Abstract
Mating behavior of animals is regulated by the sensory stimuli provided by the other sex. Sexually receptive females emit mating signals that can be inhibited by male ejaculate. The genetic mechanisms controlling the release of mating signals and encoding behavioral responses remain enigmatic. Here we present evidence of a Caenorhabditis elegans hermaphrodite-derived cue that stimulates male mating-response behavior and is dynamically regulated by her reproductive status. Wild-type males preferentially mated with older hermaphrodites. Increased sex appeal of older hermaphrodites was potent enough to stimulate robust response from mating-deficient pkd-2 and lov-1 polycystin mutant males. This enhanced response of pkd-2 males toward older hermaphrodites was independent of short-chain ascaroside pheromones, but was contingent on the absence of active sperm in the hermaphrodites. The improved pkd-2 male response toward spermless hermaphrodites was blocked by prior insemination or by genetic ablation of the ceh-18-dependent sperm-sensing pathway of the hermaphrodite somatic gonad. Our work suggests an interaction between sperm and the soma that has a negative but reversible effect on a hermaphrodite-derived mating cue that regulates male mating response, a phenomenon to date attributed to gonochoristic species only.
THE immense variety of reproductive strategies of metazoans underlie the diversity of their reproductive behaviors. To maximize its lifetime reproductive fitness, females of dioecious species release mating signals to attract males, and female mating receptiveness can be inhibited by male ejaculate (Gillott. 2003; Chasnov et al. 2007; Avila et al. 2011). Animals continuously adjust their sexual behavior according to internal reproductive needs and stimuli provided by the opposite sex. The repercussions of the evolution of self-fertility on mating cues and behavior output of the progeny-bearing parent have not been intensively investigated. The genetic mechanisms controlling the release of mating signals and regulating behavioral responses of the opposite sex remain enigmatic.
We used the nematode Caenorhabditis elegans as a model to investigate the genetic regulation of mating cues by the changing reproductive status of an animal. C. elegans is an androdioecious (male/hermaphrodite) species that evolved from gonochoristic (male/female) ancestors (Kiontke et al. 2004; Cutter et al. 2008). During larval development, C. elegans hermaphrodites produce limited amount of sperm that can self-fertilize oocytes. C. elegans hermaphrodites can also be cross-fertilized by males, although self-fertile hermaphrodites exhibit physical resistance to male mating (Garcia et al. 2001; Kleemann and Basolo. 2007). C. elegans males chemotax toward hermaphrodite-secreted cues and are retained by a signal in the hermaphrodite cuticle (Simon and Sternberg. 2002; White et al. 2007; Barrios et al. 2008). Once in physical proximity of a potential mate, males display response behavior, which is defined as the purposeful contact by male tail copulatory structures (Barr and Garcia. 2006). The hermaphrodite cue that regulates male contact-based mating response is not known. In this report, we present evidence of a hermaphrodite-derived cue that dynamically regulates male mating-response behavior in C. elegans.
Sperm has a profound effect on the reproductive physiology of the C. elegans hermaphrodite: it maintains gonadal morphological integrity and organization and positively regulates oocyte maturation and ovulation (McCarter et al. 1999; Miller et al. 2003; Mendenhall et al. 2011). The hermaphrodite gonad remains responsive to sperm and capable of producing cross-progeny for several days after self-sperm depletion (Mendenhall et al. 2011). We reasoned that this non-self-reproductive, but functional, state of the adult hermaphrodite gonad might influence her mating signals and affect male mating behavioral responses. To explore this idea, we compared male mating response toward wild-type sperm-containing, wild-type sperm-depleted, and reproductive mutant C. elegans hermaphrodites. We found that sperm-depleted hermaphrodites elicited preferential response from wild-type males and robust male response from mating-defective polycystin males. This hermaphrodite-derived cue for male response is negatively regulated by activated sperm and positively regulated by her somatic gonad tissues. The inhibitory effect of sperm-carrying females on male mating response was also observed in the dioecious Caenorhabditis remanei species, suggesting conservation of function. We suggest that the reproductive system of the C. elegans hermaphrodite regulates mating cue output to suppress or elicit mating and cross-fertilization by males.
Materials and Methods
Stocks
Nematodes were maintained using standard conditions (Brenner 1974). Males were isolated at L4 stage ≥24 hr prior to experiments and kept at 20–22° overnight. Hermaphrodites were isolated at L4 stage 1 or 3 days prior to experiments, labeled 1DA (days of adulthood) and 3DA, respectively. 3DA animals were replated on day 2 to avoid overcrowding from progeny. To achieve cross-insemination, C. elegans N2, fog-2, and C. remanei females were exposed to isogenic young males throughout their aging period. Nematodes were considered fertilized on the basis of the presence of embryos in the uterus.
Sperm content in spermatheca of 1DA and 3DA C. elegans N2, daf-22, fog-2, vab-1, ceh-18 prior to the response assays was inferred on the basis of the presence or absence of embryos in the uterus and confirmed by DAPI staining of age-matched hermaphrodites. Sperm content in spermatheca of 1DA and 3DA C. elegans spe-19, spe-8, and spe-38 was inferred on the basis of the DAPI staining of age-matched hermaphrodites.
Mating assay
Response efficiency (RE) reflects the percentage of a population of males successfully responding to hermaphrodite contact within 4 min. The reported n is the total number of males tested over the course of at least three tests performed on separate days. Response assay plates were prepared by dropping 13 μl of OP50 Escherichia coli culture onto a fresh NGM plate the night before assay, making a lawn of ∼5 mm diameter. Twenty hermaphrodites were placed onto the mating dot and allowed to acclimate for 10 min prior to the assay. Response was assayed as follows: five males of the one genotype were placed in the center of the mating lawn and observed for a 4-min time period, during which response (1/yes or 0/no) was recorded for each male. Response was scored as positive when a male made flush contact with the ventral side of his tail against the hermaphrodite and initiated scanning along her body. After 4 min, all males were removed from the assay plate and five males of the next genotype were assayed against the same hermaphrodites.
Statistical analysis
All the statistical analyses were performed using GraphPad Prism 5 software. To control for type I error rate, multiple group comparisons were done by Kruskal –Wallis test for nonparametric data with Dunn's multiple comparison post-hoc test.
Results
Aged hermaphrodites have more sex appeal
The first step of C. elegans male mating repertoire is response, the act of physical contact between a male tail copulatory structures and hermaphrodite body (Barr and Garcia 2006). Male response is mediated by nine bilateral pairs of tail rays, each composed of two sensory neurons (A and B types) and a structural cell (Sulston et al. 1980; Liu and Sternberg 1995). Male-specific polycystin genes lov-1 and pkd-2 are expressed in the B-type ray neurons, act nonredundantly in the same genetic pathway, and are required for efficient male response to hermaphrodite contact (Barr and Sternberg. 1999; Barr et al. 2001). The low RE of pkd-pathway mutant males is attributed to defects in sensory transduction, rather than structure, of the male cilia (Barr and Sternberg 1999; Barr et al. 2001).
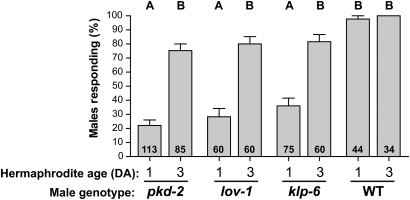
When tested with young immobilized hermaphrodites, pkd-2(sy606) male RE is 20–22% (Barr et al. 2001 and Figure 1). Strikingly, we observed that when exposed to aged hermaphrodites of the same genotype, pkd-2 male RE increases dramatically to 75% (Figure 1). Male RE of other polycystin pathway mutants, lov-1(sy582) and klp-6(my8), also significantly increased toward aged hermaphrodites (Figure 1). Because hermaphrodites in these assays were genetically immobilized unc-31(e169) mutants, we conclude that the difference in male response was not due to changes in hermaphrodites locomotory behavior, as described by Kleeman and Basolo (2007).
Figure 1 .
Male response to aged unc-31(e169) hermaphrodites. x-axis: hermaphrodite age groups (DA, days of adulthood) and male genotypes, pkd-2(sy606), lov-1(sy582), klp-6(my8), him-5(e1490). y-axis: mean ± SEM percentage of responding males out of total males tested (number within column). Letters above columns indicate results of the Kruskal–Wallis test with Dunn's Multiple Comparison post-hoc test. Columns marked with the same letter were not statistically different at P < 0.01 cutoff.
Wild-type male RE was high with either young or aged hermaphrodites, suggesting that, in the parameters of our response assay (see Materials and Methods), pkd-2 males are more sensitive to variation in hermaphrodite-derived mating cues than wild-type males (Figure 1). To determine whether wild-type males can distinguish between young and aged hermaphrodites, we developed a “choice assay,” a modification of response assay in which males are exposed to an even mixture of young and aged wild-type (N2) hermaphrodites. Of wild-type males, 66% responded to an aged hermaphrodite during the choice assay, thus showing preference for aged over young hermaphrodites (Fisher’s exact P < 0.0001, n = 125). This observation suggests that when placed in a more complex environment of choice assay, wild-type males distinguish age-dependent differences in hermaphrodite sex appeal. In conclusion, the qualitative change in aged hermaphrodites improves pkd-2 male mating behavior and may also have relevance for wild-type male behavior.
Hermaphrodite sex appeal is not dependent on short-chain ascarosides
Hermaphrodite short-chain ascarosides, glycosides of the dideoxysugar ascarylose, act as male chemoattractants at a distance (Srinivasan et al. 2008). To test whether the increased male response was dependent on ascarosides, we assayed the pkd-2 male response with daf-22(m130) hermaphrodites, which are defective in ascaroside synthesis and whose extracts have no male-attraction activity (Srinivasan et al. 2008). pkd-2 RE to daf-22 hermaphrodites was comparable to control N2 hermaphrodites in either age group (Table 1). Therefore, short-chain ascarosides are not responsible for the increased pkd-2 RE toward aged hermaphrodites.
Table 1 . pkd-2(sy606) males’ response to mutant hermaphrodites.
| H. Genotypea | H. Ageb | Male REc | nd | 1DA vs. 3DAe |
|---|---|---|---|---|
| N2 | 1DA | 16 ± 4 | 95 | P < 0.001 |
| N2 | 3DA | 65 ± 5 | 85 | P < 0.001 |
| daf-22(m130) | 1DA | 7 ± 3 | 56 | P < 0.001 |
| daf-22(m130) | 3DA | 66 ± 6 | 56 | P < 0.001 |
| fog-2(q71) | 1DA | 63 ± 4* | 133 | NS |
| fog-2(q71) | 3DA | 63 ± 6 | 72 | NS |
| spe-19(hc41) | 1DA | 56 ± 5* | 100 | NS |
| spe-19(hc41) | 3DA | 53 ± 6 | 60 | NS |
| spe-8(hc40) | 1DA | 58 ± 6* | 60 | NS |
| spe-8(hc40) | 3DA | 60 ± 6 | 60 | NS |
| spe-38(eb44) | 1DA | 8 ± 3 | 95 | NS |
| spe-38(eb44) | 3DAf | 32 ± 5* | 95 | NS |
| vab-1(dx31) | 1DA | 23 ± 6 | 60 | P < 0.001 |
| vab-1(dx31) | 3DA | 78 ± 5 | 60 | P < 0.001 |
| ceh-18(mg57) | 1DA | 23 ± 4 | 88 | NS |
| ceh-18(mg57) | 3DA | 28 ± 4* | 108 | NS |
| fog-2; ceh-18 | 1DA | 18 ± 4** | 106 |
P < 0.001 vs. same-age N2, Kruskal–Wallis test with Dunn's posthoc, **P < 0.001 vs. same-age fog-2, Kruskal–Wallis test with Dunn's posthoc.
Hermaphrodite genotype.
Hermaphrodite age; DA, days of adulthood, number of days since L4 stage.
RE, response efficiency (mean ± SE), percentage of pkd-2 males responding.
Number of observations.
Analysis of statistical significance between age groups of same genotype.
Some spe-38 hermaphrodites retained self-sperm at 3DA age.
Hermaphrodite sex appeal is inhibited by active spermatozoa
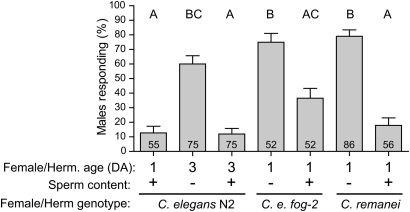
The quantity of self-sperm in a C. elegans hermaphrodite is finite. The self-reproductive period spans roughly 3–5 days following last larval molt, depending on the temperature, during which about 300 oocytes are fertilized and deposited (Maupas 1900; Argon and Ward 1980). We observed that the onset of increased pkd-2 male RE toward aging hermaphrodites temporally coincided with their self-sperm depletion (supporting information, Figure S1), which led us to the hypothesis that self-sperm was a negative regulator of hermaphrodite sex appeal during early adulthood. To test this hypothesis, we used fog-2(q71) mutants, which do not produce self-sperm and are essentially female (Schedl and Kimble 1988). Young fog-2 females elicited high RE from pkd-2 males, comparable to aged N2 (Table 1). When spermless hermaphrodites (either aged N2 or young fog-2) were inseminated prior to the response assay, pkd-2 male RE was reduced (Figure 2). We conclude that the presence of active sperm reduces hermaphrodite mating receptiveness, and the absence of sperm due to age-dependent depletion or genetic mutation triggers a physiological change in the hermaphrodite body that stimulates male response. The robust sex appeal of the spermless hermaphrodite is dynamic and can be reversed by sperm transferred from the male.
Figure 2 .
Response of pkd-2 males to virgin and fertilized partners. x-axis: hermaphrodite/female age (DA, days of adulthood), sperm status, and genotype. y-axis: mean ± SEM percentage of responding males out of total males tested (number within column). Letters above columns indicate results of the Kruskal–Wallis test with Dunn's Multiple Comparison post-hoc test. Genotypes marked with the same letter were not statistically different at P < 0.01 cutoff.
To test whether the sperm activity or the mere presence of sperm inhibits hermaphrodite sex appeal, we assayed pkd-2 male response to spe-19(hc41) mutant hermaphrodites whose spermatids fail to activate and do not form motile spermatozoa (Geldziler et al. 2005). Young spe-19 hermaphrodites enhanced pkd-2 male RE to levels similar to fog-2 females and spermless aged N2 hermaphrodites (Table 1). These results were confirmed with spe-8(hc40) mutant hermaphrodites of the same spermatid activation pathway (Table 1) (L'Hernault et al. 1988; Geldziler et al. 2005). Because young spe-19 and spe-8 hermaphrodites bearing inactive spermatids elicited significantly higher response than young N2 hermaphrodites, we conclude that spermatid activation is necessary for inhibition of mating receptiveness in young hermaphrodites.
We reasoned that reduced mating receptiveness of sperm-containing hermaphrodites might be not due to sperm, but rather due to the presence of embryos in the reproductive tract. To determine whether internal fertilization inhibits hermaphrodite sex appeal, we assayed pkd-2 RE toward spe-38(eb44) hermaphrodites whose spermatozoa fail to fertilize oocytes despite being morphologically indistinguishable from wild type (Chatterjee et al. 2005). Despite the lack of embryos in the uterus, young adult spe-38 hermaphrodites elicited low RE from pkd-2 males, comparable to RE toward wild-type N2 hermaphrodites (Table 1). Many aged spe-38 hermaphrodites retained self-sperm in the spermatheca and elicited low pkd-2 RE at an age when wild-type N2 hermaphrodites become highly attractive to pkd-2 males (Table 1). These results suggest that active sperm, but not embryos, in the reproductive system of the hermaphrodite negatively regulate her mating receptiveness to male response.
Hermaphrodite sex appeal is dependent on the CEH-18 sperm-sensing pathway
In the C. elegans hermaphrodite, sperm positively regulate oocyte maturation and ovulation by antagonizing the inhibitory signaling of parallel and partially redundant sperm-sensing pathways in the gonad represented by Eph receptor protein–tyrosine kinase VAB-1 and POU-class transcription factor CEH-18 (Greenstein et al. 1994; McCarter et al. 1999; Miller et al. 2003). To test whether sperm regulation of the hermaphrodite mating cue occurs via VAB-1 or CEH-18 sperm sensing pathways, we assayed pkd-2 RE toward vab-1(dx31) and ceh-18(mg57) hermaphrodites. Male response to vab-1 was comparable to N2 hermaphrodites of either age group, but ceh-18 failed to elicit elevated male RE at a sperm-depleted age, indicating that ceh-18 is a positive genetic regulator of the hermaphrodite attractiveness after self-sperm depletion. The dependence of the hermaphrodite mating cue on ceh-18 pathway was confirmed by the complete suppression of fog-2 attraction phenotype in fog-2;ceh-18 double mutants (Table 1). Cumulatively, the data indicate that sperm regulate the hermaphrodite mating cue at least in part via the ceh-18 sperm-sensing pathway.
Sperm regulation of the response cue is evolutionarily conserved
Caenorhabditis species may be gonochoristic (such as male–female C. remanei) as well as androdioecious (male–hermaphrodite C. elegans). To determine whether sperm-based inhibition of the response cue occurs in other Caenorhabditis species, we tested the ability of C. remanei females to elicit response from the sensitized C. elegans pkd-2 males. Similar to sperm-depleted N2 and feminized fog-2 C. elegans, young spermless C. remanei females elicited high RE from pkd-2 males; the RE reduced dramatically when C. remanei females were inseminated prior to assay (Figure 2). Hence, the mechanism of sperm-dependent negative regulation of female–hermaphrodite response cue is likely to be conserved in the Caenorhabditis genus.
Discussion
Here we show that the self-fertilizing C. elegans hermaphrodites play an active regulating role in their sexual attractiveness and male mating-response behavior. In the C. elegans literature, the term “mating cue” has been applied to other hermaphrodite-derived chemicals that act as distant chemoattractants or modulators of male locomotion (Simon and Sternberg. 2002; Srinivasan et al. 2008). It is important to distinguish cues that regulate social behaviors and development (e.g., short-chain ascarosides) from the cues that regulate physical aspects of male mating. In this report, we present evidence of a hermaphrodite cue that regulates male mating response, is correlated to hermaphrodite reproductive status, and does not depend on known sex pheromones or behavioral changes in locomotion. At this point, we cannot distinguish whether this cue is secreted by the hermaphrodite or represents a cuticle-bound modification.
The polycystin family of proteins play important and evolutionarily conserved roles in mating and fertilization. Polycystins have been shown to be required for mouse sperm competition (Sutton et al. 2008), Drosophila sperm guidance (Watnick et al. 2003; Kottgen et al. 2011), self-recognition of ascidian gametes (Harada et al. 2008), human sperm motility (Torra et al. 2008), flagella-dependent mating in Chlamydomonas (Huang et al. 2007), and C. elegans male mating behavior (Barr and Sternberg. 1999; Barr et al. 2001). Our data illustrate that in C. elegans, male-specific polycystin pathway is particularly required for male response toward sperm-containing unreceptive hermaphrodites. We speculate that, in Caenorhabditis species, polycystins may have evolved to give selective reproductive advantage to males by enabling mating with partners with low mating signals. The observed improvement of response efficiency in polycystin mutant males toward spermless hermaphrodites is an indication that hermaphrodite mating cues are perceived by males using both polycystin-dependent and polycystin-independent pathways. Activation of a polycystin-independent pathway by a cue from sperm-depleted hermaphrodite compensates for the sensory defects of polycystin pathway and increases the efficiency of male response. The identification of this pathway(s) will be a topic of future study.
We utilized pkd-2 mutant males as a sensitized background to detect and quantify hermaphrodite sex cues. The experimental need for the sensitized background was based on high response efficiency of wild-type males to all hermaphrodites tested. It is important to be aware, however, of the artificially optimal conditions of male growth and chance of hermaphrodite encounter during the response assay on a small two-dimensional bacterial lawn (see Materials and Methods). In the wild, the task of male mating is more challenging. Under native conditions, C. elegans male encounters a complex three-dimensional environment, suboptimal nutrition, exposure to infectious or toxic pathogens, and other obstacles. Hence, hermaphrodite-derived cues would be a significant variable in determining wild-type male response efficiency and, ultimately, reproductive success.
We observed that activated sperm inhibits mating cue(s) of C. elegans hermaphrodites. This negative effect is reversible and does not depend on fertilization competence of sperm, but depends on a functional sperm-sensing pathway of the ceh-18 transcription factor. In Drosophila, female mating receptiveness is inhibited by a sex peptide transferred in the male seminal fluid. Male sex peptide signals by binding to the sex peptide receptor on sensory neurons that innervate female uterus and control female reproductive behaviors (Yapici et al. 2008). In C. elegans hermaphrodites, spermatids promote oocyte ovulation and uterine contractions via secreted major sperm protein (MSP) signaling (McCarter et al. 1999; Miller et al. 2003). Importantly, MSP signaling does not depend on spermatid activation or even proper spermatocyte formation (McCarter et al. 1999), which makes MSP an unlikely candidate for the spermatid activation-dependent inhibitory cue of hermaphrodite mating attractiveness. C. elegans spermatid activation involves dramatic changes in cellular morphology and includes fusion of the membranous organelles to the plasma membrane, extending a pseudopod, and generating plasma membrane flow (Ward et al. 1981; Roberts and Ward. 1982; Arduengo et al. 1998). Determination of the molecular mechanisms by which activated sperm inhibits C. elegans hermaphrodite mating cue(s) awaits further studies.
The goal of mating behavior is to maximize the lifetime reproductive success of an individual. What may be the advantage of producing positive mating cues by a self-fertile hermaphrodite? The ancestral Caenorhabditis species was gonochoristic, like most of the known Caenorhabditis species that rely on sex pheromones to attract opposite-sex partners (Chasnov et al. 2007). C. elegans evolved the ability to make self-sperm, which could relax the selective pressures on genes for mating behaviors. For example, Garcia et al. (2007) demonstrated that C. remanei females, but not C. elegans hermaphrodites, are sensitive to the soporific-inducing factor of males. However, sperm remains the limiting factor of lifetime reproductive fitness of the C. elegans hermaphrodite, as illustrated by the fact that a mated hermaphrodite can produce up to 1400 progeny as compared to ∼300 of self-progeny (Kimble and Ward. 1988). We propose that the C. elegans hermaphrodite retained male attraction ability of the ancestral gonochoristic Caenorhabditis and that genes regulating C. elegans mating cues may be under positive selection due to the longer lifetime reproductive fitness of a fertilized hermaphrodite over an unfertilized one. In addition, the ability of sperm-depleted hermaphrodites (effectively females) to elicit preferential male mating response in a mixed population may give them selective reproductive advantage over younger, sperm-containing hermaphrodites.
Why don’t C. elegans hermaphrodites release the mating cue in early adulthood? Male sperm outcompete hermaphrodite self-sperm in fertilization of oocytes (Ward and Carrel. 1979). This fertilization superiority of male sperm makes it disadvantageous for the hermaphrodite to mate while in a self-fertile stage. The inhibitory effect of the self-sperm on hermaphrodite attractiveness may act to minimize her likelihood of cross-fertilization by males. For the male, however, it is advantageous to mate with a hermaphrodite as soon as she is sexually mature. Consequently, this may create an overt sexual conflict over the timing of mating, which may drive evolutionary adaptations in C. elegans to manipulate hermaphrodite mating cues in order to increase self-reproduction. In summary, our results illustrate how males and hermaphrodites adopt different strategies to maximize their reproductive success under the condition of conflicting sexual interests.
Acknowledgments
We thank Drs. Rene Garcia, Judith Kimble, Jody Hey, Andrew Singson, and Indrani Chatterjee for stimulating discussions, experimental suggestions, and comments on the manuscript. We also thank Dr. Nathan Schroeder for advice on statistical analysis. Nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources (NCRR), and the Singson lab (Rutgers University, New Brunswick). This work was funded by NIH grant DK059418 (to M.M.B.) and Rutgers University summer undergraduate research fellowships (to L.H.).
Literature Cited
- Arduengo P. M., Appleberry O. K., Chuang P., L 'Hernault S. W., 1998. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell Sci. 111(24): 3645–3654 [DOI] [PubMed] [Google Scholar]
- Argon Y., Ward S., 1980. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics 96: 413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila F. W., Sirot L. K., LaFlamme B. A., Rubinstein C. D., Wolfner M. F., 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56: 21–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M. M., Garcia L. R., 2006. Male mating behavior. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.78.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Barr M. M., Sternberg P. W., 1999. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401: 386–389 [DOI] [PubMed] [Google Scholar]
- Barr M. M., DeModena J., Braun D., Nguyen C. Q., Hall D. H., et al. , 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11: 1341–1346 [DOI] [PubMed] [Google Scholar]
- Barrios A., Nurrish S., Emmons S. W., 2008. Sensory regulation of C. elegans male mate-searching behavior. Curr. Biol. 18: 1865–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnov J. R., So W. K., Chan C. M., Chow K. L., 2007. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc. Natl. Acad. Sci. USA 104: 6730–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee I., Richmond A., Putiri E., Shakes D. C., Singson A., 2005. The Caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development 132: 2795–2808 [DOI] [PubMed] [Google Scholar]
- Cutter A. D., Wasmuth J. D., Washington N. L., 2008. Patterns of molecular evolution in Caenorhabditis preclude ancient origins of selfing. Genetics 178: 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. R., Mehta P., Sternberg P. W., 2001. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell 107: 777–788 [DOI] [PubMed] [Google Scholar]
- Garcia L. R., LeBoeuf B., Koo P., 2007. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics 175: 1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldziler B., Chatterjee I., Singson A., 2005. The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev. Biol. 283: 424–436 [DOI] [PubMed] [Google Scholar]
- Gillott C., 2003. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48: 163–184 [DOI] [PubMed] [Google Scholar]
- Greenstein D., Hird S., Plasterk R. H., Andachi Y., Kohara Y., et al. , 1994. Targeted mutations in the Caenorhabditis elegans POU homeo box gene ceh-18 cause defects in oocyte cell cycle arrest, gonad migration, and epidermal differentiation. Genes Dev. 8: 1935–1948 [DOI] [PubMed] [Google Scholar]
- Harada Y., Takagaki Y., Sunagawa M., Saito T., Yamada L., et al. , 2008. Mechanism of self-sterility in a hermaphroditic chordate. Science 320: 548–550 [DOI] [PubMed] [Google Scholar]
- Huang K., Diener D. R., Mitchell A., Pazour G. J., Witman G. B., et al. , 2007. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179: 501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Ward S., 1988. Germ-line development and fertilization, pp. 191–213 : The Nematode Caenorhabditis elegans, edited by Wood W. B. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Kiontke K., Gavin N. P., Raynes Y., Roehrig C., Piano F., et al. , 2004. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc. Natl. Acad. Sci. USA 101: 9003–9008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann G. A., Basolo A. L., 2007. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Anim. Behav. 74: 1339–1347 [Google Scholar]
- Kottgen M., Hofherr A., Li W., Chu K., Cook S., et al. , 2011. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS ONE 6: e20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L 'Hernault S. W., Shakes D. C., Ward S., 1988. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 120: 435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. S., Sternberg P. W., 1995. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14: 79–89 [DOI] [PubMed] [Google Scholar]
- Maupas E., 1900. Modes et formes de reproduction des nématodes. Arch. Zool. Exp. Gen. 8: 463–624 [Google Scholar]
- McCarter J., Bartlett B., Dang T., Schedl T., 1999. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205: 111–128 [DOI] [PubMed] [Google Scholar]
- Mendenhall A. R., Wu D., Park S. K., Cypser J. R., Tedesco P. M., et al. , 2011. Genetic dissection of late-life fertility in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 66: 842–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Ruest P. J., Kosinski M., Hanks S. K., Greenstein D., 2003. An eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 17: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Ward S., 1982. Membrane flow during nematode spermiogenesis. J. Cell Biol. 92: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T., Kimble J., 1988. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119: 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. M., Sternberg P. W., 2002. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 1598–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J., Kaplan F., Ajredini R., Zachariah C., Alborn H. T., et al. , 2008. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454: 1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Albertson D. G., Thomson J. N., 1980. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev. Biol. 78: 542–576 [DOI] [PubMed] [Google Scholar]
- Sutton K. A., Jungnickel M. K., Florman H. M., 2008. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl. Acad. Sci. USA 105: 8661–8666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torra R., Sarquella J., Calabia J., Marti J., Ars E., et al. , 2008. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 3: 790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Carrel J. S., 1979. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev. Biol. 73: 304–321 [DOI] [PubMed] [Google Scholar]
- Ward S., Argon Y., Nelson G. A., 1981. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J. Cell Biol. 91: 26–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick T. J., Jin Y., Matunis E., Kernan M. J., Montell C., 2003. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 13: 2179–2184 [DOI] [PubMed] [Google Scholar]
- White J. Q., Nicholas T. J., Gritton J., Truong L., Davidson E. R., et al. , 2007. The sensory circuitry for sexual attraction in C. elegans males. Curr. Biol. 17: 1847–1857 [DOI] [PubMed] [Google Scholar]
- Yapici N., Kim Y. J., Ribeiro C., Dickson B. J., 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451: 33–37 [DOI] [PubMed] [Google Scholar]