.7 vs. 13.9%; P < 0.001), a history of heart failure (9.9 vs. 16.7%; P < 0.001), and a history of chronic lung disease (14.0 vs. 19.1%; P < 0.001). Compared with eligible patients with a documented serum glucose level, these 1,910 excluded patients were also less likely to have a pulse ≥110/min (10.9 vs. 18.6%; P < 0.001), a systolic blood pressure <100 mmHg (7.2 vs. 10.9%; P < 0.001), a respiratory rate ≥30/min (7.9 vs. 15.5%; P < 0.001), a body temperature <36°C (14.3 vs. 16.9%; P = 0.005), an altered mental status (5.9 vs. 7.5%; P = 0.01), and an arterial oxygen saturation <90% (3.6 vs. 8.6%; P < 0.001) at presentation.
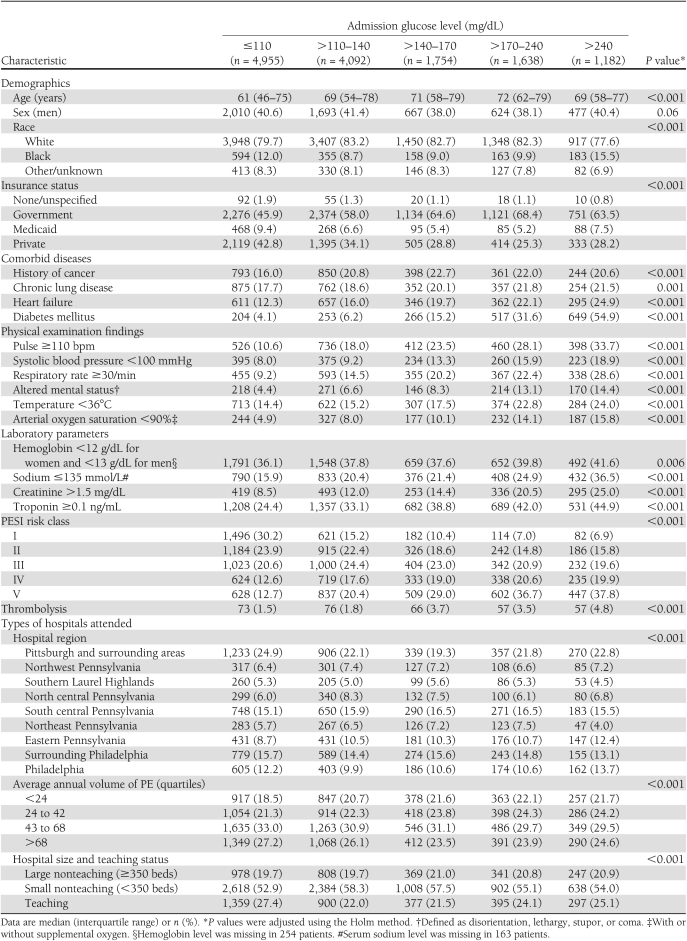
Comparison of baseline patient characteristics by admission serum glucose level
Patients with higher admission serum glucose levels were older and more likely to have comorbid diseases (cancer, chronic lung disease, heart failure, and diabetes mellitus) and clinical and biological signs of disease severity (tachycardia, hypotension, tachypnea, altered mental status, hypothermia, hypoxemia, anemia, hyponatremia, and elevated creatinine and troponin values) (Table 1). There was a higher proportion of patients in PESI risk classes IV and V among patients with higher serum glucose levels.
Table 1.
Baseline patient characteristics by level of admission glucose
Association of admission serum glucose level and 30-day mortality
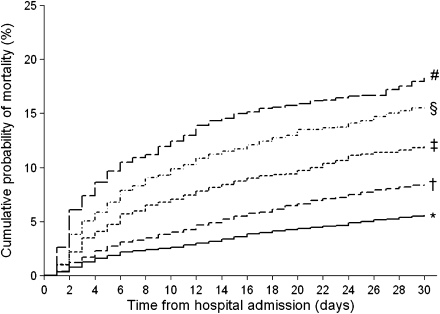
Overall, 1,301 of 13,621 patients (9.6%) died at 30 days. Higher admission serum glucose levels were associated with an increasing risk of mortality. Patients with a serum glucose level ≤110, >110–140, >140–170, >170–240, and >240 mg/dL had a cumulative 30-day mortality of 5.6, 8.4, 12.0, 15.6, and 18.3%, respectively (P for trend <0.001) (Fig. 1).
Figure 1.
Cumulative mortality by serum glucose level. Kaplan-Meier estimates of 30-day mortality were 5.6, 8.4, 12.0, 15.6, and 18.3% for patients with serum glucose level ≤110, >110–140, >140–170, >170–240, and >240 mg/dL, respectively (P for trend <0.001). Admission glucose level (mg/dL): *≤110, †>110–140, ‡>140–170, §>170–240, and #>240.
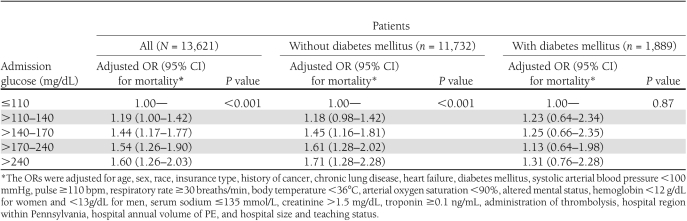
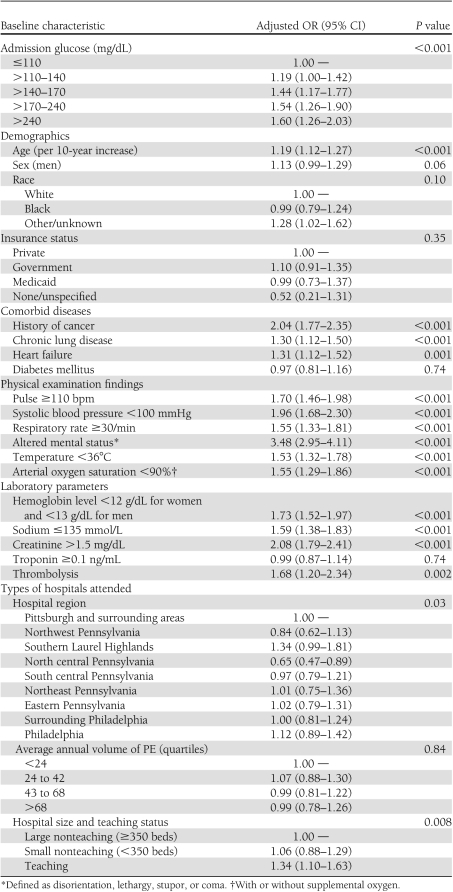
After adjustment, elevated admission serum glucose levels remained significantly associated with 30-day mortality compared with patients with an admission serum glucose ≤110 mg/dL (Table 2). The odds ratio (OR) of dying increased from 1.19 (95% CI 1.00–1.42) for patients with a serum glucose level >110–140 mg/dL to 1.60 (1.26–2.03) for patients with a serum glucose level >240 mg/dL. Although higher admission serum glucose levels were significantly associated with an increase in the odds of dying in patients without diabetes, this association was not observed in patients with diabetes (Table 2). Other characteristics that were independently associated with higher adjusted odds of death included increasing age, the presence of comorbid diseases (history of cancer, chronic lung disease, and heart failure), abnormal physical examination findings (systolic arterial blood pressure <100 mmHg, pulse ≥110/min, respiratory rate ≥30 breaths/min, body temperature <36°C, arterial oxygen saturation <90%, and altered mental status), abnormal laboratory parameters (hemoglobin level <12 g/dL for women and <13 g/dL for men, sodium ≤135 mmol/L, and creatinine >1.5 mg/dL), the administration of thrombolytics, and hospital region within Pennsylvania, size, and teaching status (Table 3).
Table 2.
Independent association of admission glucose level with 30-day mortality in patients with and without diabetes mellitus
Table 3.
Independent associations of baseline characteristics with 30-day mortality (N = 13,621)
Association of admission serum glucose level and 30-day readmission
We estimated the 30-day readmission rate in 12,656 patients, after the exclusion of 836 patients who died in the hospital, 94 who were still hospitalized at 30 days after admission, and 35 with unknown readmission status. Of these, 1,600 (12.6%) were readmitted within 30 days. Kaplan-Meier estimates of 30-day readmission were 11.6, 12.6, 14.6, 13.7, and 14.7% for patients with a serum glucose level ≤110, >110–140, >140–170, >170–240, and >240 mg/dL, respectively (P for trend = 0.001).
The adjusted odds of 30-day readmission was slightly higher in patients with admission serum glucose level >140–170 mg/dL (OR 1.21 [95% CI 1.02–1.44]) but similar in patients with an admission serum glucose level >110–140 mg/dL (1.05 [0.91–1.20]), >170–240 mg/dL (1.06 [0.88–1.28]), and >240 mg/dL (1.10 [0.87–1.38]) compared with patients with a serum glucose level of ≤110 mg/dL. Overall, the adjusted odds of readmission did not differ across serum glucose categories (P = 0.31).
CONCLUSIONS
Our results demonstrate that a substantial proportion of patients with PE (63.6%) have an elevated serum glucose level at the time of presentation. After adjusting for potential patient- and hospital-related confounders, and the administration of thrombolytic therapy, we found that patients with elevated serum glucose levels had a significantly higher short-term mortality. Our findings are consistent with a retrospective analysis demonstrating an independent association between elevated mean glucose levels and inpatient mortality in intensive care unit patients with PE (16). In contrast, we observed no significant association between serum glucose levels and the rate of hospital readmission.
Several mechanisms may explain the association between elevated serum glucose levels and mortality in patients with PE. First, elevated serum glucose levels have a procoagulant effect and decrease fibrinolysis (8,17–19). Second, hyperglycemia is often accompanied by hyperinsulinemia, which may further inhibit fibrinolysis and increase the prothrombotic effect of hyperglycemia (8,18). Finally, it is possible that hyperglycemia is not a causal factor for adverse clinical outcomes but merely a marker of increased stress and severity of illness. In acute illnesses, such as PE, stress hormones (i.e., catecholamine, growth hormone, cortisol, and cytokines) are released, increasing hepatic glucose production and insulin resistance (20).
Although our study demonstrated a significant association between increasing glucose levels and mortality in patients without a diagnosis of diabetes mellitus, we did not find such an association in patients with known diabetes. A similar phenomenon has been observed in patients with stroke, with acute myocardial infarction, and in a mixed intensive care unit setting (4,11,21). Whether chronic hyperglycemia is protective of acute hyperglycemia-mediated damage or a lower intensity of stress is required to produce a similar degree of hyperglycemia in patients with diabetes mellitus remains unknown (5).
Our findings have both clinical and research implications. Clinically, patients with PE who have hyperglycemia at presentation carry a higher risk of short-term mortality and may therefore potentially benefit from more intensive surveillance in the hospital and after discharge. Whether laboratory markers of coagulation and fibrinolysis are correlated with the admission glucose level in patients with PE should be further examined. Further research is also warranted to determine whether glucose-lowering treatment with insulin is associated with improved outcomes for patients with PE. Currently, there is no evidence that insulin therapy to strictly control blood glucose in critically ill patients or patients with myocardial infarction or stroke improves mortality (22).
Our study has potential limitations. First, patients in the study sample were identified by use of ICD-9-CM codes for PE rather than standardized radiographic criteria, and patient eligibility may therefore be subject to study selection biases due to hospital coding procedures. In a prior study, 96% of patients with specific codes for PE had objectively documented disease on the basis of chart review criteria (23). Second, our sample excluded 10.8% of younger, healthier, and less severely ill patients in whom serum glucose level was not measured at the time of admission. However, the exclusion of these lower risk patients, of whom probably a low proportion had hyperglycemia, is unlikely to change our study results. Third, because measures of coagulation and fibrinolysis were not available in our database, we could not examine whether these factors are correlated with admission glucose levels. Fourth, we were not able to adjust our results for other potential confounders that may influence glucose level and prognosis, such as concomitant inflammatory or infectious diseases, known metabolic syndrome, glucose intolerance, impaired fasting glucose, and HbA1c levels. Moreover, we had no information about insulin use or other glucose-lowering treatments during hospitalization and its effect on mortality. Fifth, we had no information on serum glucose level after hospital admission and discharge; thus, the prognostic implication of transient versus persistent elevated serum glucose level could not be analyzed. Finally, we could detect only associations, not causality, from our data. Thus, we cannot determine whether hyperglycemia has a direct effect on patient prognosis or is a mere marker of severity of illness and stress.
In conclusion, in this large sample of patients hospitalized with acute PE, hyperglycemia at the time of presentation was associated with a significantly higher risk of 30-day mortality. Elevated serum glucose levels may be a potential therapeutic target, and future studies should examine whether glucose-lowering treatments could improve outcomes in hyperglycemic patients with PE.
Acknowledgments
This study was partially supported by National Heart, Lung, and Blood Institute grant 1-R21-HL075521-01A1 and by Swiss National Science Foundation grant 33CSCO-122659.
No potential conflicts of interest relevant to this article were reported.
N.S. wrote and edited the manuscript and takes responsibility for the contents of the article. J.L. contributed to the study concept and design, performed the analysis, contributed to writing the manuscript, and reviewed the final manuscript. D.A. acquired data, created the study concept and design, discussed the study results, contributed to writing the manuscript, and reviewed the manuscript. M.M. wrote and reviewed the manuscript. All authors had full access to data, take responsibility for its integrity, and have read and agreed to the manuscript as written.
Parts of this study were presented in poster form at the Annual Conference of the Swiss Society of General Internal Medicine, Lausanne, Switzerland, 11–13 May 2011.
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–778 [DOI] [PubMed] [Google Scholar]
- 2.Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med 2006;166:1613–1619 [DOI] [PubMed] [Google Scholar]
- 3.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care 2005;28:810–815 [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426–2432 [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med 2010;363:2540–2546 [DOI] [PubMed] [Google Scholar]
- 6.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13 2007;165:1–209 [PubMed] [Google Scholar]
- 7.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost 2010;8:1663–1669 [DOI] [PubMed] [Google Scholar]
- 9.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102 [DOI] [PubMed] [Google Scholar]
- 10.Hermanides J, Cohn DM, Devries JH, et al. Venous thrombosis is associated with hyperglycemia at diagnosis: a case-control study. J Thromb Haemost 2009;7:945–949 [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 2005;111:3078–3086 [DOI] [PubMed] [Google Scholar]
- 12.MacMahon B. The National Death Index. Am J Public Health 1983;73:1247–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams BC, Demitrack LB, Fries BE. The accuracy of the National Death Index when personal identifiers other than social security number are used. Am J Public Health 1992;82:1145–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol 2002;12:462–468 [DOI] [PubMed] [Google Scholar]
- 15.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs. Holm methods. Am J Public Health 1996;86:726–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009;37:3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao AK, Chouhan V, Chen X, Sun L, Boden G. Activation of the tissue factor pathway of blood coagulation during prolonged hyperglycemia in young healthy men. Diabetes 1999;48:1156–1161 [DOI] [PubMed] [Google Scholar]
- 18.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006;55:202–208 [PubMed] [Google Scholar]
- 19.Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006;55:480–486 [DOI] [PubMed] [Google Scholar]
- 20.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med 2008;36:2249–2255 [DOI] [PubMed] [Google Scholar]
- 22.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011;154:260–267 [DOI] [PubMed] [Google Scholar]
- 23.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res 2010;126:61–67 [DOI] [PubMed] [Google Scholar]