Abstract
Introduction
Both cardiovascular disease and osteoporosis are important causes of morbidity and mortality in the elderly. The co-occurrence of cardiovascular disease and osteoporosis prompted us to review the evidence of an association between cardiovascular (CV) disease and osteoporosis and potential shared common pathophysiological mechanisms.
Methods
A systematic literature search (Medline, Pubmed and Embase) was conducted to identify all clinical studies that investigated the association between cardiovascular disease and osteoporosis. Relevant studies were screened for quality according to guidelines as proposed by the Dutch Cochrane Centre and evidence was summarized.
Results
Seventy studies were included in this review. Due to a large heterogeneity in study population, design and outcome measures a formal meta-analysis was not possible. Six of the highest ranked studies (mean n = 2,000) showed that individuals with prevalent subclinical CV disease had higher risk for increased bone loss and fractures during follow-up compared to persons without CV disease (range of reported risk: hazard ratio (HR) 1.5; odds ratio (OR) 2.3 to 3.0). The largest study (n = 31,936) reported a more than four times higher risk in women and more than six times higher risk in men. There is moderate evidence that individuals with low bone mass had higher CV mortality rates and incident CV events than subjects with normal bone mass (risk rates 1.2 to 1.4). Although the shared common pathophysiological mechanisms are not fully elucidated, the most important factors that might explain this association appear to be, besides age, estrogen deficiency and inflammation.
Conclusions
The current evidence indicates that individuals with prevalent subclinical CV disease are at increased risk for bone loss and subsequent fractures. Presently no firm conclusions can be drawn as to what extent low bone mineral density might be associated with increased cardiovascular risk.
Introduction
Cardiovascular (CV) disease and osteoporosis are both important causes of morbidity and mortality in aging men and women. They share common risk factors, such as increased age and inactivity, and are frequently found in the same individuals, suggesting a possible relationship. Results from epidemiological studies indicate an association between CV disease and osteoporosis. Prevalent CV disease and subclinical atherosclerosis have been found to be related to low bone mass and increased fracture risk [1-4]. Similarly, low bone mineral density (BMD) has been related to increased cardiovascular risk [5-8]. This relationship is often regarded as a result of aging; however, recent evidence suggests a direct association, independent of age and traditional cardiovascular risk factors and accumulating evidence from experimental research indicates a shared pathogenesis. A variety of factors that influence bone metabolism are involved in the development of vascular disease, for example, atherosclerosis and vascular calcification. Interestingly, several bone-related proteins are implicated in the calcification process resulting in mineral deposition [9]. This is important as calcification of the arterial wall may be a marker for CV disease and was shown to predict CV events [10]. Given the importance of identifying a person at risk for CV events or fractures, evidence for an association of CV disease with osteoporosis might have implications for screening decisions in patients with low bone mass and vice versa. This review aims to summarize all the present clinical literature about the association between CV disease and osteoporosis and to describe common pathophysiological mechanisms. The results of this review are grouped into two topics: clinical results, discussing the relationship between 1) cardiovascular disease and osteoporosis and 2) vice versa. In addition, the possible pathophysiological links of CV disease and osteoporosis will be discussed.
Materials and methods
Search strategy
A systematic search (in Medline, Pubmed and Embase) was conducted to identify all clinical studies from 1966 to January 2010 (last updated 8 June 2010) that investigated the association between cardiovascular disease and osteoporosis. The following search terms for cardiovascular disease were used: cardiovascular diseases, cerebrovascular diseases and peripheral vascular diseases. These searches were each combined with an osteoporosis search block and duplicates were removed. Searches were limited to human studies in the English, Dutch and German languages. The complete Medline search is available in Additional file 1. In addition, references from the retrieved articles were scanned for additional relevant studies.
Selection criteria
Abstracts were screened by one reviewer (DdU) and studies were included in the review if they fulfilled the following inclusion criteria: epidemiological studies (including prospective, cross-sectional, case-control, or retrospective studies) reporting the association between CV disease and osteoporosis in the general population or in patients with prevalent CV disease or low bone mass. Cardiovascular disease was defined as coronary heart disease (CHD) (myocardial infarction, angina pectoris, coronary insufficiency or ischemic heart disease), cerebrovascular disease (stroke, transient ischemic attacks), peripheral arterial disease (PAD) (lower extremity claudication, arterial thrombosis/embolism, ankle brachial index (ABI) <0.90) or subclinical atherosclerosis measured as intima media thickness (IMT) or vascular calcification. In addition, bone mass had to be assessed as bone mineral density or bone quality, and osteoporosis was defined as low bone mass (T-score ≤-2.5) or increased fracture risk (vertebral and non-vertebral). Exclusion criteria were: reviews, letters, case-reports, intervention studies and biomechanical studies. Studies in patients with co-morbidity other than osteoporosis or CV disease were also excluded. Finally, investigations using risk factors of CV disease or osteoporosis as outcome measurements, such as hypertension, metabolic syndrome, atrial fibrillation, bone markers, and calcium supplementation were not included.
Assessment of study quality
The quality of each manuscript was systematically assessed with a checklist for cohort studies as proposed by the Dutch Cochrane Collaboration [11] (Additional file 2). Quality assessment included a scoring of the following components: definition of study population, the likelihood of bias, adequate blinding, the accuracy of outcome measurements, duration of follow-up and selective loss-to follow-up, the appropriateness of the statistical analysis and the clinical relevance. All items had the following answer options: yes/no/too little information to answer the question. We considered incomplete information or data important criteria for study quality. Therefore, if the answer could not be given because the study provided too little information, a negative score (for example, "no") was given. Each "no" was scored and an equal weight was given to each item. A maximum of 10 points could be given. The scores of each study are given in Tables 1 and 2.
Table 1.
Prospective studies investigating relationship CV disease and low BMD
| Study | Study population (years follow-up) | Number of cases (% women) | Postmenopausal women | CV disease excluded | Mean age | Outcome CV disease | Outcome bone mass | Results # | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Sennerby, 2009 [13] | Population-based (20) |
31,936 (NA) |
NA | Yes | 67.9 to 74.4 | CV disease by National patient registry, ICD 9 codes | Incident hip fracture by National patient registry, ICD 9 codes | Women: HR: 4.42 (95% CI 3.49 to 5.61) Men: HR: 6.65 (95% CI 4.82 to 9.19) |
3 |
| Szulc, 2008 [14] | Population-based (10) |
781 (0%) |
No | No | 65 | AC by X-spine | Incident fracture by hospital records or X-ray | OR: 2.54 to 3.04 (P < 0.005 to 0.001) | 3 |
| Naves, 2008 [4] | Population-based (4) |
624 (51%) |
NA | No | 65 | AC by X-spine | BMD lumbar spine and femur by DXA Incident fracture by hospital record or X-ray |
Change BMD spine in progression AC vs no progression AC: -1.48% vs 1.43% (P <.0001) Change BMD hip in progression AC and no progression AC: -0.48% vs 0.23% (P = 0.315) Incident fracture: OR: 2.13 (95% CI 0.85 to 5.31) |
3 |
| Von Muhlen, 2009 [15] | Population-based (4) |
1,332 (60%) |
NA | No | 73.8 | PAD by ABI | BMD lumbar spine and hip by DXA and incident fracture by X-ray | Women: Change BMD in PAD vs no PAD: 59.2% vs 43.5% (P < 0.05) Incident non-vert fracture: OR: 0.84 (95% CI 0.31 to 2.26) Men : Change BMD in PAD vs no PAD : 43.5% vs 35.5% (P = 0.20) Incident non-vert fracture: OR: 1.52 (95% CI 0.30 to 7.45) |
3 |
| Collins, 2009 [2] | Population-based (5.4) |
4,302 (0%) |
NA | No | 73.5 | PAD by ABI | BMD hip by DXA Incident fractures by x-ray and hospital records |
Change BMD in PAD vs no PAD: -0.60% vs -0.32% (P < 0.001 PAD and non-vert fracture risk: HR = 1.47 (95% CI 1.07 to 2.04) |
3 |
| Hak, 2000 [3] | Population-based (9) |
236 (100%) |
No (100%) | No | 49 | AC by X-spine | MCA by radiogrammetry | MCA in patients with AC progression vs no AC progression -3.5 mm vs -2.0 mm (P < 0.01) |
3 |
| Samelson, 2007 [12] | Population-based (21) |
2,499 (58%) |
No | 61 | AC by X-spine | Incident hip fracture by hospital records and death certificates | Women: HR: 1.4 (0.8 to 2.3) Men: HR: 1.2 (0.2 to 5.7) |
4 | |
| Bagger, 2006 [1] | Population-based (7.5) |
2,262 (100%) |
Yes (100%) | No | 65 | AC by X-spine | BMD lumbar spine and hip and incident fractures by hospital records or X-ray | Change hip BMD AC score ≥3 vs <3: -0.38% vs -0.25% (P < 0.001) AC and hip fracture: OR: 2.3 (95% CI 1.1 to 4.8) AC and vert fracture: OR: 1.2 (95% CI 1.0 to 1.5) |
4 |
| Schulz, 2004 [17] | Clinic-based (8) |
228 (100%) |
Yes | No | 65.2 | AC by CT-scan of spine | BMD spine by CT-scan | Change BMD AC vs no AC: -5.3% vs -1.3% (P < 0.001) |
6 |
#adjusted for confounders; NA, not available; AC, aortic calcification; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; PAD, peripheral arterial disease; ABI, ankle brachial index; MCA, metacarpal cortical area.
Table 2.
Prospective studies investigating relationship low BMD and CV disease
| Study | Study population (years follow-up) | Number of cases (% women) | Postmenopausal women | CV disease excluded | Mean age (years) | Race | Outcome osteoporosis | Outcome CV disease | Results # | Quality (x nee) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mussolino, 2007 [69] | Population-based (9) |
5,272 (NA) | NA | Yes | 60.9 to 69.4 | Caucasian (NA%), black and Mexican-American | BMD proximal femur by DXA | CV and stroke mortality by death certificates | Women: BMD and CV mortality RR: 1.26 (95% CI 0.88 to 1.80) BMD and stroke mortality: RR: 1.34 (95% CI 0.86 to 2.07) Men: BMD and CV mortality: RR: 1.05 (95% CI 0.79 to 1.39) BMD and stroke mortality: RR; 0.73 (95% CI 0.43 to 1.23) |
3 |
| Farhat, 2007 [6] | Population-based (5.4) |
2,310 (55%) | Yes | Yes | 73.5 | Caucasian (58%) and black | BMD total hip, femoral neck and trochanter by DXA BMD spine by CT-scans |
Incident CV disease by hospital records and death certificates | Women: BMD fem neck and incident CV disease: HR: 1.24 (95% CI 1.02 to 1.52) Men: BMD fem neck and incident CV disease: HR: 1.04 (95% CI 0.89 to 1.21) |
3 |
| Tamaki, 2009 [75] | Population-based (10) |
609 (100%) | Yes (60%) | No | 55.9 | Japanese | BMD lumbar spine and total hip by DXA | IMT values | <10 YSM: IMT OP vs normal bone mass: 1.55 vs 1.19 (P < 0.05) ≥YSM: IMT OP vs normal bone mass: 1.53 vs 1.28 (P < 0.05) |
3 |
| Browner, 1991 [5] | Population-based (2.8) |
9,704 (100%) | Yes | No | NA | Caucasian (99%) and Asian | BMD distal radius, prox radius and calcaneus by single photon absorptiometry | Overall mortality and CV mortality by death certificates | BMD and risk overall mortality: RR: 1.22 (95% CI 1.01 to 1.47) BMD and stroke mortality: RR: 1.75 (95% CI 1.15 to 2.65) BMD and CV mortality: RR: 1.17 (95% CI 0.92 to 1.51) |
3 |
| Trone, 2007 [68] | Population-based (7.6) |
1,580 (60%) | Yes (NA %) | No | 71.9 | Caucasian | Prevalence vertebral fracture by lateral spine radiographs | Overall mortality by death certificates | Women: prevalent vertebral fracture and overall mortality: HR: 1.15 (95% CI 0.83 to 1.59) Men: prevalent vertebral fracture and overall mortality: HR: 0.98 (95% CI 0.55 to 1.46) |
3 |
| Kado, 2000 [64] | Population-based (3.5) |
6,018 (100%) | Yes | No | 76.5 | Caucasian | BMD total hip by DXA | Overall and CV mortality by death certificates | BMD and overall mortality: RH: 1.3 (95% CI 1.1 to 1.4) BMD and CV mortality: RH: 1.3 (95% CI 1.0 to 1.9) |
4 |
| Trivedi, 2001 [67] | Population-based (6.7) |
1,002 (0%) | No women included | No | 69.7 | NA | BMD total hip by DXA | Overall and CV mortality by death certificates | BMD and overall mortality: RR: 0.79 (95% CI 0.65 to 0.97) BMD and CV mortality: RR: 0.72 (95% CI 0.56 to 0.93) |
4 |
| Tanko, 2005 [76] | Clinic-based (4) |
2,576 (100%) | Yes | No | 66.5 | NA | BMD lumbar spine and femoral neck by DXA | Incidence CV events self-reported and confirmed by primary documents | HR: 3.9 (95% CI 2.0 to 7.7) | 4 |
| Pinheiro, 2006 [66] | Population-based (5) |
208 (100%) | Yes | No | 75.1 | Caucasian | BMD lumbar spine, femoral neck and trochanter by DXA | Overall and CV mortality by death certificates | BMD and overall mortality: HR: 1.44 (95% CI 1.06 to 2.21) BMD and CV mortality: HR: 1.28 (95% CI 1.08 to 2.26) |
4 |
| Johansson, 1998 [7] | Population-based (7) |
1,468 (56%) | Yes | No | 74.0 | Caucasian | BMD calcaneus by DPA | Overall mortality by death certificates | Women: RR: 1.19 (95% CI 1.02 to 1.39) Men: RR: 1.23 (95% CI 1.10 to 1.41) |
4 |
| Mussolino, 2003 [65] | Population-based (18.5) |
3,402 (NA) | NA | Yes | NA | Caucasian (87%) and black | BMD phalangeal by single photon absorption | Stroke mortality by death certificates | Women: RR: 1.01 (95% CI 0.86 to 1.19) Men: RR: 1.13 (95% CI 0.93 to 1.38) Blacks: RR : 0.93 (95% CI 0.72 to 1.21) |
4 |
| Samelson, 2004 [70] | Population-based (30) |
2,059 (60%) | Yes (85,3-94%) | Yes | 60.2 | NA | Second MCA by radiogrammatry | Incidence coronary heart disease by hospital records and death certificates | Women: HR: 0.73 (95% CI 0.53 to 1.00) Men: HR: 1.14 (95% CI 0.84 to 1.56) |
4 |
| Kiel, 2001 [77] | Population-based (25) |
554 (66%) | NA | No | 54.4 | NA | Second MCA by radiogrammetry | AC by radiograph of the lumbar spine | Women: Sign association % change in MCA and change AC index (P = 0.01) Men: No association % change MCA and change AC index (P = 0.50) |
4 |
| Browner, 1993 [62] | Population-based (1.98) |
4,024 (100%) | Yes | Yes | NA | Caucasian | BMD distal radius and calcaneus by single photon absorptiometry | Incident strokes by hospital records and death certificates | HR: 1.31 (95% CI 1.03 to 1.67) | 5 |
| Von der Recke, 1999 [8] | Clinic-based (17) |
1,063 (100%) | Yes | Yes | 50 and 70 | NA | BMD distal forearm by single photon absorptiometry with 125I source | CV mortality by death certificates, hospital records and autopsy reports | Early menopause: RR: 2.3 (95% CI 1.0 to 5.3) Late menopause: RR: 1.3 (95% CI 0.9 to 1.8) |
5 |
| Silverman, 2004 [71] | Clinic-based (3) |
2,565 (100%) | Yes | No | 67 | Caucasian (95.8%) | Prevalence vertebral fracture by lateral spine radiographs | Incident CV event self-reported and confirmed by primary documents | CV event rate women with prevalent vertebral fracture vs no vertebral fracture: 15.1 vs 8.3 (P = 0.55) | 5 |
| Varosy, 2003 [73] | Clinic-based (4.1) |
2,763 (100%) | Yes | Yes | NA | NA | Prevalent and incident skeletal fracture self-reported. Incident fractures were confirmed by radiological reports | Incident coronay event by hospital records | HR: 0.75 (95% CI 0.57 to 0.98) | 5 |
| Gonzales-Macias, 2009 [63] | Clinic-based (3) |
5,201 (100%) | Yes | No | 72.3 | Caucasian | eBMD calcaneus by QUS | Overall and CV mortality by medical records | eBMD and overall mortality: HR: 1.19 (95% CI 0.97 to 1.45) eBMD and CV mortality: HR: 1.39 (95% CI 1.15 to 1.66) |
6 |
#adjusted for age; AC, aortic calcification; BMD, bone mineral density; DPA, dual photon absorptiometry; DXA, dual-energy x-ray absorptiometry; IMT, intima media thickness; MCA, metacarpal relative cortical area; NA, not available; QUS, quantitative ultrasonography; YSM, years since menopause.
Statistical analysis
A formal meta-analysis of the prospective studies investigating the association between bone mass and risk for cardiovascular events and mortality was not possible due to extended heterogeneity between studies with respect to the study population and methods used. Furthermore, the number of prospective studies that were eligible for pooling was too small for analysis. For this reason, narrative summaries are provided in the results section and quantitatively presented in Tables 1 and 2. The heterogeneity between studies in terms of study population and outcome measures is shown in Tables 1 and 2. Moreover, cross-sectional studies are shown in Table 3.
Table 3.
Cross-sectional studies investigating relationship CV disease and low BMD
| Study | Study population | Number of cases | % women | Outcome bone mass | Outcome CV disease | Main results # |
|---|---|---|---|---|---|---|
| Frye, 1992 [35] | Population-based | 200 | 100% | BMD lumbar spine and hip by single photon absorptiometry | AC by x-ray | Association AC and BMD lumbar spine: β-2.213 (P < 0.05) Association AC and BMD hip: β-0.661 (NS) |
| Barengolts, 1998 [32] | Clinic-based | 45 | 100% | BMD lumbar spine and hip by DXA | Coronary calcium score by EBT | Correlation BDM hip and calcium score: r-0.34 (P = 0.022) Correlation BMD spine and calcium score: r-0.28 (P = 0.056) |
| Jorgensen, 2001 [27] | Clinic-based | 63 | 52% | BMD femoral neck by DXA | Incident stroke | Women: OR: 6.6 (95% CI 1.8 to 24.8) Men: OR: 0.6 (95% CI 0.1 to 2.3) |
| Aoyagi, 2001 [40] | Population-based | 524 | 100% | BMD distal and proximal radius, calcaneus single photon absorptiometry by sinlge photon absorptiometry | AC by x-ray | BMD distal radius and AC: OR: 1.1 (95% CI 0.9 ro 1.3) BMD calcaneus and AC: OR: 1.1 (0.9 to 1.3) |
| Van der Klift, 2002 [29] | Population-based | 5,268 | 57% | BMD lumbar spine and hip by DXA | PAD by ABI | Women: PAD and BMD hip: OR: 1.35 (95% CI 1.02 to 1.79) Men: PAD and BMD hip: OR: 0.89 (95% CI 0.64 to 1.23) |
| Tanko, 2003 [39] | Population-based | 963 | 100% | BMD hip and lumbar spine by DXA | AC by x-ray | AC and BMD hip: β-0.10, 9 (P = 0.004) |
| Hirose, 2003 [56] | Clinic-based | 7,865 | 9% | OSI calcaneus | baPWV | Women: β-0.11 (P < 0.01) Men: β-0.07 (P < 0.01) |
| Pennisi, 2004 [50] | Clinic-based | 36 | 44% | BMD total body, lumbar spine, and hip by DXA and calcaneus by QUS | IMT and presence of plaque in carotid artery | 63% patients with BMD spine T <-1 93% patients with BMD hip T <-1 |
| Jorgensen, 2004 [47] | Population-based | 5,296 | 52% | BMD distal radius by single x-ray absorptiometry | IMT and prevalent plaque | BMD and IMT: NS BMD and prevalent plaque: OR: 0.90 (95% CI 0.75 to 1.07) BMD and echogenic plaque: OR: 0.51 (95% CI 0.31 to 0.83) |
| Montalcini, 2004 [49] | Clinic-based | 157 | 100% | BMD calcaneus by QUS | IMT | BMD and IMT: NS |
| Magnus, 2005 [23] | Population-based | 5,050 | 36% | BMD hip by DXA | Self reported CV events | Women: OR: 1.22 (0.80 to 1.86) Men: OR: 1.39 (95% CI 1.03 to 1.87) |
| Bakhireva, 2005 [31] | Population-based | 366 | 51% | BMD lumbar spine and hip by DXA | CAC by CT scan | Women: BMD hip and CAC: OR: 0.69 (95% CI 0.51 to 0.93) Men: BMD hip and CAC: OR: 1.03 (0.75 to 1.41) |
| Wong, 2005 [30] | Population-based | 3,998 | 50% | BMD lumbar spine and hip by DXA | PAD by ABI | Per SD increase in ABI sign associated with hip BMD: 0.5 (95% CI 0.02 to 0.9) |
| Yamada, 2005 [53] | Clinic-based | 260 | 59% | BMD lumbar spine by DXA and OSI calcanues | IMT carotid artery and femoral artery | BMD lumbar spine and FA-IMT: ρ-0.117 (P < 0.005) |
| Farhat, 2006 [34] | Population-based | 490 | 100% | vBMD spine by CT scan | AC and CAC by CT scan | AC and BMD: OR: 1.68 (95% CI 1.06 to 2.68) CAC and BMD: OR: 1.19 (95% CI 0.81 to 1.74) |
| Farhat, 2006 [19] | Population-based | 1,489 | 51% | BMD hip by DXA vBMD lumbar spine by QCT |
Prevalent CV disease self reported Prevalent PAD by ABI | Women: Prevalent CV disease and BMD hip: OR: 1.22 (95% CI 1.03 to 1.43) PAD and BMD hip: NS Men: Prevalent CV disease and BMD hip: NS PAD and BMD hip: OR: 1.39 (95% CI 1.03 to 1.84) |
| Yamada, 2006 [54] | Population-based | 149 | 100% | BMD lumbar spine by DXA and vBMD calcaneus by QCT | IMT and PWV | FA-IMT and BMD spine: β-0.067 (P < 0.05) PWV and BMD spine: NS |
| Sumino, 2006 [60] | Clinic-based | 315 | 100% | BMD lumbar spine by DXA | baPWV | Association baPWV and BMD: β-0.265 (P = 0.002) |
| Sinnot, 2006 [43] | Clinic-based | 480 | 65% | BMD lumbar spine by QCT | Calcium score by CT-scan | No correlation CAD and BMD in women and men |
| Shaffer, 2007 [51] | Population-based | 870 | 61% | BMD lumbar spine, hip and distal radius by DXA | IMT | Women >60 years: IMT and BMD spine: β-73.0 (P < 0.001) IMT and BMD hip: β-62.4 (P < 0.001) Men >60 years: IMT and BMD radius: β-27.0 (P < 0.001) |
| Sumino, 2007 [61] | Clinic-based | 85 | 100% | BMD lumbar spine by DXA | Brachial arterial endothelial function (FMD) | Correlation FMD and BMD: r .034 (P < 0.01) Association FMD and BMD: β 0.40 (P < 0.01) |
| Hyder, 2007 [36] | Clinic-based | 365 | 64% | BMD lumbar spine by CT-scan | Atherosclerotic calcium in carotid, coronary and iliac arteries by CT-scan | Women: Calcium score aorta and BMD: OR: 3.14 (95% CI 1.55 to 6.38) Calcium score iliac arteries and BMD: OR: 2.20 (95% CI 1.13 to 4.29) Men: Calcium score carotid and BMD: OR: 2.85 (95% CI 1.02 to 7.96) Calcium score aorta and BMD: OR: 5.90 (95% CI 1.78 to 19.6) |
| Shen, 2007 [42] | Population-based | 682 | 56% | BMD lumbar spine and hip by DXA | CAC by CT scan | CAC and BMD spine: -0.105 ± 0.132 (NS) CAC and BMD hip: 0.022 ± 0.142 (NS) |
| Sioka, 2007 [24] | Clinic-based | 21 | 0% | BMD lumbar spine and hip by DXA | CAD by angiography | BMD in severe CAD vs no CAD: 77.8% vs 37.5%, P =? |
| Sumino, 2008 [52] | Clinic-based | 175 | 100% | BMD lumbar spine by DXA | IMT | BMD and IMT β-0.313 (P = 0.001) |
| Kim, 2008 [48] | Clinic-based | 194 | 100% | BMD lumbar spine and hip by DXA Prevalent vertebral fracture |
IMT and prevalent plaque | BMD and IMT: NS BMD and plaque: NS Vertebral fracture and plaque: OR: 2.8 (95% CI 1.17 to 7.12) |
| Frost, 2008 [45] | Clinic-based | 54 | 100% | Lumbar spine and hip by DXA | IMT and PWV | BMD spine and IMT: r -.025 (P = 0.26) BMD hip and IMT: r-0.17 (NS) BMD and PWV: NS |
| Mangiafico, 2008 [57] | Clinic-based | 182 | 100% | BMD lumbar spine and hip DXA | PWA (AIx and PWV) | BMD hip and AIx: β-5.46 (P < 0.0001) BMD spine and Aix: β-3.29 (P < 0.0001) |
| Tekin, 2008 [25] | Clinic-based | 227 | 100% | BMD lumbar spine by DXA | Prevalence CAD | CAD and low BMD: OR: 0.68 (95% CI 0.39 to 1.28) |
| Broussard, 2008 [18] | Population-based | 3,881 | 51% | BMD total femur by DXA | Framingham CHD risk score by Framingham CHD prediction model | Women: moderate CHD risk and low BMD: OR: 1.45 (95% CI 1.03 to 2.06) high CHD risk and low BMD: OR: 1.73 (95% CI 1.12 to 2.66) Men: NS |
| Chow, 2008 [41] | Population-based | 693 | 54% | vBMD lumbar spine and hip by QCT and vBMD distal radius by HRpQCT | AC by CT-scan | Women: NS Men: NS |
| Hyder, 2009 [37] | NA | 1,909 | 50% | vBMD lumbar spine by CT scan | CAC and AAC score | Women: vBMD and CAC (P-trend <0.002) vBMD AND AAC (P-trend <0.004) Men: vBMD and CAC (P-trend <0.034) vBMD and AAC (P-trend <0.001) |
| Hmamouchi, 2009 [46] | Clinic-based | 72 | 100% | BMD lulmbar spine and hip by DXA | IMT in carotid artery and femoral artery | CA-IMT and BMD hip: r-0.330 (P < 0.05) FA-IMT and BMD hip: NS IMT and BMD lumbar spine: NS |
| Mikumo, 2009 [58] | Clinic-based | 143 | 100% | BMD lumbar spine by DXA | PWV | BMD and PWV: r-99.78 (NS) |
| Marcowitz, 2005 [20] | Clinic-based | 209 | 88% | Lumbar spine, hip and distal radius by DXA | CAD | Osteoporosis: OR: 5.58 (95% CI 2.59 to 12.0) for CAD |
| Ness, 2006 [38] | Clinic-based | 1,000 | 100% | Diagnosis osteoporosis or osteopenia by electronic medical records | AVD | Prevalence AVD osteoporotis vs osteopenia: 60% vs 35% (P < 0.001) Prevalence AVD osteoporis vs normal bone mass: 60% vs 22% (P < 0.001) |
| Gupta, 2006 [78] | Clinic-based | 101 | 100% | BMD lumbar spine and total hip by DXA | Prevalent CV disease | Prevalent CV disease in low BMD vs normal BMD: 61% vs 38% (P < 0.025) |
| Mangifico, 2006 [28] | Clinic-based | 345 | 100% | BMD lumbar spine and femoral neck by DXA | PAD by ABI | PAD and BMD lumbar spine: OR: 1.01 (95% CI 0.97 to 1.05) PAD and BMD hip: OR: 0.20 (95% CI 0.05 to 0.70) |
| Erbilen, 2007 [33] | Clinic-based | 74 | 0% | BMD lumbar spine and hip by DXA | CAD | Association BMD and CAD: OR: 5.4 (95% CI 1.66 to 17.49) |
| Sennerby, 2007 [21] | Clinic-based | 1,327 | 100% | Incident hip fracture by X-ray and hospital record | Prevalent CV disease by questionnaire | OR: 2.38 (95% CI 1.92 to 2.94) |
| Varma, 2008 [22] | Clinic-based | 198 | 74% | Lumbar spine and hip by DXA | Obstructive CAD | Prevalence CAD osteoporosis vs osteopenia: 76% vs 68% (P < 0.01) Prevalence CAD osteoporosis vs normal bone mass: 76% vs 47% (P < 0.005) |
| Seo, 2009 [59] | Clinic-based | 253 | 100% | BMD lumbar spine and hip by DXA | baPWV | Sign association BMD hip and baPWV: Β-0.123 (P < 0.05) |
| Pouwels, 2009 [16] | Clinic-based | 6,763 | 73% | Incident hip fracture | Incident stroke by ICD 9 code | Risk hip fracture after stroke Women: OR: 2.12 (95% CI 1.73 to 2.59) Men: OR: 1.63 (95% CI 1.17 to 2.28) |
#adjusted for confounders; BMD, bone mineral density; AC, aortic calcification; DXA, dual-energy x-ray absorptiometry; PAD, peripheral arterial disease; ABI, ankle brachial index; OSI, osteosono assessment index; baPWV, brachial-ankle pulse wave velocity; IMT, intimal medial thickness; CAC, coronary artery calcium; QCT, quantitative computerized tomography; PWV, pulse wave velocity; CAD, coronary artery disease; PWA, pulse wave analysis; AIx, augmentation index; CHD, coronary hearth disease; AVD, atherosclerotic vascular disease.
Results
Studies included
Our search strategy resulted in 2,886 references. The search strategy resulted in 70 relevant articles, including 9 studies prospectively assessing the relationship between CV disease and osteoporosis and 18 prospective studies about the inverse relationship. Figure 1 shows the flow-chart of included and excluded studies.
Figure 1.
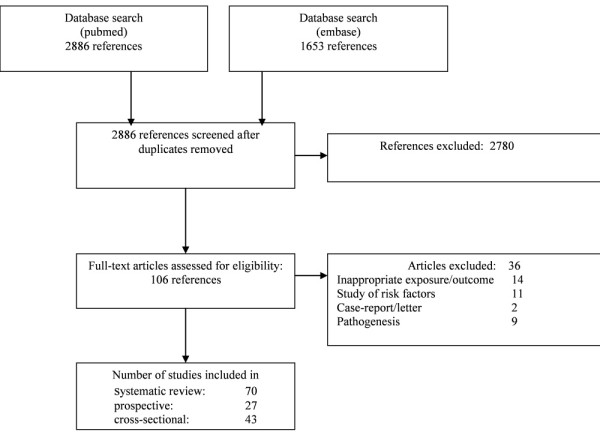
Flow-chart of the systematic review.
Study results
The relationship between CV disease and osteoporosis
Cardiovascular disease and fracture risk
Seven population-based cohort studies assessed the relationship between CV disease and fracture risk [1,2,4,12-15] (Table 1). An increased risk of incident fractures was observed in four studies with risk rates ranging from 1.2 to 6.7 [1,2,13,14].
The largest study included more than 30,000 twins with a follow-up duration of 20 years [13]. In this study, twins, without prevalent CV disease, were included at the age of 50 years and followed up until a first hip fracture, death or end of follow-up period. Twins were considered unexposed until the first CV event. An increased hip fracture risk was found after all diagnoses of CV disease in both men (hazard ratio (HR) 6.65; 95% CI 4.82 to 9.19) and women (HR 4.42; 95% CI 3.49 to 5.61).
Furthermore, this study showed that CHD was associated with an increased fracture risk (HR 2.32; 95% CI 1.91 to 2.84) as was cerebral vascular disease (HR 5.09 95% CI 4.18 to 6.20) [13]. This was confirmed in a large population case-control study. This case-control study was conducted using the Dutch PHARMO Record Linkage System database. Patients (n = 6,763) with a hip fracture were compared with age- and sex-matched patients without a hip fracture (n = 26,341), with the objective to evaluate the association between stroke and risk of hip fracture [16]. The prevalence of stroke was 3.3% in cases versus 1.5% in control patients. The risk for a hip fracture was increased in patients who experienced a stroke before the index date (OR 1.96; 95% CI 1.65 to 2.33).
Three studies looked at the association between PAD and fracture risk. PAD was associated with increased risk for non-vertebral fractures (HR 1.47; 95% CI 1.07 to 2.04) [2] and hip fractures (HR 3.20; 95% CI 2.28 to 4.50) [13]. In contrast, a smaller study in men and women, with shorter follow-up time, did not find an association between PAD and non-vertebral fracture risk [15]. Time of follow-up might be an important factor explaining different results, for the risk of fractures was highest more than 10 years after the diagnosis of PAD [13].
Longitudinal analysis in healthy postmenopausal women (n = 2,262) showed that aortic calcifications (AC) represented a strong predictor for fragility fractures: AC predicted a 2.3-fold increased risk for hip fracture [1]. Not only women, but also men with advanced AC have a two- to three-fold increased fracture risk [14]. However, a large population-based study with 21 years follow-up, found no evidence that severity of vascular calcification, measured as AC, is associated with an increased risk of incident hip fracture [12]. Conflicting results might be due to differences in population and methodology. The incident fracture rates were equal in comparison to the other studies.
Hence, although heterogeneity makes it difficult to draw firm conclusions, there is evidence that subjects with atherosclerotic disease are at an increased risk for frailty fractures. There are insufficient data to draw conclusions about fracture risk in patients with prevalent coronary or cerebral CV disease.
Cardiovascular disease and bone loss
Longitudinal data about CV disease and bone loss were available from six studies [1-4,15,17]. All studies showed that prevalent CV disease was associated with an increased bone loss during follow-up, independent of age and traditional risk factors. In addition, several cross-sectional studies similarly reported that prevalent CV disease is associated with low BMD [18-22]. In the next section the results are presented per subcategory of CV disease.
The association of CHD and BMD was only addressed in cross-sectional studies and all but one found an association with low BMD [20,22-25]. Several studies reported increased bone loss after an incident stroke. Particularly patients who are wheelchair-bound or have paretic limbs as a result of the stroke have significant bone loss within months after the stroke [26]. These studies were not included in this review, for the underlying pathogenesis is obvious. One study looked at bone density immediately after the stroke and found that female stroke patients have lower BMD than controls [27]. Since the BMD measurement was assessed within six days after the stroke, one may assume that the possible differences are not a result of immobilisation.
A large prospective study found that men with prevalent PAD had an increased rate of hip bone loss compared with men without PAD (-0.6% vs -0.3%, P < 0.001) [2]. In another, smaller, study the association between PAD and bone loss in women was weaker and not observed in men [15]. In addition, a number of cross-sectional studies showed that women and/or men with PAD have decreased BMD [19,28-30].
Numerous reports have looked at the association between subclinical atherosclerosis and osteoporosis. Men and women with progression of AC have significantly higher bone loss in the lumbar spine compared with subjects without AC progression (-1.5% vs 1.4%) [4]. This is in line with other studies where AC progression is associated with higher rates of bone loss in the proximal femur and metacarpal bones [1,3]. Furthermore, several studies confirmed the prospective data and showed that subjects with calcifications in the aorta, coronary arteries, carotid arteries or femoral arteries have significant lower BMD compared with controls [31-39]. Only a few studies fail to find an association [40-43]. In recent years, many studies have examined the association between atherosclerosis and osteoporosis. An increased IMT has been associated with severity of atherosclerosis and increased cardiovascular risk and considered useful in identifying subjects with increased risk [44]. An association between IMT and BMD was studied intensively and most of the studies reported an association of increased IMT with low bone density [45-54]. Endothelial dysfunction is considered to be an early phase of atherosclerosis and one way to measure this is to focus on arterial compliance. The endothelium plays an important role in determining vascular tone and dysfunction will result in increased arterial stiffness [55]. In line with earlier discussed results, an increased arterial stiffness is associated with low BMD [45,54,56-61].
Altogether, the results strongly suggest that subjects with subclinical atherosclerosis and early CV disease are at increased risk of bone loss. Again, there were insufficient data to reach conclusions about bone loss in patients with prevalent coronary or cerebral CV disease.
The relationship between osteoporosis and CV disease
Eighteen studies, most of moderate quality, reporting about the relationship between osteoporosis and CV disease were included. Results will be discussed per subcategory of CV disease, when possible.
Low bone mineral density and cardiovascular mortality
The association of osteoporosis with CV mortality was studied in 10 prospective studies [5,7,8,62-68] (Table 2). Low bone mass was inversely related with CV mortality in seven studies [5,7,8,62-64,66,67]. Postmenopausal women with a low BMD had a 1.2- to 2.3-fold increased risk of dying from CV events, independent of traditional CV risk factors [7,8,66]. Similar results were found in elderly men [7,67]. Studies in postmenopausal women with relative short follow-up periods (around three years) showed no or minimally significant elevated mortality rates [5,63,64]. Two large population-based studies in elderly men and women did not reveal a significant association between low bone mass and CV mortality [65,69]. The most recent and largest study determined the risk of CV mortality in 5,272 persons [69]. Women with low BMD had higher risk for CV mortality; however, this did not reach significance (relative risk (RR) 1.26; 95% CI 0.88 to 1.80). No association was found in men.
Focusing on the few studies that reported the results per CV subcategory, women with low bone mass had no or a small increased risk for mortality by coronary heart disease (RR 1.17; 95% CI 0.92 to 1.51) and (relative hazard 1.3; 95% CI 1.0 to 1.8), respectively [5,64] and two out of three studies showed that men and women with low BMD had a 1.3- to 1.7-fold increased risk for stroke mortality [5,62,65].
Low bone mineral density and incident cardiovascular disease
A total of six studies assessed the risk of incident CV events in persons with osteoporosis [6,62,70-73]. Most of them show a significant inverse relationship between BMD and incident CV events in women (HR 1.23 to 3.9) [6,39,62,70] but not in men [6,70]. Two studies related the prevalence of vertebral fractures with future CV events and were unable to find any association [68,71]. Surprisingly, one study showed that women with prevalent fractures and known CHD had a reduced risk for CV events [73].
Few articles assessed incident CV events separated per CV category. Three studies assessed the risk for CHD. Two studies showed an association with increased risk for CHD in postmenopausal women [72,73]. One study could not find an association in elderly men and women [70]. Cerebrovascular events were studied in two articles. Both found an increased risk for stroke in postmenopausal women with low BMD with hazard ratios of 1.31 and 4.1 [62,72].
There was a considerable heterogeneity in measurement of osteoporosis. It is shown that the specificity and sensitivity of the densitometry tests differs greatly, and the site of measurement plays an important role in diagnosing osteoporosis as well [74]. Only six studies used dual energy absorptiometry (DXA) measurements to assess BMD [6,64,66,67,69,75,76], while in the other studies BMD was measured with older techniques such as single photon absorptiometry, dual photon absorptiometry (DPA) or quantitative ultrasonography (QUS). Most studies measured BMD of the hip and lumbar spine, but also distal radius and heel were measured and in some the phalangeals.
Low bone mineral density and subclinical atherosclerosis
In addition to associations with CV events, low BMD has also been shown to be associated with surrogate markers of CV disease, such as vascular calcification. In women with the largest decrease in metacarpal cortical area during a 25-year follow-up, the most severe progression of aortic calcification was observed [77] and women with a prevalent vertebral fracture had a higher IMT measured 10 years later [75]. Moreover, results from several cross-sectional studies confirmed that both women and men with low bone mass, compared to subjects with normal bone mass, have significantly more subclinical atherosclerosis [20,28,31-34,37,38,45,48,49,51,52,78,79], increased risk of peripheral arterial disease [28,29,34,54] and other surrogate end markers for CV disease [57,60,61].
Taken together, there is some evidence that persons with low BMD are at increased risk for CV events and subsequent CV mortality. However, variations in study design, for example, study population and outcome measures, limits interpretation. Since only a few studies assessed the CV outcome divided per CV subcategory, no conclusions can be drawn concerning a relationship between osteoporosis and specific categories of CV disease.
Links between CV disease and osteoporosis
Common pathogenesis
CV disease is preceded by atherosclerosis, for example, arterial disease. Atherosclerosis is a long-term process in which deposits of cholesterol, cellular waste products and calcium accumulates in the arterial wall causing it to thicken. Clinically, atherosclerosis is manifested by coronary heart disease, cerebrovascular disease and peripheral arterial disease. Endothelial dysfunction is the first step in the pathogenesis of atherosclerosis and predicts future CV events [80]. Calcification in the aorta and coronary arteries, for example, vascular calcification, may be a surrogate marker for atherosclerosis and increased CV risk [81]. In a recent meta-analysis patients with calcifications were found to have an increased risk for CV mortality and events [10]. Presently, vascular calcification is regarded as an active process, regulated by factors known to be involved in the process of osteogenesis, such as bone morphogenetic protein (BMP), alkaline phosphatase (ALP), osteopontin (OPN) and matrix GLA protein (MGP) [82-85] (Figure 2). Accumulating evidence suggests that calcification is a consequence of active bone formation by osteoblast-like cells [86]. Vascular smooth muscle cells (VSMCs) are able to re-differentiate towards osteoblast-like cells and a subpopulation, that is, calcifying vascular cells (CVCs), were shown to form nodules and mineralisation spontaneously [87]. In vitro, these osteoblastic cells produce hydroxyapatite, a mineral important in bone formation [88]. In the following paragraphs some of the bone-related factors that are involved in vascular calcification will be discussed in more detail.
Figure 2.
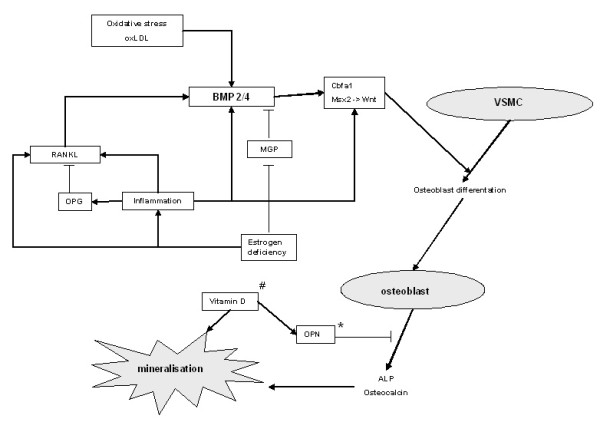
Vascular calcification. Vascular calcification is an active process regulated by factors known to be involved in the process of osteogenesis. Vascular smooth muscle cells are able to differentiate towards osteoblast-like cells, promoted by a variety of stimuli, including BMP, RANKL, oxidative stress, inflammation and estrogen deficiency. These osteoblastic cells produce osteocalcin and ALP, important factors in mineralisation. # Excessive vitamin D promotes mineralisation. * It is not clear whether OPN promotes or inhibits calcification in the arterial wall, in bone mineralisation it is a known mineralisation inhibitor. Abbreviations: ALP, alkaline phosphatase; BMP, bone morphogenetic protein; Cbfa1, core binding factor-α1; MGP, matrix GLA protein; Msx2, msh homeobox 2; OPG, osteoprotegerin; OPN, osteopontin; ox-LDL, oxidized low density lipoprotein; RANKL, receptor activator of nuclear factor-B ligand; VSMC, vascular smooth muscle cell; Wnt, combination of wingless and Int.
BMPs are members of the transforming growth factor-β superfamily and important factors in the regulation of osteoblast differentiation. BMP acts through upregulation of transcription factors important in bone metabolism, such as core binding factor-α1 (Cbfα1), also known as runt-related transcription factor 2 (Runx2), and msh homeobox 2 (Msx2). BMP appears to be an important mediator in vascular calcification. An increased expression of BMP2 and BMP4 is found in atherosclerotic lesions in endothelial cells, foam cells and VSMCs [88,89]. In vitro studies showed that several factors that are known to induce CV disease, such as oxidative stress, oxidized low-density lipoprotein (ox-LDL) and tumor necrosis factor alpha (TNF-α), are able to upregulate BMP expression in endothelial cells [90,91].
MGP is a calcium-binding protein and requires vitamin K to function. MGP is found to be expressed in areas with arterial calcification [92] and may be an important calcification inhibitor. MGP knock-out mice developed extensive calcification in coronary arteries [93]. Recently the mechanism by which MGP inhibits calcification has become clear. In vitro, MGP has been shown to inhibit calcification by binding to BMP2, thereby blocking the induction of osteoblasts [94].
OPN is a glycoprotein that accumulates in the extracellular matrix of bone tissue where it binds to hydroxyapatite and calcium. In bone, OPN is expressed by (pre-) osteoblasts and osteoclasts and is also found to be highly expressed in the atherosclerotic artery [89,92]. Whether it promotes or inhibits calcification in the arterial wall is not completely clear [95]. While high OPN serum levels are associated with vascular calcification [96] and vitamin increases OPN and subsequent calcification in bovine VSMC's [97], OPN is also shown to inhibit calcification by inhibiting de novo hydroxyapatite production [98].
ALP is found on the surface of osteoblasts and is often used as a marker for bone turnover. ALP is an enzyme that catalyses the hydrolysis of phosphate esters. Hydrolysis of pyrophosphate, which is an inhibitor of hydroxyapatite formation, is especially needed to facilitate normal mineralisation [99]. In vitro studies in VSMC's showed that the ALP expression is increased in response to inflammatory markers, LDL and oxidative stress and this increased expression was associated with increased mineralisation [100-102].
The recent identification of receptor activator of nuclear factor-kB (RANK), osteoprotegerin (OPG) and RANK ligand (RANKL) provides more insight into bone metabolism [103]. Most interestingly, there is increasing evidence that OPG is a key regulator in the pathogenesis of osteoporosis and vascular calcification. OPG production by osteoblastic cells is regulated by a number of factors, including BMP-2, inflammation, estrogen, vitamin D and oxidative stress [104]. OPG is expressed in various tissues, including the skeleton and vascular wall, and serves as a soluble decoy for RANKL [105]. Interestingly, OPG knock-out mice show, in addition to early-onset osteoporosis, increased vascular calcification [106]. In vitro studies have shown that OPG appears to be important for endothelial cell survival [107] and may inhibit active calcification [108]. Surprisingly, while experimental studies showed that OPG might protect against vascular calcification, OPG levels appear to be elevated in patients with CV disease. Several, but not all, clinical studies found a correlation of high OPG serum levels and more severe CV disease [45,50,62,109-111]. Other pathways interacting with OPG might explain this discrepant finding. Estrogen deficiency results in an increased vascular OPG/RANKL ratio with subsequent increased calcification in an animal model [112]. Furthermore, pro-inflammatory cytokines are shown to elevate OPG levels in patients with CV disease [113]. Thus, while OPG appears to play a role in the pathogenesis of atherosclerosis, the exact mechanism remains to be elucidated.
Another important mechanism linking CV disease and osteoporosis is Wnt signalling, a combination of the genes Wg (wingless) and Int. Animal models showed the important role of Wnt signalling in bone formation through lipoprotein receptor-related protein 5 (LRP5), lipoprotein receptor-related protein 6 (LRP6) and β-catenin [114]. Wnt signalling is suggested to play an important role in bone formation and bone adaptation to mechanical loading [115,116]. Interestingly, TNF-α [117], oxidative stress [118] and vitamin D [119] are shown to promote vascular calcification through the Wnt signalling pathway and this supports the hypothesis that Wnt signalling is an interesting new molecular mechanism that influences bone and vascular metabolism.
Common risk factors
CV disease and osteoporosis are both common diseases in elderly men and women. While the increased prevalence of both conditions is often attributed to aging, most of the associations found in observational studies remain significant after adjustment for age. Other important traditional risk factors are also shared, such as inactivity, smoking, estrogen deficiency and chronic inflammation, explaining part of the link between CV disease and osteoporosis [9].
Estrogen deficiency is considered an important risk factor for osteoporosis [120] and some studies suggest estrogen deficiency to be a cardiovascular risk factor [121-123]. Estrogen regulates bone turnover and the CV system directly and indirectly through the effects on the immune system, antioxidant system and other risk factors. After menopause, estrogen levels decrease rapidly resulting in an upregulated osteoclast formation and differentiation, inducing high bone turnover and accelerated bone loss [124]. Furthermore, following estrogen withdrawal the production and secretion of the pro-inflammatory cytokines interleukin-6 (IL-6), interleukin-1 and TNF-α is increased [116,125].
Presently, inflammation is considered to play an important role in the process of atherosclerosis [126,127]. Both cellular and humoral pathways of the immune response contribute to an important part in the pathogenesis of atherosclerosis [128]. Markers of inflammation, such as pro-inflammatory cytokines and C-reactive protein (CRP), are involved in the development of atherosclerosis and CRP predicts cardiovascular events independently of other CV risk factors [129,130]. There is accumulating evidence that inflammation influences bone metabolism and is considered to be the most important cause of postmenopausal osteoporosis. Pro-inflammatory cytokines enhance bone resorption directly through an induction of osteoclastogenesis or through the OPG pathway [116,131].
Recent research has identified new common mediators for vascular calcification and bone loss, such as hyperlipidemia, oxidative stress and vitamin D deficiency. An abnormal lipid profile, that is, high levels of total cholesterol, LDL and triglycerides and low levels of high-density lipoprotein (HDL), is known to play a key role in development of atherosclerosis and CV disease [132,133]. Interestingly, HDL is able to regulate the calcification of VSMCs [134]. HDL inhibited the spontaneous and cytokine induced osteogenic differentiation of CVCs in vitro. The role of lipids in the regulation of bone mass is more complicated. While experimental studies showed that ox-LDL influences bone metabolism [135], results in observational studies are contradictory [1,136-138].
Oxidative stress is believed to increase with age and is associated with hypertension and atherosclerosis [139]. Free radicals have important effects on osteoclast differentiation and function [140] and oxidative stress markers are significantly associated with BMD [141]. In vitro, minimally oxidized low-density lipoprotein (MM-LDL) enhances the differentiation of VSMC's towards osteoblastic cells. Interestingly, antioxidants inhibited these effects [100].
The prevalence of vitamin D deficiency is high among elderly men and women [142] and associated with osteoporosis and increased fracture risk [143]. Observational studies showed an inverse association of vitamin D deficiency with hypertension and CV events, suggesting a role for low vitamin D [144-148]. Proposed mechanisms are effects on myocardial gene expression, the renin-angiotensin axis or through secondary hyperparathyroidism. Important risk factors as physical condition and immobility were rarely assessed. Animal models and in vitro studies on the other hand, demonstrated that toxic levels of vitamin D induce vascular calcification [97,149]. Interestingly, osteoprotegerin has been shown to inhibit the vitamin-induced calcifications in an animal model [150]. It has been suggested that vitamin D has a biphasic relation with vascular calcification and that both vitamin D deficiency and vitamin D excess results in increased vascular calcification.
Genetic studies
In complex, multifactorial diseases genetic factors are believed to play an important role in the pathogenesis in addition to environmental influences. Identifying candidate genes offers opportunities to gain more insight into possible shared pathogenesis and common risk factors in CV disease and osteoporosis. Many candidate genes have been examined, mainly genes coding for known factors, such as cytokines, bone-associated factors and receptors. The genes that might be involved in both diseases will be discussed here.
Polymorphism in the IL-6 gene, a cytokine involved in bone metabolism and CV disease, might be an interestingly candidate gene. A G174C polymorphism in the promoter region of the IL-6 gene was shown to be associated with low bone mass in the radius in postmenopausal women [151] and with a high blood pressure and increased CV risk in men [152].
Vitamin D receptor polymorphisms have been associated in many studies with bone density [153,154]. Although this could not be replicated in a large meta-analysis, it did show that the Cdx2 polymorphism was associated with risk for vertebral fractures [155]. In addition, the BsmI polymorphism was associated with IMT and myocardial infarction (MI) [156,157], strengthening the possible role of vitamin D in linking CV disease and osteoporosis.
One of the most interesting candidate genes to mention is the OPG gene, located on chromosome 8 and several single nucleotide polymorphisms (SNPs) are identified in this gene. So far, studies were able to associate different SNPs with either bone density or vascular disease. SNPs A163G and T245G were associated with osteoporotic fractures [158]. The linked polymorphisms T950C and C1181C within the promoter region of the OPG gene were associated with an increased risk for CAD in men [159]. In addition, C1181C was also associated with first-ever intracerebral haemorrhage [160]. Furthermore, another SNP in the promoter region in the TATA box was related to vascular morphology and function [161].
A genetic defect in the Wnt signalling pathway was recently discovered in a family with features of metabolic syndrome and early onset coronary artery disease [162]. This rare mutation in the LRP6 gene is associated with dyslipidemia, hypertension and diabetes. This finding supports further research for mutations in genes involved in the Wnt signalling pathway.
Collagen type I is an important protein in the mineralisation matrix and connective tissue. Mutations in this gene are associated with low BMD and fracture risk [163]. Interestingly, besides low BMD, individuals with a SNP in the COL1A gene (rs42524) had an increased prevalence of stroke and MI [164].
The calcium-sensing receptor (CASR) is a receptor involved in the regulation of calcium homeostasis. A SNP in the CARS gene (A986S) was associated with higher serum calcium and increased prevalence of coronary artery disease (CAD) and MI [165]. This SNP was also associated with low BMD in premenopausal women [166]. However, the role in postmenopausal osteoporosis is not clear, since several studies showed no association of this SNP with BMD or fracture risk in postmenopausal women [167,168].
An interesting candidate gene to mention is the klotho gene. Defects in the klotho gene have been shown to result in arteriosclerosis and increased IMT in klotho deficient mice [169]. A SNP in this gene (G395A) was associated with CAD. Surprisingly, this same SNP was associated with bone density [170] and was suggested to be involved in the pathophysiology of bone loss. This SNP in the promoter region resulted in impaired function of the gene. What makes this gene interesting is that it might offer a new treatment approach, because the abnormalities seen in klotho-deficient mice can be reversed by restoring the klotho expression [171].
Finally, polymorphisms in the apolipoprotein E (APOE) gene has been studied intensively. It has been associated with hypertension, atherosclerotic disease and CV disease [172-174]. Furthermore, APOE gene polymorphisms have been suggested to be associated with low BMD and fracture risk. However, a recent meta-analysis was unable to show a strong and consistent association with BMD and fracture incidence [175].
Discussion
Our study is the first to systematically review the epidemiological literature about the association between CV disease and osteoporosis. An extensive literature search yielded 27 prospective studies addressing this relationship. Due to considerable heterogeneity in study design and outcome measurements the results could not be pooled. Focusing on the methodologically strongest studies (those with minimal selection bias and the appropriate assessments, that is, a methodological score of more than 3), our review indicates that the prevalent subclinical CV disease predicts future fractures and bone loss [2-4,13-15] (Table 4).
Table 4.
Summary of findings in high quality prospective studies
| Association | No association | |
|---|---|---|
| CV disease and OP | N = 6 | N = 0 |
| Bone mass and CV events | N = 3 | N = 2 |
Furthermore, there is some evidence that low bone mass predicts CV mortality and CV events [6,62,68,69,75].
Interestingly, several studies demonstrated shared risk factors, supporting the existence of a direct association between vascular calcification and bone biology.
Due to the substantial diversity of patients and study methods, pooled analysis was not considered appropriate. Although numerous efforts were made to investigate the association between CV disease and osteoporosis, a vast majority of studies used secondary outcome measurements, while a limited number of studies used primary outcome measurements such as incident CV events or osteoporosis. Furthermore, the population studied varied with respect to age, sex, baseline risk for CV events or fractures and ethnicity. Larger prospective studies in elderly persons, men and women, are needed to answer this question. To reduce heterogeneity we encourage that in new studies well-defined outcome measures should be incorporated, such as incident CV disease presented per subcategory of CV disease and measurement of BMD by DXA-scans on regular interval periods.
Conclusions
The current evidence indicates that individuals with prevalent (sub)clinical CV disease are at increased risk for bone loss and subsequent fractures. Presently, no firm conclusions can be drawn to which extent low BMD might be associated with increased cardiovascular risk. Age, estrogen deficiency and inflammation represent the most important common risk factors and the discovery of new pathways, for example, OGP/RANKL and Wnt signalling, might provide interesting new therapeutic options. Altogether our results suggest that bone density screening could be recommended in patients with prevalent CV disease.
Abbreviations
ABI: ankle brachial index; AC: aortic calcifications; ALP: alkaline phosphatase; APOE: apolipoprotein E; BMD: bone mineral density; BMP: bone morphogenetic protein; CAD: coronary artery disease; CASR: calcium-sensing receptor; Cbfa1: core binding factor-α1; CDH: coronary heart disease; CRP: C-reactive protein; CV: cardiovascular; CVC: calcifying vascular cells; DPA: dual photon absorptiometry; DXA: dual energy absorptiometry; HDL: high density lipoprotein; HR: hazard ratio; IL-6: interleukine-6; IMT: intima media thickness; LRP5: lipoprotein receptor-related protein 5; LRP6: lipoprotein receptor-related protein 6; MGP: matrix GLA protein; MI: myocardial infarction; MM-LDL: minimally oxidized low-density lipoprotein; Msx2: msh homeobox 2; OPG: osteoprotegerin; OPN: osteopontin; OR: odds ratio; ox-LDL: oxidized low density lipoprotein; PAD: peripheral arterial disease; QUS: quantitative ultrasonography; RANK: receptor activator of nuclear factor-B; RANKL: receptor activator of nuclear factor-B ligand; RR: relative risk; Runx2: runt-related transcription factor 2; SNP: single nucleotide polymorphism; TNF-α: tumour necrosis factor alpha; VSMC: vascular smooth muscle cell; Wnt: combination of wingless and Int.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DU conducted the data collection, interpretation and analysis of the data and drafted the manuscript. LT participated in interpretation and analysis of the data and helped to draft the manuscript. WL conceived of the hypothesis of the manuscript and participated in study design and coordination. MT, HR and WL helped to draft the manuscript. All authors critically reviewed, contributed to and approved the final manuscript.
Supplementary Material
Medline search. Complete medline search on 8 June 2010.
Quality assessment cohort studies. List of quality assessment of cohort studies as proposed by the Dutch Cochrane Collaboration.
Contributor Information
Debby den Uyl, Email: d.denuyl@vumc.nl.
Mike T Nurmohamed, Email: mt.nurmohamed@planet.nl.
Lilian HD van Tuyl, Email: l.vantuyl@vumc.nl.
Hennie G Raterman, Email: h.raterman@vumc.nl.
Willem F Lems, Email: wf.lems@vumc.nl.
Acknowledgements
We would like to thank Hans Ket (Clinical Library, VU medical centre, Amsterdam) for his assistance in collecting the literature for this systematic review.
References
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-C. [DOI] [PubMed] [Google Scholar]
- Farhat GN, Newman AB, Sutton-Tyrrell K, Matthews KA, Boudreau R, Schwartz AV, Harris T, Tylavsky F, Visser M, Cauley JA. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18:999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Johansson C, Black D, Johnell O, Oden A, Mellstrom D. Bone mineral density is a predictor of survival. Calcif Tissue Int. 1998;63:190–196. doi: 10.1007/s002239900513. [DOI] [PubMed] [Google Scholar]
- von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/S0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/VHRM.S4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checklist quality assessment cohort studies. Dutch Cochrane Centre; 2010. [Google Scholar]
- Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaelsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- von Mühlen D, Allison M, Jassal SK, Barrett-Connor E. Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporos Int. 2009;20:2071–2078. doi: 10.1007/s00198-009-0912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels S, Lalmohamed A, Leufkens B, de Boer A, Cooper C, van Staa T, de Vries F. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke. 2009;40:3281–3285. doi: 10.1161/STROKEAHA.109.554055. [DOI] [PubMed] [Google Scholar]
- Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Magnus JH. Coronary heart disease risk and bone mineral density among U.S. women and men. J Womens Health (Larchmt) 2008;17:479–490. doi: 10.1089/jwh.2007.0593. [DOI] [PubMed] [Google Scholar]
- Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int. 2006;79:102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–1063. doi: 10.1016/j.amjcard.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–1362. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- Varma R, Aronow WS, Basis Y, Singh T, Kalapatapu K, Weiss MB, Pucillo AL, Monsen CE. Relation of bone mineral density to frequency of coronary heart disease. Am J Cardiol. 2008;101:1103–1104. doi: 10.1016/j.amjcard.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Magnus JH, Broussard DL. Relationship between bone mineral density and myocardial infarction in US adults. Osteoporos Int. 2005;16:2053–2062. doi: 10.1007/s00198-005-1999-9. [DOI] [PubMed] [Google Scholar]
- Sioka C, Goudevenos J, Pappas K, Bougias C, Papadopoulos A, Grammatikopoulos K, Fotopoulos A. Bone mineral density and coronary atherosclerosis. Calcif Tissue Int. 2007;81:333. doi: 10.1007/s00223-007-9070-9. [DOI] [PubMed] [Google Scholar]
- Tekin GO, Kekilli E, Yagmur J, Uckan A, Yagmur C, Aksoy Y, Turhan H, Yetkin E. Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography. Int J Cardiol. 2008;131:66–69. doi: 10.1016/j.ijcard.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kuno H, Kaji M, Ohshima Y, Asoh T, Oizumi K. Increased bone resorption during the first year after stroke. Stroke. 1998;29:1373–1377. doi: 10.1161/01.str.29.7.1373. [DOI] [PubMed] [Google Scholar]
- Jorgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke. 2001;32:47–51. doi: 10.1161/01.str.32.1.47. [DOI] [PubMed] [Google Scholar]
- Mangiafico RA, Russo E, Riccobene S, Pennisi P, Mangiafico M, D'Amico F, Fiore CE. Increased prevalence of peripheral arterial disease in osteoporotic postmenopausal women. J Bone Miner Metab. 2006;24:125–131. doi: 10.1007/s00774-005-0658-8. [DOI] [PubMed] [Google Scholar]
- van der Klift M, Pols HA, Hak AE, Witteman JC, Hofman A, de Laet CE. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcif Tissue Int. 2002;70:443–449. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- Wong SY, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YY, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Ms Os, Hong Kong. Osteoporos Int. 2005;16:1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Barrett-Connor EL, Laughlin GA, Kritz-Silverstein D. Differences in association of bone mineral density with coronary artery calcification in men and women: the Rancho Bernardo Study. Menopause. 2005;12:691–698. doi: 10.1097/01.gme.0000184422.50696.ef. [DOI] [PubMed] [Google Scholar]
- Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- Erbilen E, Yazici S, Ozhan H, Bulur S, Ordu S, Yazici M. Relationship between angiographically documented coronary artery disease and low bone mass in men. Circ J. 2007;71:1095–1098. doi: 10.1253/circj.71.1095. [DOI] [PubMed] [Google Scholar]
- Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- Frye MA, Melton LJ III, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- Hyder JA, Allison MA, Criqui MH, Wright CM. Association between systemic calcified atherosclerosis and bone density. Calcif Tissue Int. 2007;80:301–306. doi: 10.1007/s00223-007-9004-6. [DOI] [PubMed] [Google Scholar]
- Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness J, Aronow WS. Comparison of prevalence of atherosclerotic vascular disease in postmenopausal women with osteoporosis or osteopenia versus without osteoporosis or osteopenia. Am J Cardiol. 2006;97:1427–1428. doi: 10.1016/j.amjcard.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- Aoyagi K, Ross PD, Orloff J, Davis JW, Katagiri H, Wasnich RD. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69:20–24. doi: 10.1007/s002230020003. [DOI] [PubMed] [Google Scholar]
- Chow JT, Khosla S, Melton LJ III, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. J Bone Miner Res. 2008;23:1601–1612. doi: 10.1359/jbmr.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Bielak LF, Streeten EA, Ryan KA, Rumberger JA, Sheedy PF, Shuldiner AR, Peyser PA, Mitchell BD. Relationship between vascular calcification and bone mineral density in the Old-order Amish. Calcif Tissue Int. 2007;80:244–250. doi: 10.1007/s00223-007-9006-4. [DOI] [PubMed] [Google Scholar]
- Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202. doi: 10.1007/s00223-005-0244-z. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- Frost ML, Grella R, Millasseau SC, Jiang BY, Hampson G, Fogelman I, Chowienczyk PJ. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int. 2008;83:112–120. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- Hmamouchi I, Allali F, Khazzani H, Bennani L, El Mansouri L, Ichchou L, Cherkaoui M, Abouqal R, Hajjaj-Hassouni N. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC Public Health. 2009;9:388. doi: 10.1186/1471-2458-9-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. Am J Epidemiol. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim YM, Cho MA, Rhee Y, Hur KY, Kang ES, Cha BS, Lee EJ, Lee HC, Lim SK. Echogenic carotid artery plaques are associated with vertebral fractures in postmenopausal women with low bone mass. Calcif Tissue Int. 2008;82:411–417. doi: 10.1007/s00223-008-9141-6. [DOI] [PubMed] [Google Scholar]
- Montalcini T, Emanuele V, Ceravolo R, Gorgone G, Sesti G, Perticone F, Pujia A. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. Am J Cardiol. 2004;94:266–269. doi: 10.1016/j.amjcard.2004.03.083. [DOI] [PubMed] [Google Scholar]
- Pennisi P, Signorelli SS, Riccobene S, Celotta G, Di Pino L, La Malfa T, Fiore CE. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int. 2004;15:389–395. doi: 10.1007/s00198-003-1550-9. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Kammerer CM, Rainwater DL, O'Leary DH, Bruder JM, Bauer RL, Mitchell BD. Decreased bone mineral density is correlated with increased subclinical atherosclerosis in older, but not younger, Mexican American women and men: the San Antonio Family Osteoporosis Study. Calcif Tissue Int. 2007;81:430–441. doi: 10.1007/s00223-007-9079-0. [DOI] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H, Takayama Y, Kanda T, Murakami M, Kurabayashi M. Relationship between carotid atherosclerosis and lumbar spine bone mineral density in postmenopausal women. Hypertens Res. 2008;31:1191–1197. doi: 10.1291/hypres.31.1191. [DOI] [PubMed] [Google Scholar]
- Yamada S, Inaba M, Goto H, Nagata M, Ueda M, Nakatuka K, Tahara H, Yokoyama H, Emoto M, Shoji T, Nishizawa Y. Significance of intima-media thickness in femoral artery in the determination of calcaneus osteo-sono index but not of lumbar spine bone mass in healthy Japanese people. Osteoporos Int. 2005;16:64–70. doi: 10.1007/s00198-004-1642-1. [DOI] [PubMed] [Google Scholar]
- Yamada S, Inaba M, Goto H, Nagata-Sakurai M, Kumeda Y, Imanishi Y, Emoto M, Ishimura E, Nishizawa Y. Associations between physical activity, peripheral atherosclerosis and bone status in healthy Japanese women. Atherosclerosis. 2006;188:196–202. doi: 10.1016/j.atherosclerosis.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A. Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab. 2003;88:2573–2578. doi: 10.1210/jc.2002-021511. [DOI] [PubMed] [Google Scholar]
- Mangiafico RA, Alagona C, Pennisi P, Parisi N, Mangiafico M, Purrello F, Fiore CE. Increased augmentation index and central aortic blood pressure in osteoporotic postmenopausal women. Osteoporos Int. 2008;19:49–56. doi: 10.1007/s00198-007-0438-5. [DOI] [PubMed] [Google Scholar]
- Mikumo M, Okano H, Yoshikata R, Ishitani K, Ohta H. Association between lumbar bone mineral density and vascular stiffness as assessed by pulse wave velocity in postmenopausal women. J Bone Miner Metab. 2009;27:89–94. doi: 10.1007/s00774-008-0014-x. [DOI] [PubMed] [Google Scholar]
- Seo SK, Cho S, Kim HY, Choi YS, Park KH, Cho DJ, Lee BS. Bone mineral density, arterial stiffness, and coronary atherosclerosis in healthy postmenopausal women. Menopause. 2009;16:937–943. doi: 10.1097/gme.0b013e3181a15552. [DOI] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Sakamaki T, Kurabayashi M. Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas. 2006;55:212–218. doi: 10.1016/j.maturitas.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H, Takayama Y, Kanda T, Murakami M, Kurabayashi M. Relationship between brachial arterial endothelial function and lumbar spine bone mineral density in postmenopausal women. Circ J. 2007;71:1555–1559. doi: 10.1253/circj.71.1555. [DOI] [PubMed] [Google Scholar]
- Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- González-Macías J, Marín F, Vila J, Carrasco E, Benavides P, Castell MV, Magaña JE, Chavida F, Díez-Pérez A. ECOSAP. Relationship between bone quantitative ultrasound and mortality: a prospective study. Osteoporos Int. 2009;20:257–264. doi: 10.1007/s00198-008-0645-8. [DOI] [PubMed] [Google Scholar]
- Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc. 2004;52:1662–1667. doi: 10.1111/j.1532-5415.2004.52458.x. [DOI] [PubMed] [Google Scholar]
- Mussolino ME, Madans JH, Gillum RF. Bone mineral density and stroke. Stroke. 2003;34:e20–e22. doi: 10.1161/01.STR.0000065826.23815.A5. [DOI] [PubMed] [Google Scholar]
- Pinheiro MM, Castro CM, Szejnfeld VL. Low femoral bone mineral density and quantitative ultrasound are risk factors for new osteoporotic fracture and total and cardiovascular mortality: a 5-year population-based study of Brazilian elderly women. J Gerontol A Biol Sci Med Sci. 2006;61:196–203. doi: 10.1093/gerona/61.2.196. [DOI] [PubMed] [Google Scholar]
- Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12:259–265. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- Trone DW, Kritz-Silverstein D, von Muhlen DG, Wingard DL, Barrett-Connor E. Is radiographic vertebral fracture a risk factor for mortality? Am J Epidemiol. 2007;166:1191–1197. doi: 10.1093/aje/kwm206. [DOI] [PubMed] [Google Scholar]
- Mussolino ME, Armenian HK. Low bone mineral density, coronary heart disease, and stroke mortality in men and women: the Third National Health and Nutrition Examination Survey. Ann Epidemiol. 2007;17:841–846. doi: 10.1016/j.annepidem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, Wilson PW, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Delmas PD, Kulkarni PM, Stock JL, Wong M, Plouffe L Jr. Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J Am Geriatr Soc. 2004;52:1543–1548. doi: 10.1111/j.1532-5415.2004.52420.x. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- Varosy PD, Shlipak MG, Vittinghoff E, Black DM, Herrington D, Hulley SB, Browner WS. Fracture and the risk of coronary events in women with heart disease. Am J Med. 2003;115:196–202. doi: 10.1016/S0002-9343(03)00330-9. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–541. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- Tamaki J, Iki M, Hirano Y, Sato Y, Kajita E, Kagamimori S, Kagawa Y, Yoneshima H. Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2009;20:53–60. doi: 10.1007/s00198-008-0633-z. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Qin G, Alexandersen P, Bagger YZ, Christiansen C. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporos Int. 2005;16:184–190. doi: 10.1007/s00198-004-1662-x. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- Gupta G, Aronow WS. Atherosclerotic vascular disease may be associated with osteoporosis or osteopenia in postmenopausal women: a preliminary study. Arch Gerontol Geriatr. 2006;43:285–288. doi: 10.1016/j.archger.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Jorgensen L, Joakimsen O, Mathiesen EB, Ahmed L, Berntsen GK, Fonnebo V, Joakimsen R, Njolstad I, Schirmer H, Jacobsen BK. Carotid plaque echogenicity and risk of nonvertebral fractures in women: a longitudinal population-based study. Calcif Tissue Int. 2006;79:207–213. doi: 10.1007/s00223-006-0071-x. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O'Fallon WM, Gentile F, Whisnant JP, Wiebers DO, Seward JB. Independent association of high blood pressure and aortic atherosclerosis: A population-based study. Circulation. 2000;102:2087–2093. doi: 10.1161/01.cir.102.17.2087. [DOI] [PubMed] [Google Scholar]
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- Mody N, Tintut Y, Radcliff K, Demer LL. Vascular calcification and its relation to bone calcification: possible underlying mechanisms. J Nucl Cardiol. 2003;10:177–183. doi: 10.1067/mnc.2003.0012. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Demer LL. Recent advances in multifactorial regulation of vascular calcification. Curr Opin Lipidol. 2001;12:555–560. doi: 10.1097/00041433-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Watson KE, Stanford WP, Demer LL. Atherosclerotic calcification: relation to developmental osteogenesis. Am J Cardiol. 1995;75:88B–91B. doi: 10.1016/0002-9149(95)80020-S. [DOI] [PubMed] [Google Scholar]
- Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 2004;320:424–427. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Osteoporosis and atherosclerosis: biological linkages and the emergence of dual-purpose therapies. QJM. 2005;98:467–484. doi: 10.1093/qjmed/hci077. [DOI] [PubMed] [Google Scholar]
- Ohmori R, Momiyama Y, Taniguchi H, Takahashi R, Kusuhara M, Nakamura H, Ohsuzu F. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis. 2003;170:333–337. doi: 10.1016/S0021-9150(03)00298-3. [DOI] [PubMed] [Google Scholar]
- Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–1306. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/S0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91:9–16. doi: 10.1161/01.RES.0000026421.61398.F2. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J Biol Chem. 2000;275:20959–20962. doi: 10.1074/jbc.C000290200. [DOI] [PubMed] [Google Scholar]
- Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omland T, Ueland T, Jansson AM, Persson A, Karlsson T, Smith C, Herlitz J, Aukrust P, Hartford M, Caidahl K. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–633. doi: 10.1016/j.jacc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- Siepi D, Marchesi S, Vaudo G, Lupattelli G, Bagaglia F, Pirro M, Brozzetti M, Roscini AR, Mannarino E. Preclinical vascular damage in white postmenopausal women: the relevance of osteoprotegerin. Metabolism. 2008;57:321–325. doi: 10.1016/j.metabol.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Choi BG, Vilahur G, Cardoso L, Fritton JC, Ibanez B, Zafar MU, Yadegar D, Speidl WS, Schaffler MB, Fuster V, Badimon JJ. Ovariectomy increases vascular calcification via the OPG/RANKL cytokine signalling pathway. Eur J Clin Invest. 2008;38:211–217. doi: 10.1111/j.1365-2362.2008.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- Shalhoub V, Shatzen E, Henley C, Boedigheimer M, McNinch J, Manoukian R, Damore M, Fitzpatrick D, Haas K, Twomey B, Kiaei P, Ward S, Lacey DL, Martin D. Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif Tissue Int. 2006;79:431–442. doi: 10.1007/s00223-006-0126-z. [DOI] [PubMed] [Google Scholar]
- Meema HE. Menopausal and aging changes in muscle mass and bone mineral content. A roentgenographic study. J Bone Joint Surg Am. 1966;48:1138–1144. [PubMed] [Google Scholar]
- Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab. 2002;87:1062–1067. doi: 10.1210/jc.87.3.1062. [DOI] [PubMed] [Google Scholar]
- van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–718. doi: 10.1016/S0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- Vogt MT, San VR, Forrest KY, Nevitt MC, Cauley JA. Bone mineral density and aortic calcification: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1997;45:140–145. doi: 10.1111/j.1532-5415.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/er.23.1.90. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–2838. doi: 10.1001/jama.256.20.2835. [DOI] [PubMed] [Google Scholar]
- Kinosian B, Glick H, Garland G. Cholesterol and coronary heart disease: predicting risks by levels and ratios. Ann Intern Med. 1994;121:641–647. doi: 10.7326/0003-4819-121-9-199411010-00002. [DOI] [PubMed] [Google Scholar]
- Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–576. doi: 10.1161/01.RES.0000036607.05037.DA. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, Battaglia E. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74:136–142. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health (Larchmt) 2006;15:261–270. doi: 10.1089/jwh.2006.15.261. [DOI] [PubMed] [Google Scholar]
- Samelson EJ, Cupples LA, Hannan MT, Wilson PW, Williams SA, Vaccarino V, Zhang Y, Kiel DP. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study. Bone. 2004;34:557–561. doi: 10.1016/j.bone.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103:255–261. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/er.22.4.477. [DOI] [PubMed] [Google Scholar]
- Gallacher SJ, McQuillian C, Harkness M, Finlay F, Gallagher AP, Dixon T. Prevalence of vitamin D inadequacy in Scottish adults with non-vertebral fragility fractures. Curr Med Res Opin. 2005;21:1355–1361. doi: 10.1185/030079905X59148. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- Kitagawa S, Yamaguchi Y, Kunitomo M, Imaizumi N, Fujiwara M. Altered vasoconstrictor responsiveness in vitamin D-induced arteriosclerotic rat aortas. Jpn J Pharmacol. 1993;61:283–289. doi: 10.1254/jjp.61.283. [DOI] [PubMed] [Google Scholar]
- Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borel O, Sornay-Rendu E, Duboeuf F, Jeffery R, Woo P, Delmas PD. Association between a functional interleukin-6 gene polymorphism and peak bone mineral density and postmenopausal bone loss in women: the OFELY study. Bone. 2002;31:43–50. doi: 10.1016/S8756-3282(02)00810-4. [DOI] [PubMed] [Google Scholar]
- Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin-6-174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J. 2001;22:2243–2252. doi: 10.1053/euhj.2001.2678. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Umbach DM. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J Bone Miner Res. 1996;11:1841–1849. doi: 10.1002/jbmr.5650111203. [DOI] [PubMed] [Google Scholar]
- Gong G, Stern HS, Cheng SC, Fong N, Mordeson J, Deng HW, Recker RR. The association of bone mineral density with vitamin D receptor gene polymorphisms. Osteoporos Int. 1999;9:55–64. doi: 10.1007/s001980050116. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Ralston SH, Brandi ML, Carey AH, Grinberg D, Langdahl BL, Lips P, Lorenc R, Obermayer-Pietsch B, Reeve J, Reid DM, Amedei A, Bassiti A, Bustamante M, Husted LB, Diez-Perez A, Dobnig H, Dunning AM, Enjuanes A, Fahrleitner-Pammer A, Fang Y, Karczmarewicz E, Kruk M, van Leeuwen JP, Mavilia C, van Meurs JB, Mangion J, McGuigan FE, Pols HA, Renner W. et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med. 2006;145:255–264. doi: 10.7326/0003-4819-145-4-200608150-00005. [DOI] [PubMed] [Google Scholar]
- Kammerer CM, Dualan AA, Samollow PB, Perisse AR, Bauer RL, MacCluer JW, O'Leary DH, Mitchell BD. Bone mineral density, carotid artery intimal medial thickness, and the vitamin D receptor BsmI polymorphism in Mexican American women. Calcif Tissue Int. 2004;75:292–298. doi: 10.1007/s00223-004-0215-9. [DOI] [PubMed] [Google Scholar]
- Ortlepp JR, Krantz C, Kimmel M, von Korff A, Vesper K, Schmitz F, Mevissen V, Janssens U, Franke A, Hanrath P, Zerres K, Hoffmann R. Additive effects of the chemokine receptor 2, vitamin D receptor, interleukin-6 polymorphisms and cardiovascular risk factors on the prevalence of myocardial infarction in patients below 65 years. Int J Cardiol. 2005;105:90–95. doi: 10.1016/j.ijcard.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Miner Res. 2002;17:1245–1255. doi: 10.1359/jbmr.2002.17.7.1245. [DOI] [PubMed] [Google Scholar]
- Soufi M, Schoppet M, Sattler AM, Herzum M, Maisch B, Hofbauer LC, Schaefer JR. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J Clin Endocrinol Metab. 2004;89:3764–3768. doi: 10.1210/jc.2003-032054. [DOI] [PubMed] [Google Scholar]
- Strand M, Soderstrom I, Wiklund PG, Hallmans G, Weinehall L, Soderberg S, Olsson T. Polymorphisms at the osteoprotegerin and interleukin-6 genes in relation to first-ever stroke. Cerebrovasc Dis. 2007;24:418–425. doi: 10.1159/000108431. [DOI] [PubMed] [Google Scholar]
- Brandstrom H, Stiger F, Lind L, Kahan T, Melhus H, Kindmark A. A single nucleotide polymorphism in the promoter region of the human gene for osteoprotegerin is related to vascular morphology and function. Biochem Biophys Res Commun. 2002;293:13–17. doi: 10.1016/S0006-291X(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann V, Ralston SH. Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone. 2003;32:711–717. doi: 10.1016/S8756-3282(03)00087-5. [DOI] [PubMed] [Google Scholar]
- Lindahl K, Rubin CJ, Brandstrom H, Karlsson MK, Holmberg A, Ohlsson C, Mellstrom D, Orwoll E, Mallmin H, Kindmark A, Ljunggren O. Heterozygosity for a coding SNP in COL1A2 confers a lower BMD and an increased stroke risk. Biochem Biophys Res Commun. 2009;384:501–505. doi: 10.1016/j.bbrc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Marz W, Seelhorst U, Wellnitz B, Tiran B, Obermayer-Pietsch B, Renner W, Boehm BO, Ritz E, Hoffmann MM. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab. 2007;92:2363–2369. doi: 10.1210/jc.2006-0071. [DOI] [PubMed] [Google Scholar]
- Lorentzon M, Lorentzon R, Lerner UH, Nordstrom P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur J Endocrinol. 2001;144:257–261. doi: 10.1530/eje.0.1440257. [DOI] [PubMed] [Google Scholar]
- Bollerslev J, Wilson SG, Dick IM, Devine A, Dhaliwal SS, Prince RL. Calcium-sensing receptor gene polymorphism A986S does not predict serum calcium level, bone mineral density, calcaneal ultrasound indices, or fracture rate in a large cohort of elderly women. Calcif Tissue Int. 2004;74:12–17. doi: 10.1007/s00223-002-0066-1. [DOI] [PubMed] [Google Scholar]
- Takacs I, Speer G, Bajnok E, Tabak A, Nagy Z, Horvath C, Kovacs K, Lakatos P. Lack of association between calcium-sensing receptor gene "A986S" polymorphism and bone mineral density in Hungarian postmenopausal women. Bone. 2002;30:849–852. doi: 10.1016/S8756-3282(02)00741-X. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H, Inoue S, Nabeshima Y, Nakamura K, Kuro-o M, Kawaguchi H. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Niu W, Qi Y, Qian Y, Gao P, Zhu D. The relationship between apolipoprotein E epsilon2/epsilon3/epsilon4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens Res. 2009;32:1060–1066. doi: 10.1038/hr.2009.164. [DOI] [PubMed] [Google Scholar]
- Paternoster L, Martinez-Gonzalez NA, Charleton R, Chung M, Lewis S, Sudlow CL. Genetic effects on carotid intima-media thickness: systematic assessment and meta-analyses of candidate gene polymorphisms studied in more than 5000 subjects. Circ Cardiovasc Genet. 2010;3:15–21. doi: 10.1161/CIRCGENETICS.108.834366. [DOI] [PubMed] [Google Scholar]
- Xin XY, Song YY, Ma JF, Fan CN, Ding JQ, Yang GY, Chen SD. Gene polymorphisms and risk of adult early-onset ischemic stroke: A meta-analysis. Thromb Res. 2009;124:619–624. doi: 10.1016/j.thromres.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Peter I, Crosier MD, Yoshida M, Booth SL, Cupples LA, Dawson-Hughes B, Karasik D, Kiel DP, Ordovas JM, Trikalinos TA. Associations of APOE gene polymorphisms with bone mineral density and fracture risk: a meta-analysis. Osteoporos Int. 2010. in press . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Medline search. Complete medline search on 8 June 2010.
Quality assessment cohort studies. List of quality assessment of cohort studies as proposed by the Dutch Cochrane Collaboration.


