Abstract
In aerobic organisms, protection from oxidative damage involves the combined action of enzymatic and nonproteinaceous cellular factors that collectively remove harmful reactive oxygen species. One class of nonproteinaceous antioxidants includes small molecule complexes of manganese (Mn) that can scavenge superoxide anion radicals and provide a backup for superoxide dismutase enzymes. Such Mn antioxidants have been identified in diverse organisms; however, nothing regarding their physiology in the context of cellular adaptation to stress was known. Using a molecular genetic approach in Bakers’ yeast, Saccharomyces cerevisiae, we report that the Mn antioxidants can fall under control of the same pathways used for nutrient sensing and stress responses. Specifically, a serine/threonine PAS-kinase, Rim15p, that is known to integrate phosphate, nitrogen, and carbon sensing, can also control Mn antioxidant activity in yeast. Rim15p is negatively regulated by the phosphate-sensing kinase complex Pho80p/Pho85p and by the nitrogen-sensing Akt/S6 kinase homolog, Sch9p. We observed that loss of either of these upstream kinase sensors dramatically inhibited the potency of Mn as an antioxidant. Downstream of Rim15p are transcription factors Gis1p and the redundant Msn2/Msn4p pair that typically respond to nutrient and stress signals. Both transcription factors were found to modulate the potency of the Mn antioxidant but in opposing fashions: loss of Gis1p was seen to enhance Mn antioxidant activity whereas loss of Msn2/4p greatly suppressed it. Our observed roles for nutrient and stress response kinases and transcription factors in regulating the Mn antioxidant underscore its physiological importance in aerobic fitness.
ADAPTATION to life in oxygen has entailed evolution of numerous enzymatic and nonenzymatic methods for detoxifying reactive oxygen species and repairing damage. Much is known about the widely spread superoxide dismutase (SOD) enzymes that disproportionate superoxide anion into oxygen and hydrogen peroxide (McCord and Fridovich 1969; Abreu and Cabelli 2010). Less understood is the class of small molecule nonproteinaceous manganese (Mn) containing complexes that can substitute for SOD in a variety of organisms. So-called “Mn antioxidants” were first identified in Lactobaccilus plantarum that lacks SOD enzymes, but is aerobically viable due to intracellular accumulation of millimolar Mn (Archibald and Fridovich 1981a,b, 1982a,b; Archibald and Duong 1984). High levels of Mn have also been shown to substitute for SOD in strains of Escherichia coli (Al-Maghrebi et al. 2002), Neisseria gonorrheae (Tseng et al. 2001), and the yeast Saccharomyces cerevisiae (Chang and Kosman 1989; Sanchez et al. 2005; Reddi et al. 2009), engineered to lack SOD enzymes. Elegant work by Daly and colleagues has shown that tolerance to radiation and oxidative stress in a variety of bacterial species is due to accumulation of high levels of intracellular Mn, but low Fe (Daly 2006, 2009; Daly et al. 2007, 2010; Gross 2007; Granger et al. 2011). In higher organisms, Mn treatment can prolong the life span and oxidative stress resistance in the simple metazoan, Caenorhabditis elegans, and also defend against reactive oxygen species (ROS) in the cryopreservation of sperm (Lin et al. 2006; Bansal and Kaur 2009; Cheema et al. 2009).
The biochemical nature of the Mn antioxidant has been subject to much investigation. It has been defined as a Mn-dependent but SOD-independent superoxide scavenging activity in bacterial and yeast lysates. This activity is EDTA sensitive, dialyzable, heat and protease resistant, and was proposed to represent Mn complexes of small molecule cellular metabolite(s) (Archibald and Fridovich 1981a; Chang and Kosman 1989). A number of Mn–carboxylato complexes (Archibald and Fridovich 1982b), as well as Mn-orthophosphate (Pi) (Barnese et al. 2008), have been shown to exhibit superoxide scavenging activity in vitro, and the extreme radioresistance of Deinococcus radiodurans is associated with certain Mn–Pi and -peptide complexes (Daly et al. 2010). Moreover, our in vivo studies in the Baker’s yeast have demonstrated a role for Mn–Pi as an important backup for Cu/Zn containing SOD (SOD1) (McNaughton et al. 2010).
Using ENDOR spectroscopy to monitor Mn speciation in intact yeast cells, we observed a close correlation between the cellular level of Mn–Pi in various yeast strains and their resistance to oxygen toxicity (McNaughton et al. 2010; Szuromi 2010). Of the strains tested, the most severe oxygen toxicity was observed when phosphate control was disrupted through mutations in the phosphate-sensing kinase complex Pho80p/Pho85p (Kaneko et al. 1982; Ogawa et al. 2000; Wykoff and O’shea 2001; Carroll and O’shea 2002; Lee et al. 2007; Wykoff et al. 2007). Deletion of either pho80 or pho85 rendered sod1∆ mutants lacking Cu/Zn SOD1 inviable in air and cells seemed nearly devoid of Mn antioxidant protection (Reddi et al. 2009). By ENDOR spectroscopy, Mn–Pi levels were lowered in this strain (McNaughton et al. 2010), although not to a degree expected for the profound oxygen toxicity observed. Ablation of Pho80/Pho85p inhibited Mn antioxidant activity through a mechanism other than simple loss of Mn–Pi.
In the context of cellular physiology, what dictates formation of Mn antioxidants? Are these spontaneously formed complexes of Mn that incidentally scavenge superoxide, or does the cell tightly regulate Mn antioxidant activity in concert with protein-based antioxidants? Using the Bakers’ yeast S. cerevisiae as a model organism, we provide evidence for the latter. Namely, we find that the ability of cells to utilize Mn as an antioxidant can fall under control of Rim15p, a PAS-kinase that integrates phosphate, nitrogen, and carbon-nutrient sensing (Pedruzzi et al. 2003; Cameroni et al. 2004; Roosen et al. 2005; Wanke et al. 2005; Swinnen et al. 2006; Smets et al. 2010; Yang et al. 2010). Hyperactivation of Rim15p from genetic ablation of its Pho80p/Pho85p (phosphate sensing) or Sch9p (nitrogen sensing) upstream regulators results in strong downregulation of the Mn antioxidant, helping to explain the profound oxygen toxicity in the pho80 (or pho85) sod1 null mutants. In addition, transcription factors downstream of Rim15p, which include Gis1p and the redundant pair Msn2p and Msn4p (hereafter referred to as Msn2/4p) that typically regulate stress-response factors, likewise play a role in titrating the activity of the Mn antioxidant. Overall, this work demonstrates that the Mn antioxidant is controlled by the same pathways that sense nutrients and respond to stress.
Materials and Methods
Yeast strains, plasmids, and growth conditions
All yeast strains for this study were derived from BY4741 (MATa, leu2∆0, met15∆0, ura3∆0, his3∆1). A full listing, including descriptions on their source or construction, is included in Supporting Information, File S1. Details on the construction of the msn2::HIS3 disruption plasmid, pAR007, and the sch9::HIS3 disruption plasmid, pAR009, are in File S1. All strains were verified by PCR and DNA sequencing.
In general, yeast cells were grown at 30° in enriched yeast extract, peptone, dextrose medium (YPD), or synthetic complete (SC) medium lacking lysine as needed (Sherman 1991). For anaerobic growth, YPD or SC media was supplemented with 15 mg/liter ergosterol and 0.5% Tween-80 (YPDE or SCE, respectively). All experiments employed cells freshly obtained from frozen stocks and cultured on YPDE in oxygen-depleted, CO2-enriched culture jars (GasPak, Becton-Dickinson). Growth tests to assay oxygen resistance in SC complete medium were conducted under well-aerated (shaking at 220 rpm) conditions as described (Reddi et al. 2009); tests for aerobic lysine auxotrophy and Mn toxicity were conducted under micro-aerobic conditions (not shaking) as described (Reddi et al. 2009; Rosenfeld et al. 2010).
Biochemical assays
For all biochemical assays including phosphate and manganese measurements, superoxide scavenging activity and Fe/S enzyme assays, cells were pregrown in triplicate cultures in SCE media ≈16 hr under an anaerobic N2 atmosphere prior to dilution to o.d.600nm = 0.25 and growth for 6 hr in air, shaking at 220 rpm. Cells were then harvested, washed three times in ice-cold TE buffer (10 mM Tris-hydrochloride and 1 mM EDTA, pH 8.0), and three times in ice-cold MiliQ water prior to further analyses.
Total cellular phosphates (orthophosphate and polyphosphates) were measured using the molybdate reactivity method (Ames 1966; Reddi et al. 2009; McNaughton et al. 2010). Cells were lysed by glass bead homogenization in 500 ml of 0.1% Triton X-100. Total phosphate was measured from boiling 3–30 μg of whole-cell lysates for 10 min in 1 N H2SO4. Phosphate was quantified using a calibration curve of 0–300 µM phosphoric acid. Total cellular manganese was measured using atomic absorption spectroscopy (AAS) as described previously (Reddi et al. 2009).
For superoxide scavenging-activity assays, cells were lysed in 50 mM MES, 100 mM KCl, 0.1% Triton X-100, pH 7.0 by glass bead homogenization and 10–300 μg of cell lysate protein was subject to the xanthine/xanthine oxidase/XTT (3′-1-(phenylamino)-carbonyl-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate) assay for superoxide generation and detection (Ukeda et al. 1997). The increase in absorbance at 470 nm over 60 min upon addition of xanthine oxidase, corresponding to the rate of reduction of XTT by superoxide, was read in a Biotek HT Synergy plate reader or in a Beckman-Coulter UV/vis spectrophotometer. The rate of XTT reduction by lysates in the absence of xanthine oxidase was subtracted from all measurements. One unit of superoxide scavenging activity was defined as a 50% decrease in the rate of XTT reduction.
For aconitase (Aco1p) and isopropylmalate isomerase (Leu1p) activity assays, cells were subjected to glass bead lysis in 50 mM MES, 100 mM KCl, 0.1% Triton X-100, pH 7.0 under a nitrogen atmosphere in a COY chamber. Aco1p and Leu1p activity was determined spectrophotometrically using a Biotek HT Synergy plate reader (Wallace et al. 2004). The assay mixture contained 50–300 µg of lysate protein in 200 µl of a buffer containing 50 mM tris(hydroxymethyl)aminomethane (Tris)-HCl, pH 7.4, and 100 mM NaCl and supplemented with either 0.5 mM cis-aconitate (Aco1p activity) or 0.5 mM citraconitate (Leu1p activity). Activities were determined by monitoring the disappearance of cis-aconitate (Aco1p) or citraconitate (Leu1p) at 240 or 235 nm, respectively, over the course of 3 min. These species were quantified by generating calibration curves of standardized concentrations of cis-aconitate or citraconitate. In both cases, 1 U of activity is defined as 1 nmol of substrate consumed per minute per milligram of protein.
Results
Phosphate signaling through Pho4p is not responsible for the aerobic lethality of sod1Δ pho80Δ mutants
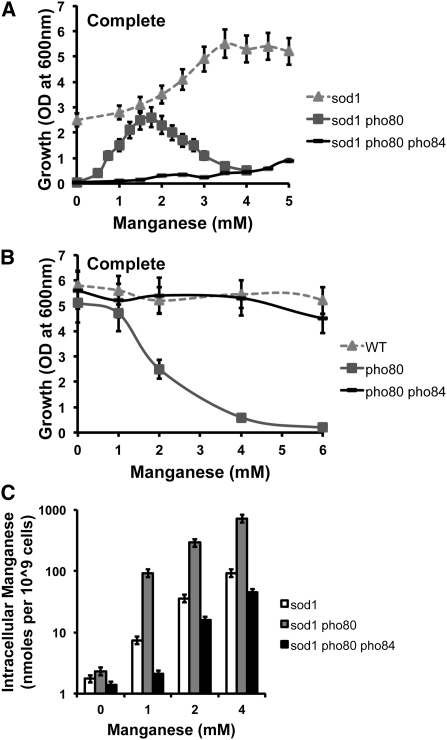
A loss of phosphate control in S. cerevisiae through disruption of PHO80 or PHO85 renders sod1∆ cells inviable in air and unable to effectively utilize Mn for oxidative stress suppression (Reddi et al. 2009; McNaughton et al. 2010; Rosenfeld et al. 2010). As seen in Figure 1A, Mn supplements to the growth medium only partly restore aerobic growth to a sod1∆pho80∆ double mutant. The limited efficacy of Mn in this regard might be explained by the intolerance of pho80 mutants to Mn toxicity (Figure 1B). These mutants accumulate very high Mn (Figure 1C, note log scale) due to uncontrolled uptake of Mn–Pi by the Pho84p transporter (Wykoff and O'shea 2001; Jensen et al. 2003; Reddi et al. 2009; Rosenfeld et al. 2010). To remove effects of Mn toxicity, we disrupted PHO84, which reversed both the high Mn accumulation (Figure 1C) and Mn sensitivity (Figure 1B) of the sod1∆pho80∆ mutant. However, the pho84 mutation did not restore aerobic growth and most striking, Mn supplementation was ineffective at reversing the aerobic lethality of a sod1∆pho80∆pho84∆ mutant (Figure 1A). Hence, sod1∆pho80∆ cells require massive accumulation of Mn to rescue any oxygen toxicity, consistent with the low efficacy of Mn as an antioxidant in this strain.
Figure 1 .
Loss of the Mn–phosphate transporter Pho84p enhances oxygen toxicity in a sod1∆ pho80∆ strain. (A) The indicated sod1∆ strains were seeded in synthetic complete (SC) media at an optical density at 600 nm (o.d.600nm) of 0.05, and grown shaking for 16 hr with the indicated concentrations of MnCl2. Total growth was determined by measuring the o.d.600nm. (B) Manganese toxicity of the indicated strains was determined by growing cells in SC medium supplemented with the indicated concentrations of Mn as described in A under nonshaking conditions. (C) Manganese accumulation of the indicated sod1∆ strains was determined by atomic absorption spectroscopy (AAS) with cells grown in SC medium treated for 6 hr with the indicated concentrations of MnCl2 as described in Materials and Methods. Manganese content is represented as nanomoles per 109 cells. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: WT, BY4741; pho80∆, LR237; pho80∆ pho84∆, LR154; sod1∆, AR203; sod1∆ pho80∆, LR156; sod1∆ pho80∆ pho84∆, LR178.
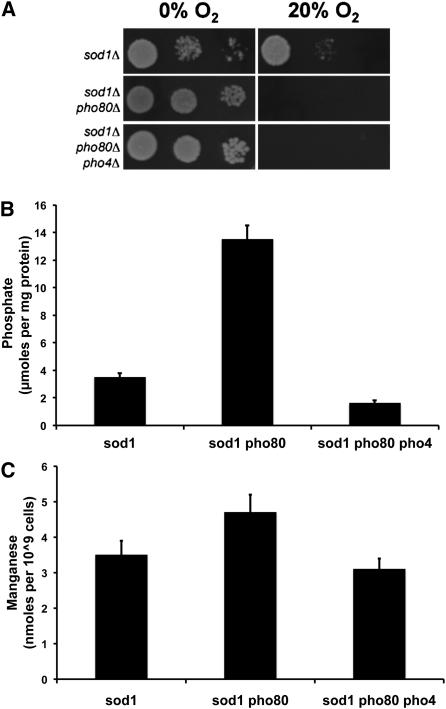
The Pho80p/Pho85p kinase pair negatively regulates the Pho4p transcription factor for the induction of phosphate uptake and storage genes such as PHO84 (Kaneko et al. 1982; Ogawa et al. 2000; Wykoff and O’shea 2001; Lee et al. 2007; Wykoff et al. 2007). We addressed whether hyperactive Pho4p in pho80 mutants accounts for the severe oxygen toxicity of sod1∆pho80∆ cells. As seen in Figure 2A, deletion of PHO4 did not reverse the aerobic lethality of sod1∆pho80∆ cells, despite lowering phosphate (Figure 2B) and intracellular (Mn) (Figure 2C) to levels that approximated the sod1∆ control. Hence, hyperactivated Pho4p cannot account for the aerobic lethality of sod1∆pho80∆ cells and another downstream effector molecule must be responsible.
Figure 2 .
Activation of the Pho4p transcription factor is not responsible for the aerobic lethality of sod1∆ pho80∆ strains. (A) The effect of a pho4∆ mutation on the aerobic lethality of sod1∆ pho80∆ cells was tested by spotting 104, 103, and 102 cells of the indicated strains onto SCE plates and by growing in air or anaerobically for 3 days. (B) Phosphate content of the indicated strains was measured by molybdate reactivity of the indicated strains grown in SC medium as described in Materials and Methods. (C) Manganese content of the indicated strains was measured by AAS precisely as described in Figure 1C. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: sod1∆, AR203; sod1∆ pho80∆, LR156; sod1∆ pho80∆ pho4∆, AR106.
Rim15p as a regulator of Mn antioxidant activity
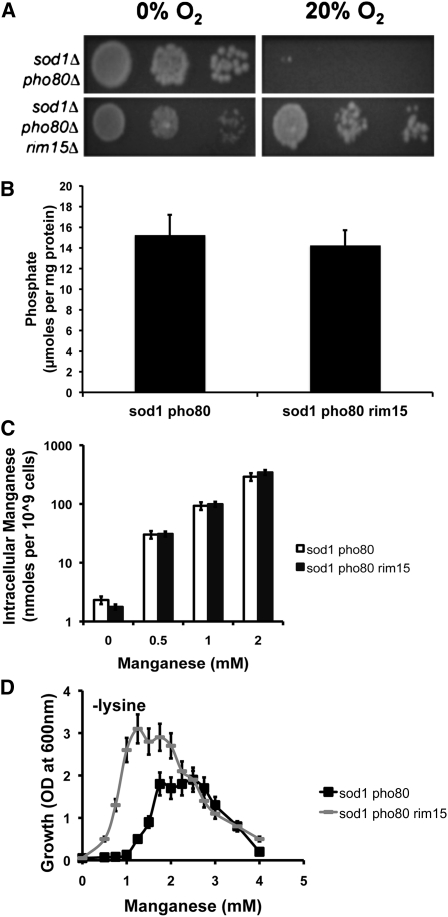
A second target of the Pho80p–Pho85p cyclin–CDK complex is the PAS-kinase, Rim15p (Wanke et al. 2005; Swinnen et al. 2006). As with Pho4p, Rim15p is hyperactivated in cells lacking pho80 or pho85. A deletion of rim15 had no effect on the elevated phosphate (Figure 3B) or manganese (Figure 3C) of a sod1∆pho80∆ mutant. Nevertheless, loss of Rim15p restored aerobic viability to these cells (Figure 3A). Thus, hyperactive Rim15p appears responsible for the severe oxidative stress of sod1∆pho80∆ mutants under atmospheric oxygen.
Figure 3 .
RIM15 is responsible for the aerobic lethality and poor utility of Mn as an antioxidant in sod1∆ pho80∆ strains. Shown are the effects of a rim15 mutation on (A) aerobic lethality of sod1∆ pho80∆ strains, (B) phosphate accumulation, and (C) Mn accumulation, as described in Figure 2, A, B, and C, respectively. (D) Manganese rescue of the sod1∆-linked aerobic lysine auxotrophy was tested in SC medium lacking lysine and supplemented with the indicated concentrations of MnCl2. Cells were grown as in A except under nonshaking conditions. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: sod1∆ pho80∆, LR156; sod1∆ pho80∆ rim15∆, AR110.
Another marker of sod1∆-linked oxidative stress is an aerobic lysine auxotrophy resulting from superoxide damage to lysine biosynthetic enzyme(s) (Bilinski et al. 1985; Wallace et al. 2004). This defect can be reversed by Mn supplementation (Chang and Kosman 1989; Sanchez et al. 2005; Reddi et al. 2009). In comparing the dose response to Mn, the aerobic lysine auxotrophy of the sod1∆pho80∆rim15∆ triple mutant was more effectively rescued by Mn than the sod1∆pho80∆ double mutant (Figure 3D), despite accumulating identical levels of the metal (Figure 3C). Thus, deletion of RIM15 in sod1∆pho80∆ cells not only promotes aerobic growth, but also lowers the intracellular dose of Mn required to protect against oxidative damage.
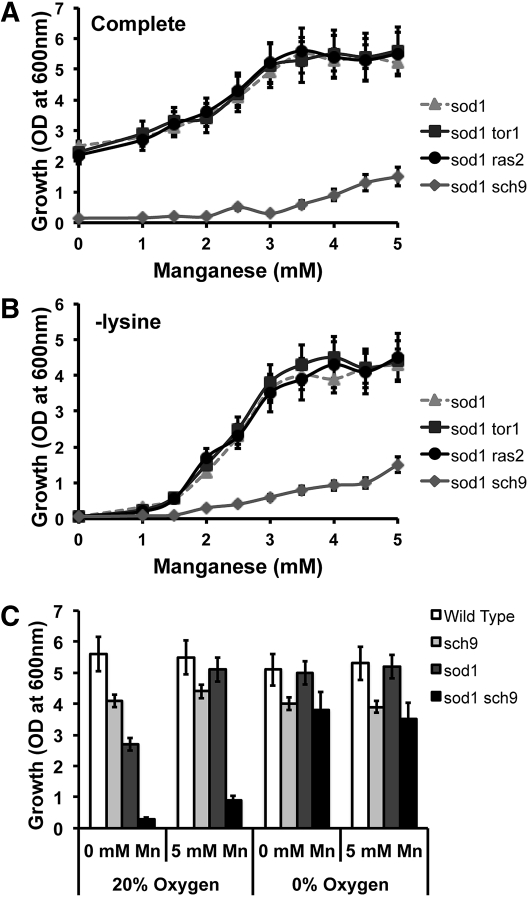
Rim15p is negatively regulated by a number of kinases downstream of nutrient sensing and signaling circuits. In addition to the aforementioned Pho80p–Pho85p cyclin–CDK pair (phosphate sensing), these kinases include Tor1p and the yeast Akt/S6K homolog, Sch9p, which are both involved in nitrogen sensing, and PKA, which senses carbon sources (Pedruzzi et al. 2003; Cameroni et al. 2004; Roosen et al. 2005; Wanke et al. 2005; Swinnen et al. 2006; Smets et al. 2010; Yang et al. 2010). We sought to determine if ablating the activities of any of these kinases in sod1 cells phenocopied the effects of a pho80∆ mutation. Toward this end, we deleted TOR1, RAS2, a positive regulator of PKA activity, and SCH9 in sod1∆ cells. Of these, the sch9 mutation conferred a severe aerobic growth defect to sod1∆ cells that was particularly profound in liquid cultures and was poorly rescued by Mn supplements (Figures 4A and 4B). This sch9-growth defect was indeed due to oxygen toxicity as it was abolished under anaerobic conditions or in strains with WT SOD1 (Figure 4C). Moreover, sch9∆ conferred oxygen sensitivity to sod1∆ nulls without global changes in cellular phosphates or Mn (Figure S1A and Figure S1B). The ability of sch9 mutations to phenocopy pho80 mutations in terms of sod1∆ oxidative stress supports a role for Rim15p in negatively regulating the Mn antioxidant.
Figure 4 .
Deleting sch9 renders sod1∆ cells oxidatively stressed and unable to utilize Mn for antioxidant protection. (A and B) The effect of sch9∆, tor1∆, and ras2∆ deletions on Mn promotion of aerobic growth was tested in (A) well-aerated SC media and (B) media lacking lysine, as was done as in Figures 1A and 3D, respectively. (C) The effect of sch9∆, sod1∆, and sod1∆ sch9∆ mutations on growth in SCE media in the absence and presence of oxygen and manganese was determined by measuring the o.d.600nm values of shaking cultures after 16 hr of growth, as described in Materials and Methods. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: sod1∆, AR203; sod1∆ sch9∆, AR163; sch9∆, AR164. sod1∆ tor1∆, AR120; sod1∆ ras2∆, AR158.
The downstream transcription factors Gis1p and Msn2/4p work in opposite to regulate the Mn antioxidant as a scavenger of superoxide in the cytosol
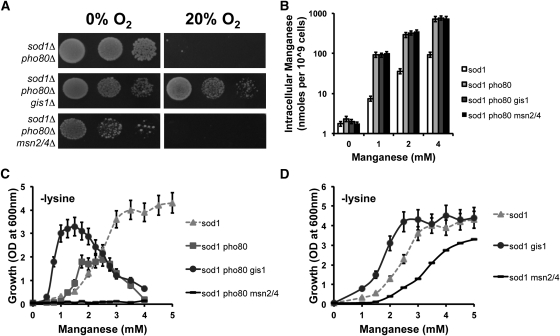
The downstream targets of Rim15p activation include the transcription factors Gis1p and the redundant pair Msn2/4p. All three transcription factors, which share ∼95% of their gene targets, are thought to work in concert to activate cellular stress defense pathways, as well as metabolic adaptations to nutrient deficiency (Cameroni et al. 2004; Swinnen et al. 2006; Smets et al. 2010). We tested whether Gis1p and/or Msn2/4p activation was responsible for the aerobic lethality of the sod1∆pho80∆ strain. As shown in Figure 5A, deletion of gis1 rescued the aerobic lethality of sod1∆pho80∆ cells, while msn2/4 deletions did not. Moreover, in a dose-response study, extracellular Mn was more effective in suppressing the aerobic growth defect (Figure S2A) and aerobic lysine auxotrophy (Figure 5C) of sod1∆pho80∆ cells when gis1 was deleted, even though intracellular Mn (Figure 5B) and phosphate (Figure S2B) levels were unchanged. At equivalent intracellular concentrations of ∼90 nmol Mn per 109 cells achieved when sod1∆ cells are treated with 4 mM Mn or when sod1∆pho80∆gis1∆ cells are treated with 1 mM Mn (Figure 5B), the aerobic lysine auxotrophy of both strains is rescued by Mn to nearly the same degree (Figure 5C). Overall, the deletion of gis1 phenocopies the effect of a rim15 deletion in sod1∆pho80∆ cells in terms of reversing the aerobic lethality and promoting Mn antioxidant capacity.
Figure 5 .
GIS1 and MSN2/4 have opposing affects on Mn antioxidant protection. The effect of gis1 and msn2/4 mutations on the (A) aerobic lethality of sod1∆ pho80∆ cells, (B) Mn accumulation in sod1∆ pho80∆ cells, and (C and D) Mn-mediated rescue of the sod1∆-linked aerobic lysine auxotrophy in sod1∆ pho80∆ (C) and sod1∆ (D) cells were tested precisely as in Figure 3, A, C, and D, respectively. Details on culture conditions and growth are described in Materials and Methods. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: sod1∆, AR203; sod1∆ pho80∆, LR156; sod1∆ pho80∆ gis1∆, AR138; sod1∆ pho80∆ msn2/4∆, AR300; sod1∆ gis1∆, AR121; sod1∆ msn2/4∆, AR155.
In stark contrast to a gis1 deletion, the msn2/4 mutations dramatically inhibited the ability of Mn to promote aerobic growth. Mn supplements were completely ineffective in suppressing the aerobic lysine auxotrophy of sod1∆pho80∆msn2/4∆ cells (Figure 5C) and also poorly reversed the aerobic lethality of sod1∆pho80∆msn2/4∆ in complete medium (Figure S2A). The differential effects of gis1 vs. msn2/4 mutations on the efficacy of Mn as an antioxidant were not due to differences in Mn accumulation (Figure 5B) or Mn toxicity (Figure S2C) or intracellular phosphate concentrations (Figure S2B). As a potential caveat to these studies, sod1∆pho80∆msn2/4∆ cells also grow poorly under anaerobic conditions (Figure 5A), due to a synthetic defect of combining pho80 mutations with msn2∆msn4∆. However, our other studies (see Figures 5D and 6) demonstrate that the inhibitory effects of msn2∆msn4∆ mutations on the Mn antioxidant are also visible in a PHO80+ strain.
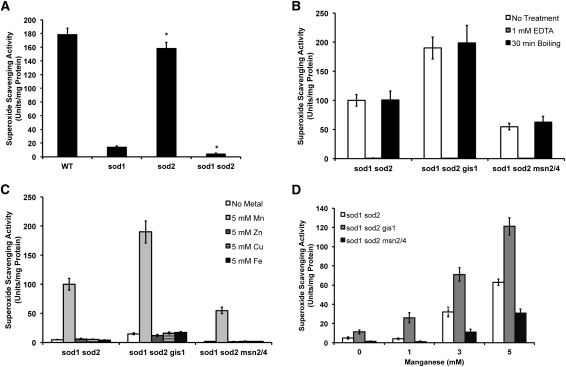
Figure 6 .
GIS1 and MSN2/4 differentially affect manganese-dependent superoxide scavenging activity in SOD-deficient cells. The indicated cells were grown for 6 hr in SC medium that was either (A) not supplemented with metals, or supplemented with (B) 5 mM MnCl2, (C) 5 mM of MnCl2, ZnCl2, CuCl2, or FeCl2, or (D) the indicated concentrations of MnCl2. Whole-cell lysates were prepared and analyzed for total cellular superoxide scavenging activity by the xanthine/xanthine oxidase/XTT assay described in Materials and Methods. One unit is defined as a 50% decrease in the rate of XTT reduction per milligram lysate protein. (A) The asterisk denotes a P < 0.05 and reflects the statistical significance in loss of activity upon a sod2∆ mutation. (B) Where indicated, lysates were incubated for 30 min at room temperature with 1 mM EDTA or at 100° prior to superoxide scavenging activity assays to demonstrate metal chelator sensitivity and heat resistance of SISS activity. Details on culture conditions and the superoxide scavenging assays are described in Materials and Methods. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: WT, BY4741; sod1∆, AR203; sod1∆ gis1∆, AR121; sod1∆ msn2/4∆, AR155; sod1∆ sod2∆, AR142; sod1∆ sod2∆ gis1∆, AR161; sod1∆ sod2∆ msn2/4∆, AR160.
We next assessed whether the effects of gis1 and msn2/4 mutations on the Mn antioxidant were evident without hyperactivation of Rim15p, i.e., in a PHO80+ strain. For these studies, sod1∆gis1∆ double and sod1∆msn2∆msn4∆ triple mutants were generated. As seen in Figure 5D, the ability of Mn to support sod1∆ aerobic growth on media lacking lysine was significantly enhanced with a gis1∆ mutation and repressed with msn2/4∆ mutations without global changes in cellular phosphates or manganese (Figure S3). Even in the absence of hyperactivation conditions, these factors control the efficacy of Mn as an antioxidant.
To more directly study the differential effects of Gis1p and Msn2/4p on the Mn antioxidant, we used a biochemical assay to probe Mn-dependent superoxide scavenging activity. Figure 6A shows the superoxide scavenging activity of cell lysates prepared from WT, sod1∆, sod2∆, and sod1∆sod2∆ cells, based on the xanthine/xanthine oxidase/XTT assay for in vitro superoxide generation and detection (Ukeda et al. 1997). By comparing results from the different sod null strains, we estimated the activities of Sod1p and Sod2p to be ∼154 and ∼20 U/mg protein, respectively, as well as SOD-independent superoxide scavenging (SISS) activity as ∼5 U/mg protein. Previous studies have implicated this SISS to be that of the “Mn antioxidant” (Chang and Kosman 1989). Indeed, consistent with previous studies, we determined SISS activity in the background of cells lacking the cytosolic Cu/Zn SOD1 and the mitochondrial Mn–SOD2 is specific for Mn and is EDTA sensitive and heat resistant (Figures 6B and 6C). We observed that the gis1 and msn2/4 mutations differentially affect Mn-dependent SISS activity in vitro. As shown in Figure 6D, the gis1 mutation endows cell extracts with approximately twofold greater superoxide scavenging activity (∼11 U/mg protein), whereas the msn2/4 mutations reduce it by nearly the same factor (∼2 U/mg protein). In addition, Mn supplemented to cells during growth is more effectively utilized by the gis1 mutant and poorly utilized by the msn2/4 mutant for superoxide scavenging (Figure 6D).
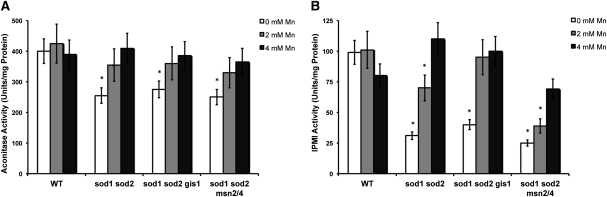
Finally, we sought to determine the cellular localization of Gis1p and Msn2/4p-dependent Mn antioxidant activity. To address this, we took advantage of the fact that Fe/S cluster proteins are specific targets of superoxide toxicity (Wallace et al. 2004) and examined activity of two highly homologous dehydratase Fe/S enzymes that are differentially localized, namely aconitase (Aco1p, mitochondrial matrix) and isopropylmalate isomerase (IPMI) (Leu1p, cytosol). Figures 7A and 7B show that deletion of the SOD encoding genes result in ∼30% and 75% reductions in aconitase and IPMI activities, respectively, and these losses in activities are fully restored by Mn supplements. Although gis1 and msn2/4 mutations had no effect on the Mn rescue of mitochondrial aconitase (Figure 7A), these same mutations had a significant impact on Mn-mediated protection of cytosolic IMPI activity (Figure 7B). The gis1 mutation enhanced IPMI activity at 2 mM Mn, whereas the msn2/4 mutations reduced IPMI activity at all Mn levels tested. Hence, gis1 and msn2/4 mutations affect Mn antioxidant activity in the cytosol.
Figure 7 .
GIS1 and MSN2/4 differentially affect the ability of manganese to protect cytosolic but not mitochondrial Fe/S proteins. The indicated strains were grown in SC medium that was supplemented for 6 hr with the designated concentrations of MnCl2 prior to cell lysate preparation and analysis of (A) aconitase and (B) isopropylmalate isomerase (IPMI) activity as described in Materials and Methods. Asterisk denotes a P < 0.05 and reflects the statistical significance between 4 mM Mn treatment and 0 or 2 mM Mn treatment. All reported values are averages based on triplicate cultures, with the errors representing the standard deviations. Strains employed: WT, BY4741; sod1∆ sod2∆, AR142; sod1∆ sod2∆ gis1∆, AR161; sod1∆ sod2∆ msn2/4∆, AR160.
Discussion
Intracellular Mn has long been known to suppress oxidative stress in a variety of organisms. In organisms that do not express SODs and/or naturally accumulate high Mn and low Fe (e.g., L. plantarum and D. radiodurans), there is good evidence for nonproteinaceous complexes of Mn such as Mn–Pi serving as physiological antioxidants, primarily by protecting against protein oxidation (Daly et al. 2004, 2007, 2010; Daly 2006, 2009; Fredrickson et al. 2008). Mn may also act as an antioxidant by functionally substituting for some Fe enzymes, thereby mitigating the potential for deleterious Fenton reactions at enzyme active sites (Anjem et al. 2009; Sobota and Imlay 2011). Regardless of the mechanism, it was unclear whether the protective effect of Mn is part of the cell’s tightly regulated battery of antioxidant responses or is a passive unregulated process. Using S. cerevisiae as a model organism, we provide the first line of evidence that the Mn antioxidant is a component of the oxidative stress defense that is regulated through nutrient sensing pathways. Specifically, the nutrient signaling kinases Pho80p, Sch9p, and Rim15p as well as downstream regulators Msn2/4p and Gis1p can all regulate the efficacy of the Mn antioxidant in yeast cells.
We show herein that loss of gis1 promotes the Mn antioxidant whereas mutations in msn2/4 inhibit it; hence Gis1p and Msn2/4p negatively and positively regulate the potency of Mn as an antioxidant, respectively. Traditionally, these transcription factors are known for their regulation of genes involved in the response to stress and nutrients as well as genes for promoting longevity in response to calorie restriction (Reinders et al. 1998; Pedruzzi et al. 2000, 2003; Hasan et al. 2002; Cameroni et al. 2004; Fabrizio et al. 2004, 2001; Roosen et al. 2005; Swinnen et al. 2006; Medvedik et al. 2007; Wei et al. 2008; Zhang et al. 2009; Smets et al. 2010). The Mn antioxidant can now be added to this list of stress resistance and metabolism factors that are governed by such nutrient and stress signaling pathways.
Gis1p and Msn2/4p are reported to share a bulk of their gene targets (Cameroni et al. 2004; Swinnen et al. 2006; Smets et al. 2010); thus it is surprising that they have opposing effects on activity of the Mn antioxidant. It is possible that Gis1p and Msn2/4p negatively and positively regulate the same downstream target or coordinately regulate two distinct targets that have opposing actions on Mn as an antioxidant. While we still do not know the identity of the downstream factor(s), our data tentatively point to cytosolic target(s) for oxidative stress suppression. Specifically, gis1 and msn2/4 mutations affect the ability of Mn to protect the activity of the cytosolic Fe/S protein, Leu1p but not the homologous mitochondrial Fe/S protein, Aco1p. It is possible that the nature of the Mn antioxidant in the cytosol is distinct from that in the mitochondria and Gis1p and Msn2/4p signaling pathways only regulate the former.
Since Mn–Pi has been implicated as a key Mn antioxidant in yeast cells (Barnese et al. 2008; McNaughton et al. 2010) it is possible that Gis1p and Msn2/4p affect cellular phosphate interactions with Mn. However, we observed that gis1 or msn2/4 mutations have no effect on total phosphate or Mn. Moreover, we previously proposed that the lowering of Mn–Pi in sod1∆pho85∆ mutants could not by itself explain the extreme oxidative stress of this strain (McNaughton et al. 2010). The activation of Rim15p and Gis1p in this mutant appears to inhibit the Mn antioxidant independent of Mn–Pi effects. Mn complexes to carboxylates (e.g., lactate, succinate) can also act as antioxidants (Archibald and Fridovich 1982b) and it is possible that such compounds are targets for regulation by Rim15p, Gis1p, and Msn2/4p. This study also raises the intriguing possibility that phosphate can promote Mn antioxidant activity through Pho80p-mediated inhibition of Rim15p and Gis1p independently of phosphate-dependent chemical scavenging of superoxide by Mn–Pi complexes.
In conclusion, we demonstrate that Rim15p, Gis1p, and Msn2/4p, which are downstream of conserved nutrient sensing pathways, can regulate cytosolic Mn-mediated superoxide scavenging activity. Unlike previous studies that have implicated Gis1p and Msn2/4p working in parallel gene activation pathways (Cameroni et al. 2004; Swinnen et al. 2006; Smets et al. 2010), we provide evidence that these factors can work in opposite with regard to the Mn antioxidant: Gis1p negatively regulates Mn antioxidant activity whereas Msn2/4p positively regulates it. In this manner, the two factors provide checks and balances for one another and together titrate the precise degree of oxidative stress protection required.
Acknowledgments
We thank Dr. Leah Rosenfeld for providing yeast strains. This work was supported by the Johns Hopkins University National Institute of Environmental Health Sciences center and by National Institutes of Health (NIH) RO1 grant ES 08996. A.R.R. was supported by NIH NIGMS fellowship F32 GM 093550.
Literature Cited
- Abreu I. A., Cabelli D. E., 2010. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim. Biophys. Acta 1804: 263–274 [DOI] [PubMed] [Google Scholar]
- Al-Maghrebi M., Fridovich I., Benov L., 2002. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch. Biochem. Biophys. 402: 104–109 [DOI] [PubMed] [Google Scholar]
- Ames B. N., 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8: 115–118 [Google Scholar]
- Anjem A., Varghese S., Imlay J. A., 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72: 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N., 1984. Manganese Acquistion by Lactobacillus plantarum. J. Bacteriol. 158: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I., 1981a Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145: 422–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I., 1981b Manganese, superoxide dismutase and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 146: 928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I., 1982a Investigations on the state of the manganese in Lactobacillus plantarum. Arch. Biochem. Biophys. 215: 589–596 [DOI] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I., 1982b The scavenging of superoxide radical by manganous complexes in vitro. Arch. Biochem. Biophys. 214: 452–463 [DOI] [PubMed] [Google Scholar]
- Bansal A. K., Kaur A. R., 2009. Cooperative functions of manganese and thiol redox system against oxidative stress in human spermatozoa. J. Hum. Reprod. Sci. 2: 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnese K., Gralla E. B., Cabelli D. E., Valentine J. S., 2008. Manganous phosphate acts as a superoxide dismutase. J. Am. Chem. Soc. 130: 4604–4606 [DOI] [PubMed] [Google Scholar]
- Bilinski T., Krawiec Z., Liczmanski L., Litwinska J., 1985. Is hydroxyl radical generated by the fenton reaction in vivo? Biochem. Biophys. Res. Commun. 130: 533–539 [DOI] [PubMed] [Google Scholar]
- Cameroni E., Hulo N., Roosen J., Winderickx J., De Virgilio C., 2004. The novel yeast PAS kinase Rim 15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle 3: 462–468 [PubMed] [Google Scholar]
- Carroll A. S., O’shea E. K., 2002. Pho85 and signaling environmental conditions. Trends Biochem. Sci. 27: 87–93 [DOI] [PubMed] [Google Scholar]
- Chang E. C., Kosman D. J., 1989. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J. Biol. Chem. 264: 12172–12178 [PubMed] [Google Scholar]
- Cheema R. S., Bansal A. K., Bilaspuri G. S., 2009. Manganese provides antioxidant protection for sperm cryopreservation that may offer new consideration for clinical fertility. Oxid. Med. Cell Longev. 2: 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. J., 2006. Modulating radiation resistance: insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin. Lab. Med. 26: 491–504 x [DOI] [PubMed] [Google Scholar]
- Daly M. J., 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7: 237–245 [DOI] [PubMed] [Google Scholar]
- Daly M. J., Gaidamakova E. K., Matrosova V. Y., Vasilenko A., Zhai M., et al. , 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306: 1025–1028 [DOI] [PubMed] [Google Scholar]
- Daly M. J., Gaidamakova E. K., Matrosova V. Y., Vasilenko A., Zhai M., et al. , 2007. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 5: e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. J., Gaidamakova E. K., Matrosova V. Y., Kiang J. G., Fukumoto R., et al. , 2010. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE 5: e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D., 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292: 288–290 [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Pletcher S. D., Minois N., Vaupel J. W., Longo V. D., 2004. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 557: 136–142 [DOI] [PubMed] [Google Scholar]
- Fredrickson J. K., Li S. M., Gaidamakova E. K., Matrosova V. Y., Zhai M., et al. , 2008. Protein oxidation: Key to bacterial desiccation resistance? Isme J. 2: 393–403 [DOI] [PubMed] [Google Scholar]
- Granger A. C., Gaidamakova E. K., Matrosova V. Y., Daly M. J., Setlow P., 2011. Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl. Environ. Microbiol. 77: 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L., 2007. Paradox resolved?: the strange case of the radiation-resistant bacteria. PLoS Biol. 5: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan R., Leroy C., Isnard A. D., Labarre J., Boy-Marcotte E., et al. , 2002. The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol. Microbiol. 45: 233–241 [DOI] [PubMed] [Google Scholar]
- Jensen L. T., Ajua-Alemanji M., Culotta V. C., 2003. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 278: 42036–42040 [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Toh-E A., Oshima Y., 1982. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol. Cell. Biol. 2: 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Mulugu S., York J. D., O’shea E. K., 2007. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. T., Hoang H., Hsieh S. I., Rangel N., Foster A. L., et al. , 2006. Manganous ion supplementation accelerates wild type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radic. Biol. Med. 40: 1185–1193 [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I., 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244: 6049–6055 [PubMed] [Google Scholar]
- McNaughton R. L., Reddi A. R., Clement M. H., Sharma A., Barnese K., et al. , 2010. Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci. USA 107: 15335–15339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedik O., Lamming D. W., Kim K. D., Sinclair D. A., 2007. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 5: e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N., Derisi J., Brown P. O., 2000. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 11: 4309–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi I., Burckert N., Egger P., De Virgilio C., 2000. Saccharomyces cerevisiae Ras/cAMP pathway controls post-diauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 19: 2569–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi I., Dubouloz F., Cameroni E., Wanke V., Roosen J., et al. , 2003. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol. Cell 12: 1607–1613 [DOI] [PubMed] [Google Scholar]
- Reddi A. R., Jensen L. T., Naranuntarat A., Rosenfeld L., Leung E., et al. , 2009. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic. Biol. Med. 46: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A., Burckert N., Boller T., Wiemken A., De Virgilio C., 1998. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 12: 2943–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosen J., Engelen K., Marchal K., Mathys J., Griffioen G., et al. , 2005. PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability. Mol. Microbiol. 55: 862–880 [DOI] [PubMed] [Google Scholar]
- Rosenfeld L., Reddi A. R., Leung E., Aranda K., Jensen L. T., et al. , 2010. The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J. Biol. Inorg. Chem. 15: 1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez R. J., Srinivasan C., Munroe W. H., Wallace M. A., Martins J., et al. , 2005. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J. Biol. Inorg. Chem. 10: 913–923 [DOI] [PubMed] [Google Scholar]
- Sherman F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21 [DOI] [PubMed] [Google Scholar]
- Smets B., Ghillebert R., De Snijder P., Binda M., Swinnen E., et al. , 2010. Life in the midst of scarcity: adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 56: 1–32 [DOI] [PubMed] [Google Scholar]
- Sobota J. M., Imlay J. A., 2011. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. USA 108: 5402–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen E., Wanke V., Roosen J., Smets B., Dubouloz F., et al. , 2006. Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div. 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuromi P., 2010. Manganese sightings. Science 329: 1442 [Google Scholar]
- Tseng H. J., Srikhanta Y., McEwan A. G., Jennings M. P., 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40: 1175–1186 [DOI] [PubMed] [Google Scholar]
- Ukeda H., Maeda S., Ishii T., Sawamura M., 1997. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-1-(phenylamino)-carbonyl-3, 4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal. Biochem. 251: 206–209 [DOI] [PubMed] [Google Scholar]
- Wallace M. A., Liou L. L., Martins J., Clement M. H., Bailey S., et al. , 2004. Superoxide inhibits 4Fe-4S cluster enzymes involved in amino acid biosynthesis. Cross-compartment protection by CuZn-superoxide dismutase. J. Biol. Chem. 279: 32055–32062 [DOI] [PubMed] [Google Scholar]
- Wanke V., Pedruzzi I., Cameroni E., Dubouloz F., De Virgilio C., 2005. Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J. 24: 4271–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Fabrizio P., Hu J., Ge H., Cheng C., et al. , 2008. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 4: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., O’shea E. K., 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159: 1491–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., Rizvi A. H., Raser J. M., Margolin B., O’shea E. K., 2007. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 27: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Geng J., Yen W. L., Wang K., Klionsky D. J., 2010. Positive or negative roles of different cyclin-dependent kinase Pho85-cyclin complexes orchestrate induction of autophagy in Saccharomyces cerevisiae. Mol. Cell 38: 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wu J., Oliver S. G., 2009. Gis1 is required for transcriptional reprogramming of carbon metabolism and the stress response during transition into stationary phase in yeast. Microbiology 155: 1690–1698 [DOI] [PubMed] [Google Scholar]